Abstract
Aluminum is usually regarded as the determining factor for plant growth in acid soils and nutrient deficiencies are often additional growth-limiting factors in tropical acid soils. Taking into account the potential interactions between Al toxicity and nutrient deficiencies, the present study investigated sorghum (Sorghum bicolor Moench [L.]) and maize (Zea mays L.) cultivar differences for: (1) Al tolerance (relative growth in a one-fifth strength nutrient solution [low-nutrient medium, ionic strength: 4.5 mmol L−1] with Al and without Al), (2) low-nutrient tolerance (relative growth in a low-nutrient medium compared with growth in a full-strength nutrient solution), (3) combined tolerance (relative growth in a low-nutrient medium containing Al compared with a full-strength medium lacking Al). The goal of the present study was to identify the predominant growth-limiting factor using a solution culture medium that simulates the nutrient status of tropical acid soils. Differential Al tolerance among 15 cultivars of sorghum and 10 cultivars of maize in short-term assays (2.5 or 20 µmol L−1 AlCl3 in 0.2 mmol L−1 CaCl2 at pH 5.0 or 4.9, respectively, for 24 h) was positively correlated with Al tolerance in long-term cultures (11.1 or 42.6 µmol L−1 soluble Al in the low-nutrient medium at pH 4.5 or 4.3, respectively, for 29 days). However, the level of Al tolerance in the short-term assays was not correlated with the combined tolerance, suggesting that a short-term screening technique may not be practically useful for estimating cultivar adaptation to a combination of stress factors in tropical acid soils. In sorghum, a less Al-tolerant plant species, higher Al tolerance was associated with less Al absorption by the roots and greater K translocation into the shoots. In maize, a more Al-tolerant plant species, there was no correlation between the accumulation or transport of elements and Al tolerance. Standardized partial regression coefficients suggested that low-nutrient tolerance contributed more to combined tolerance than Al tolerance under most conditions (except for Al-sensitive sorghum at 42.6 µmol L−1 AlCl3). A greater combined tolerance was associated with a higher K shoot concentration in sorghum and a higher Ca shoot level in maize. Plant nutritional characteristics linked to low-nutrient tolerance should be evaluated as an important strategy for plant production in tropical acid soils, both for Al-tolerant plant species and for Al-sensitive plant species under low-Al conditions.
INTRODUCTION
There are many causes for the poor growth of plants in acid soils. The common and primary stress factors are: (1) H+ toxicity/low pH, (2) Al and Mn toxicities, (3) a deficiency of essential nutrients (N, P, K, Ca, Mg, Mo and B) (CitationRao 2001; CitationRao et al. 1993). The major problems of acid soils in South American Savannas are the low content of cations, the toxicity of exchangeable Al and/or soluble Al and low levels of phosphorus and low silicon availability as a result of extensive weathering (CitationOkada and Fischer 2001; CitationRao 2001; CitationRao et al. 1993). Many studies have focused on the identification of major factors that cause a decrease in plant production in solution culturing with or without Al and its related mechanisms (CitationOfei-Manu et al. 2001; CitationPavan et al. 1982; CitationPintro and Taylor 2004; CitationWright et al. 1989). However, the number of studies that have simultaneously considered the two major factors (high Al and low nutrients) in tropical acid soils is limited (CitationWenzl et al. 2003). In high-nutrient solution, Al toxicity is alleviated by a physicochemical interaction between Al and other ions, the formation of non-toxic complexes with OH− and SO2− 4, and the precipitation of Al phosphates from high ionic strength solutions (CitationBlamey et al. 1983, Citation1991; CitationWheeler and Edmeads 1995). The ionic strength of Savanna soil solutions varies from 1.3 to 1.7 mmol L−1 for unfertilized samples and increases to 5.4–13.4 mmol L−1 after fertilization (CitationWenzl et al. 2003). CitationWenzl et al. (2001) found that root growth in Brachiaria ruziziensis, a relatively Al-sensitive grass, was reduced in a solution containing toxic concentrations of Al and a low amount of nutrients compared with Brachiaria decumbens. An inadequate supply of nutrients may be one of the main factors that contributes to the poor persistence of B. ruziziensis in infertile acid soils. Aluminum activities in a solution of tropical acid soil ranged from 2.26 to 196.5 µmol L−1 (CitationPintro et al. 1999). CitationBlamey et al. (1991) reported that realistic root growth inhibition of Lotus could be obtained from a low ionic strength solution and a high Al concentration at similar levels to those in acid soils. CitationWatanabe and Okada (2005) investigated the difference in Al tolerance between Indica and Japonica cultivars under low ionic strength conditions. To the best of our knowledge, no comprehensive studies on the identification of the primary inhibitory factor for plant production have been reported. Specifically, clarification of plant nutritional characteristics for better plant production using two plant species each composed of a wide variety of cultivars has not been reported. We aimed to examine different Al tolerances and plant nutritional characteristics using a solution culture that simulates the nutrient status and concentration of soluble Al in tropical acid soils. The proposal of a method to isolate the primary factor from complicated tropical acid soil factors is urgent. In the present study, we report a greater contribution of low-nutrient tolerance and related plant nutritional characteristics for the improved production of two gramineous plant species, sorghum (Sorghum bicolor Moench [L.]) and maize (Zea mays L.), under low-nutrient and high-Al experimental conditions.
MATERIALS AND METHODS
Source of the seeds and seed germination
For sorghum, the following cultivars were used: Lucky-1, King, Takii, Toumitsu-1, Lucky-2 and Meter (Takii Seeds, Kyoto, Japan); Sudax-1, Super sugar, Hybrid-1, Little, Sudax-306 and Kaneko (Kaneko Seeds, Gunma, Japan); Koutoubun, Hazuki and Green (Snow Brand Seeds, Tokyo, Japan). For maize, the following cultivars were used: Royaldent TX115, Royaldent-110, Royaldent TH472 (Takii Seeds); Golddent KD500, Golddent KD850, Golddent KD777, Golddent KD459, Golddent KD670, Golddent KD620 and Golddent KD520 (Kaneko Seeds). Seeds were soaked in tap water under aeration for 24 h at 27°C in a growth room and germinated under fluorescent white light (80.7 µmol m−2 s−1), spread on a nylon screen and placed on a container that was filled with 9 L of tap water. The tap water contained 8.0, 2.92 and 1.95 mg L−1 of Ca, Mg and K, respectively. Temperature, light intensity and aeration were unchanged throughout the experiment.
Screening for short-term aluminum tolerance
Seedlings with roots 3–4 cm in length were selected and treated with 0.2 mmol L−1 CaCl2 for 6 h (pH 5.0 or 4.9 for low or high Al, respectively). After measuring the root length of the longest root with a ruler, the roots of the seedlings were transferred to a 0.2 mmol L−1 CaCl2 solution (control treatment, pH 5.0 or 4.9 for low or high Al, respectively) and either a 2.5 µmol L−1 (low Al) or 20 µmol L−1 (high Al) AlCl3 solution containing 0.2 mmol L−1 CaCl2 (Al treatment). The pH of the solutions was adjusted to 5.0 or 4.9 for low Al or high Al, respectively. At least 10 seedlings in the control and in each of the Al treatments were used for the short-term screening experiment. After 24 h the root length of the longest root for each seedling was re-measured.
Culturing and treatments in the long-term experiment
The elemental composition and pH of the long-term culturing medium is shown in . All seeds were soaked and spread on a nylon screen for germination in the same way as the short-term experiment. Just after germination, the seedlings were transferred to a glasshouse for preculturing in tap water for 5 days. Seedlings of equal size were selected and transplanted into 40 L of low-nutrient (LN) solution (one-fifth strength of the full-nutrient [FN] solution) for 2 days. Thereafter, all seedlings were cultured with daily pH maintenance for 29 days as follows: (1) Control (FN, pH 5.2), (2) FN under low-Al conditions (11.1 µmol L−1 soluble Al, pH 4.5), (3) FN under high-Al conditions (42.6 µmol L−1 soluble Al, pH 4.3), (4) LN (pH 5.2), (5) LN under low-Al conditions (pH 4.5), (6) LN under high-Al conditions (pH 4.3). Ionic strength for the FN solution was calculated as 22.6 mmol L−1 based on the method of CitationWada and Seki (1994). Because P and Al co-precipitate, their soluble concentration in the solution was measured as follows. After mixing 35 µmol L−1 AlCl3 with a culture solution containing 55 µmol L−1 NaH2PO4 at pH 4.5 or mixing 370 µmol L−1 AlCl3 with a culture solution containing 230 µmol L−1 NaH2PO4 at pH 4.3 with frequent pH adjustment for 1 day, culture solutions were collected just after the pH adjustment and filtered through a membrane filter (0.2 µm in pore size), after which the soluble concentrations of Al and P were measured. Finally, the mean concentrations of 11.1 or 42.6 µmol L−1 soluble Al and 5 µmol L−1 soluble P were obtained. Phosphorus was measured colorimetrically using the molybdenum blue method (CitationJackson 1958) and a spectrophotometer (U-2900; Hitachi, Japan) at 660 nm and Al was measured using an inductively coupled plasma-atomic emission spectrophotometer (ICP-AES) (Varian-2000; Varian Australia, Melbourne, Australia). In the long-term culture, the pH was adjusted daily and the culture solution was collected just after pH adjustment and the soluble concentration of P was measured at this time. When the solution P concentration was below the required level (5 µmol L−1), the requisite amount of P was added to maintain the recommended concentration as shown in and this daily adjustment of the soluble concentration of P was continued throughout the experimental period. In another experiment, the elements in the culture solutions were measured after the fourth week of treatment by ICP-AES and elemental decrease was negligible (the decrease was within 3–11% for all major nutrients [K, Ca, Mg, Fe and Mn] in all treatments). The culture solutions were renewed weekly in the first 3 weeks of culturing and then every 5 days after that to maintain the nutritional demand of the growing seedlings. The water culturing was replicated three times and there were two seedlings of each cultivar in each container. At harvest three seedlings of similar size were selected, separated into shoots and roots, thoroughly washed and dried for 3 days at 70°C in a draft oven and weighed.
Table 1 Elemental composition (mmol L−1 or µmol L−1) and pH of the long-term solution culture medium
Calculation of the tolerances
The stress tolerances of the respective crops were calculated as the percentage relative growth with respect to the plant dry weight, that is,
Analysis of the elements in the plant samples
Homogenized dried shoot and root samples were digested using an electric digestion apparatus (Fujiwara, Japan) at 200 W with an acid mixture (HNO3:60% HClO4 = 5:3 v/v). The concentrations of each element in the plant samples were measured by flame (K, Ca, Mg, Fe and Mn) or flameless (Al) atomic absorption spectrophotometry (Zeeman 5000; Hitachi) in 1,000 p.p.m. La.
Calculation of the ionic activities
The ionic activities of Al were calculated using a computer program developed by CitationWada and Seki (1994).
Statistical analysis
Significant differences among the cultivars in the short-term experiment were determined using Fisher's least significant difference (LSD) test (CitationFisher 1958). Significant correlations were tested using simple linear correlation coefficients. To compare the influence of the explanatory variable on the dependent variable, we calculated standardized partial regression coefficients using the STB option in the REG procedure of SAS (CitationSAS Institute 1988).
RESULTS
Differential aluminum tolerances among sorghum and maize cultivars under short-term screening conditions
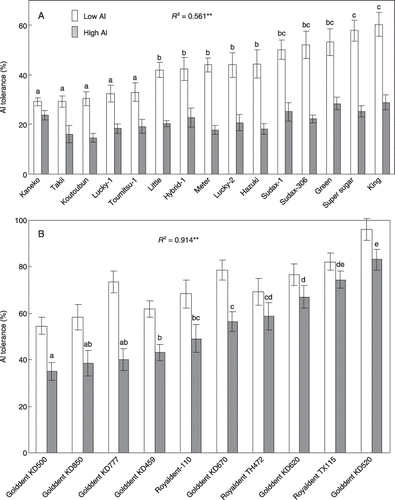
The mean Al tolerance among all sorghum cultivars in the short-term experiment under low-Al conditions was higher (43%) than that under high-Al conditions (21%) (). Cultivars Super sugar and King were the most tolerant and cultivars Kaneko and Takii were the most sensitive to Al under low-Al conditions (57.8, 60.3, 29.2 and 29.2%, respectively). For maize the mean Al tolerance in the short-term under low-Al conditions was 71%, and the tolerance under high-Al conditions was 55% (). Among the maize cultivars, Golddent KD520 was the most tolerant and cultivar Golddent KD500 was the most sensitive to Al (83.1 and 39.4% in the high-Al conditions, respectively).
Aluminum tolerance under low-Al conditions was positively correlated with that under high-Al conditions for both plant species, that is, differential Al tolerance among cultivars was the same irrespective of the Al concentration in the media for both plant species (R 2 = 0.561** [**P < 0.001] for sorghum and R 2 = 0.914** for maize). Larger variations in Al tolerance were observed under low-Al conditions for sorghum (29.2–60.3%) and under high-Al conditions for maize (34.9–83.1%).
Aluminum tolerance, low-nutrient tolerance and combined tolerance among sorghum and maize cultivars under long-term culturing conditions
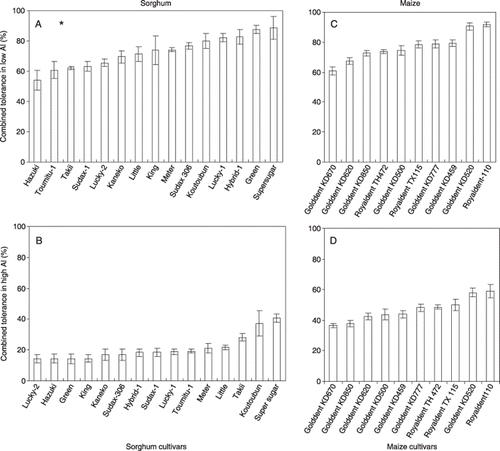
In low-Al conditions the combined tolerance for sorghum shoots was in the range of 54.0–88.8% and the mean value was 72.8% and under high Al conditions (sorghum whole plant) tolerances ranged from 14.2 to 40.7% with a mean value of 21% (). For maize (whole plant) under low-Al conditions the combined tolerance ranged from 60.8 to 91.7% with a mean value of 76.8% and under high-Al conditions tolerances ranged from 36.5 to 59.1% with a mean value of 46.9% ().
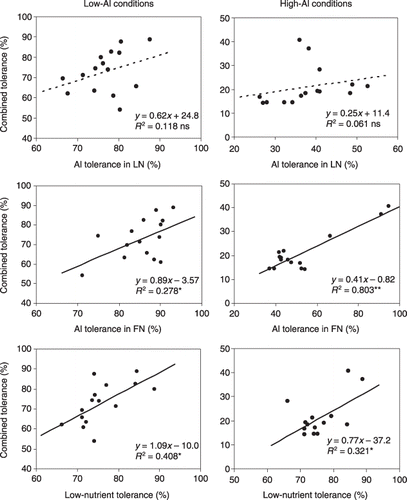
Relationships for each tolerance with combined tolerance for sorghum are indicated in . For sorghum (whole plant) in low-Al conditions, Al tolerance in the LN ranged from 66.5 to 87.6% with a mean value of 76.9%. Aluminum tolerance in the FN condition ranged from 71.2 to 93.2% with a mean value of 85.5%. For the whole plant, LN tolerance ranged from 66.2 to 88.8% and the mean value was 75.9%. The combined tolerance was positively correlated with the Al tolerance in the FN condition (R 2 = 0.278* [*P < 0.05]) and with LN tolerance (R 2 = 0.408*). In high-Al conditions, Al tolerance in the LN condition ranged from 26.3 to 52.6% and the mean value was 37.9%. Aluminum tolerance in the FN condition ranged from 37.2 to 94.6% with a mean value of 53.0%. The combined tolerance was positively correlated with Al tolerance in the FN condition (R 2 = 0.803**) and with LN tolerance (R 2 = 0.321*). However, Al tolerance in LN conditions (in both low and high Al) did not show any relationship with combined tolerance. ().
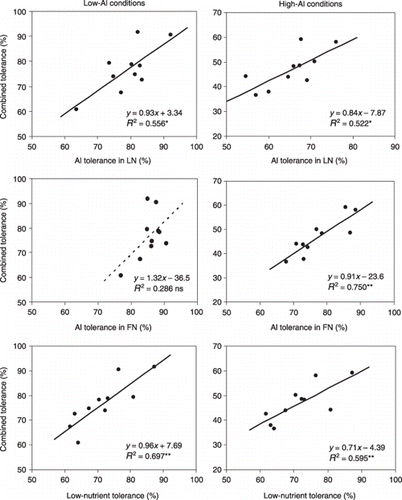
Relationships for each tolerance with combined tolerance for maize are indicated in . In maize low-Al conditions, Al tolerance under LN conditions ranged from 63.6 to 92.2% and the mean value was 79.1%. Aluminum tolerance in the FN condition ranged from 76.9 to 90.6% with a mean value of 85.7%. The LN tolerance ranged from 61.7 to 87.2% with a mean value of 71.7%. The combined tolerance was positively correlated with Al tolerance in the LN condition (R 2 = 0.556*) and with LN tolerance (R 2 = 0.697**). In high-Al conditions, Al tolerance in LN ranged from 57.0 to 76.0% with a mean value of 65.4%. Aluminum tolerance in the FN condition ranged from 67.9 to 88.6% with a mean value of 77.5%. The combined tolerance was positively correlated with Al tolerance in the LN condition (R 2 = 0.522*) and with LN tolerance (R 2 = 0.595**) ().
Changes in growth and nutritional status after long-term culturing with a low-nutrient solution or with aluminum
Focusing on the characteristic nutritional aspect after lowering the nutrient concentration of the medium and adding Al (to simulate tropical acid soils), the remarkable results in are as follows: LN decreased considerably the P and Mg concentrations in the shoots of both plant species, and for maize LN considerably decreased the concentration of K in both plant parts. In the roots, LN decreased considerably the concentrations of P, K and Fe in both plant species. In both plant parts for both plant species the considerable decrease in P under FN conditions with both concentrations of Al was considered to result from the lower concentration of soluble P in the media.
Figure 1 Short-term Al tolerance for cultivars of (A) sorghum and (B) maize. Low Al, 2.5 µmol L−1 AlCl3 in 0.2 mmol L−1 CaCl2 for 24 h (pH 5.0); high Al, 20 µmol L−1 AlCl3 in 0.2 mmol L−1 CaCl2 for 24 h (pH 4.9). The Al tolerance is expressed as the net root elongation of the longest root in the Al treatment/net root elongation of the control. Data are mean ± standard error (n ≥ 10). Mean values with the same letter(s) are not significantly different at P < 0.05 (Fisher's least significant difference test). *P < 0.05; **P < 0.01. The R2 value is the determination coefficient between Al tolerance in low Al and in high Al.
Compared with the results after Al addition to FN, Al addition to LN further decreased the dry weight (DW) and concentration of K in both plant parts and the concentration of Ca in the roots of sorghum; for maize it further decreased the concentrations of Fe and Ca in the shoots and the concentration of K in the roots.
Figure 2 Combined tolerances (%) of (A,B) sorghum and (C,D) maize in (A,C) low-Al and (B,D) high-Al conditions. Tolerances were calculated based on the dry weight after culturing in different treatment solutions as shown in Table 1 for 29 days with a daily pH adjustment. The combined tolerance was defined as the relative dry weight in the low-nutrient solution with Al (LN + Al) to that in the full nutrient (FN) solution. All tolerance values were calculated for the whole plant. Asterisk indicates the combined tolerance of sorghum under low-Al conditions for the shoot part only.
Relationship between aluminum tolerance and elemental characteristics
For sorghum, the Al in the roots was negatively correlated with the Al tolerance in the LN treatment under high-Al conditions (R 2 = 0.278*) (). In maize, however, no such correlation was observed (data not shown). For sorghum, shoot K was positively correlated with Al tolerance in the LN treatment under high-Al conditions (R 2 = 0.284*) (). No other significant correlations were observed between nutrient status and Al tolerance for sorghum (data not shown). For maize, no correlations were observed between nutrient status and Al tolerance. In addition, no correlations were observed between nutrient status in LN conditions and low nutrient tolerance in either plant species (data not shown).
DISCUSSION
Maize (Z. mays) is the third most important cereal crop grown in the world (CitationBaligar and Fageria 1997). In South and Central America, maize is mostly grown in acidic soils where yields are limited by deficient levels of available P, Ca and Mg and toxic levels of Al and Mn (CitationBaligar and Fageria 1997). Sorghum (S. bicolor) is the fifth most important cereal crop in the world (CitationSere and Estrada 1987). In South America, sorgham is grown mainly in acidic soils (4.6 million hectares) and sorghum production in South America is limited by deficient levels of available P, Ca, Mg and micronutrients as well as toxic levels of Al and Mn (CitationSere and Estrada 1987).
Figure 3 Relationship for Al tolerance in the low-nutrient (LN) solution, Al tolerance in the full nutrient (FN) solution and low-nutrient tolerance with combined tolerance for sorghum under low-Al and high-Al conditions. Tolerances were calculated based on the dry weight after culturing in different treatment solutions as shown in Table 1 for 29 days with a daily pH adjustment. Combined tolerance has been defined in Fig. 2. Al tolerance in LN, relative dry weight in LN + Al to that in LN; Al tolerance in FN, relative dry weight in FN + Al to that in FN; LN tolerance, relative dry weight in LN to that in FN. Dotted lines indicate non-significant relationships. ns, not significant; *P < 0.05; **P < 0.01.
We initially screened for short-term Al tolerance using 15 cultivars of sorghum and 10 cultivars of maize. A wide range of Al tolerance was observed among the sorghum and maize cultivars (). We also investigated long-term Al tolerance in the presence of all nutrients using the same cultivars for both plant species. In sorghum, the relationship between long-term and short-term Al tolerance was R 2 = 0.267* for the whole plant under low-nutrient and low-Al conditions (). For maize, the relationship between long-term and short-term Al tolerance was R 2 = 0.462* for the whole plant under low-nutrient and high-Al conditions (). This suggests that the short-term (24 h) screening technique for Al tolerance may be useful for estimating Al tolerance in long-term culturing with nutrients.
Figure 4 Relationship for Al tolerance in the low-nutrient (LN) solution, Al tolerance in the full nutrient (FN) solution and low-nutrient tolerance with combined tolerance for maize under low-Al and high-Al conditions. Treatments, materials and definitions are the same as Fig. 3. All tolerance values were calculated for the whole plant. Dotted line indicates non-significant relationship. ns, not significant; *P < 0.05; **P < 0.01.
No correlations were found between short-term Al tolerance and the combined tolerance: R 2 = 0.106 and 0.002 for high-Al and low-Al conditions, respectively, for sorghum. For the whole maize plant in high-Al and low-Al conditions, R 2 = 0.172 and 0.035, respectively (data not shown). Although investigations based on similar short-term screening techniques have been reported (CitationIshikawa et al. 2001; CitationKhan et al. 2008; CitationKobayashi et al. 2004; CitationMa et al. 2002; CitationWagatsuma et al. 2005; CitationYou et al. 2005), our results suggest that a short-term screening technique may not be practically useful for estimating cultivar adaptation to the combination of stress factors found in tropical acid soils.
The Al in the nutrient solutions decreased the DW and also decreased the concentrations of all of the nutrients measured (P, K, Ca, Mg, Fe and Mn) in sorghum more than maize (). A less inhibitory effect of Al on the DW in maize indicates greater Al tolerance for maize compared with sorghum. The activity of all Al ion species (![]() ) in the low-nutrient medium was higher than that in the full-nutrient medium as determined by the calculation of CitationWada and Seki (1994). The equation for the regression line of the mean Al concentration in the roots of all cultivars (µg g−1[y]) and the activity of the Al ions in the medium (x) for sorghum under four different medium conditions, that is, FN + low Al, FN + high Al, LN + low Al and LN + high Al, was y = 159x − 303 (R
2 = 0.982**) (Al concentrations ranged between 402 and 4359 µg g−1). The equation for the regression line between the above two factors for maize was y = 66.7x − 40 (R
2 = 0.965*) (Al concentrations ranged between 202 and 1905 µg g−1) (). Comparing each slope of the regression line for sorghum and maize, that is, 159 and 66.7, the absorbability of the Al ions in sorghum roots was estimated to be more than twice that of maize roots. Together with the negative correlation between Al tolerance and Al in the roots (R
2 = 0.278*; ), the lower level of Al tolerance for sorghum results from the greater absorbability of Al ions by roots. This negative correlation between Al tolerance and Al concentrations agrees with previous reports (CitationOfei-Manu et al. 2001; CitationPiñeros et al. 2005; CitationWagatsuma et al. 1995).
) in the low-nutrient medium was higher than that in the full-nutrient medium as determined by the calculation of CitationWada and Seki (1994). The equation for the regression line of the mean Al concentration in the roots of all cultivars (µg g−1[y]) and the activity of the Al ions in the medium (x) for sorghum under four different medium conditions, that is, FN + low Al, FN + high Al, LN + low Al and LN + high Al, was y = 159x − 303 (R
2 = 0.982**) (Al concentrations ranged between 402 and 4359 µg g−1). The equation for the regression line between the above two factors for maize was y = 66.7x − 40 (R
2 = 0.965*) (Al concentrations ranged between 202 and 1905 µg g−1) (). Comparing each slope of the regression line for sorghum and maize, that is, 159 and 66.7, the absorbability of the Al ions in sorghum roots was estimated to be more than twice that of maize roots. Together with the negative correlation between Al tolerance and Al in the roots (R
2 = 0.278*; ), the lower level of Al tolerance for sorghum results from the greater absorbability of Al ions by roots. This negative correlation between Al tolerance and Al concentrations agrees with previous reports (CitationOfei-Manu et al. 2001; CitationPiñeros et al. 2005; CitationWagatsuma et al. 1995).
Figure 5 Relative and average values of the dry weight and mineral concentration for (A) 15 cultivars of sorghum and (B) 10 cultivars of maize grown in long-term culturing. Low nutrient (LN) or full nutrient (FN) solution without Al(○), dry weight or mineral concentration in LN (% of FN)(□), LN under low Al conditions (% of LN)(•), LN under high Al conditions (% of LN)(▴), FN under low Al conditions (% of FN)(○), FN under high Al conditions (% of FN)(▵).
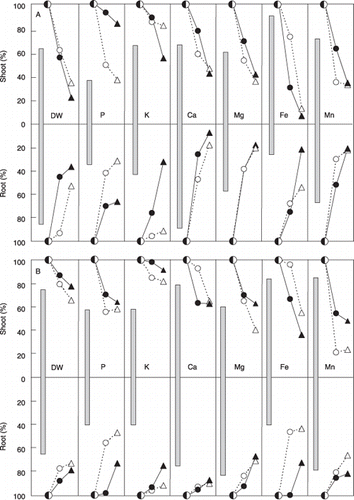
Figure 6 (A) Relationship between Al tolerance in the low-nutrient (LN) solution and Al concentration in the roots or (B) between Al tolerance and K concentration in the shoots for sorghum in long-term culturing. Al tolerance in LN was calculated as the ratio of growth in LN under high-Al conditions to that in LN. *P < 0.05.
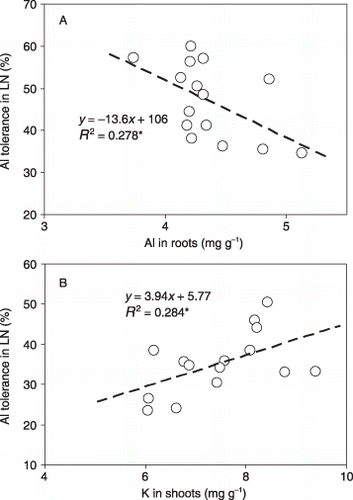
A positive correlation was also observed between Al tolerance and K in sorghum shoots (). No correlations were, however, observed between Al tolerance and all other nutrients for sorghum or between Al tolerance and all elements, including Al, for maize (data not shown). These results suggest the possible significance of higher K absorption/translocation for better growth of sorghum in the medium with Al.
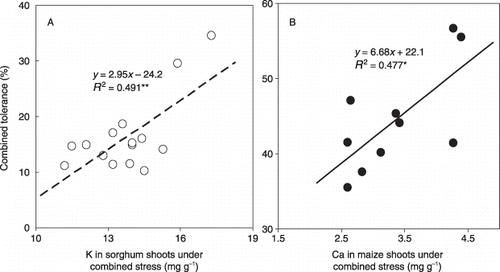
In all experimental conditions, the combined tolerance was significantly correlated both with Al tolerance and low-nutrient tolerance for both plant species (,,; ). To evaluate the relative importance of these two factors on the combined tolerance we conducted a multiple regression analysis and further calculated a standardized partial regression equation (to treat both factors equally) among the combined tolerance, Al tolerance and low-nutrient tolerance (). Although we did not find any correlation of combined tolerance for sorghum whole plants in the low-Al condition, the shoots (i.e. the harvested plant parts) showed a relationship with the other tolerances that constituted almost three-quarters of the whole plant. A greater contribution of low-nutrient tolerance than Al tolerance under most conditions was evident (0.46 > 0.39 for sorghum under low-Al conditions, 0.69 > 0.57 for maize under low-Al conditions and 0.69 > 0.64 for maize under high-Al conditions). The exception was for the Al-sensitive sorghum under high-Al conditions (0.67 > 0.02). From these findings it could be suggested that Al tolerance for low Al is more important for sorghum and that Al tolerance for high Al is more important for maize, depending on the relative tolerance to Al of each plant species (; ). Although similar results have already been reported (CitationOkada and Fischer 2001; CitationWenzl et al. 2003), our finding can be considered definitive evidence because it is based on a more comprehensive experiment. In sorghum, the combined tolerance was positively correlated with the tissue concentration of K under combined stress conditions (R 2 = 0.491*; A). In maize, the same correlation was recognized between the combined tolerance and the tissue concentration of Ca in shoots under combined stress conditions (R 2 = 0.477*; B). No correlations were observed between the combined tolerance and any other nutrients, including Al (data not shown). A greater potential for K translocation in sorghum and Ca translocation in maize is suggested as a strategy for better plant production in tropical acid soils.
Figure 7 Relationship between short-term Al tolerance and long-term Al tolerance in (A) sorghum and (B) maize. The Al tolerance for sorghum is under low-Al conditions and the Al tolerance for maize is under high-Al conditions. *P < 0.05.
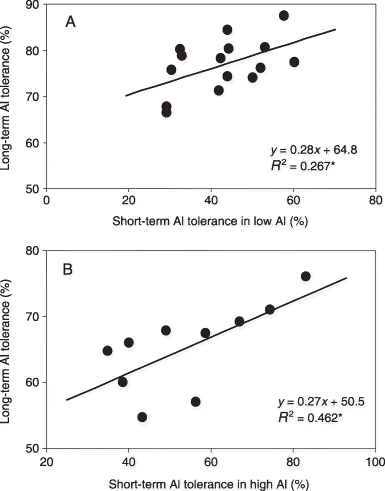
Figure 8 Relationship between the activity of the whole Al ion species in the medium and the mean Al concentrations in the roots for all sorghum cultivars (open symbols) and all maize cultivars (closed symbols). ○, •, FN + low Al; ⋄, ♦, FN + high Al; ▵, ▴, LN + low Al; □, ▪, LN + high Al. Values are mean ± standard deviation (n = 3). *P < 0.05; **P < 0.01.
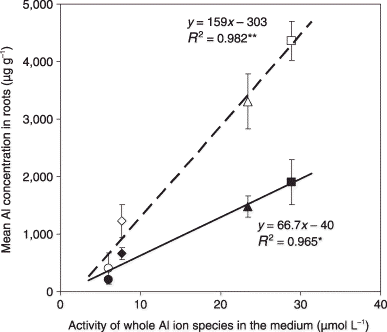
Figure 9 Relationship between combined tolerance and (A) K concentration for sorghum shoots and (B) Ca concentration for maize shoots in low-nutrient (LN) solution under high-Al conditions. *P < 0.05; **P < 0.01.
Although the soluble P concentration was rather low at 5 µmol L−1, no positive correlations were observed between shoot P and any type of shoot tolerance (R 2 = 0.116, 0.017, 0.166, 0.191 and 0.256 for Al tolerance [low Al], Al tolerance [high Al], LN tolerance, combined tolerance [low Al] and combined tolerance [high Al] in sorghum, respectively; and R 2 = 0.095, 0.334, 0.061, 0.037 and 0.396 for Al tolerance [low Al], Al tolerance [high Al], LN tolerance and combined tolerance [low Al] and combined tolerance [high Al] in maize, respectively) (data not shown). The soluble concentration of P in the medium is, therefore, not considered to be a determining factor for plant growth in the present study.
Table 2 Multiple regression equation (M), standardized partial regression equation (S) and probability (P) on the relationship between combined tolerance, Al tolerance and low-nutrient tolerance
Some of the nutritional characteristics of plants grown in the presence of Al have already been reported (CitationFoy and Brown 1964; CitationHãussler et al. 2006; CitationMariano and Keltjens 2005). We propose that the plant nutritional characteristics linked to low-nutrient tolerance demonstrated in the present investigation should be evaluated as an important strategy for plant production in tropical acid soils in both Al-tolerant plant species and Al-sensitive plant species under low-Al conditions. Aluminum tolerance and low-nutrient tolerance for plant production in these soils may fluctuate depending on the plant nutritional characteristics that are related to Al tolerance and low-nutrient tolerance. The soluble Al concentration and nutrient status of these soils are also important. A short-term screening technique that can be applied to these soils should, alternatively, be established in the future.
Our investigation was carried out using gramineous plant species. The recommended plant nutritional characteristics required to cope with low-nutrients containing Al may be different between plant species, for example, for dicotyledonous plants. Further research is needed using other plant species, such as other popular and important crop plant species grown in tropical areas.
ACKNOWLEDGMENT
This study was supported by a Grant-in-Aid for Scientific Research to T. Wagatsuma (No. 18208008) from the Japan Society for the Promotion of Science.
REFERENCES
- Baligar , VC and Fageria , NK . 1997 . “ Nutrient use efficiency in acid soils: nutrient management and plant use efficiency ” . In Plant–Soil Interactions at Low pH , Edited by: Monig , AC , Furlani , AMC , Schafferst , RE , Fageria , NK , Roselem , CA and Cantarella , H . 75 – 95 . Compinas : Brazilian Soil Science Society .
- Blamey , FPC , Edmeades , DC , Asher , CJ , Edwards , DG and Wheeler , DM . 1991 . “ Evaluation of solution culture techniques for studying aluminum toxicity in plants ” . In Plant–Soil Interactions at Low pH , Edited by: Wright , RJ , Baligar , VC and Murrmann , RP . 905 – 912 . Dordrecht : Kluwer Academic Publishers .
- Blamey , FPC , Edwards , DG and Asher , CJ . 1983 . Effect of aluminum, OH:Al and P:Al ratios, and ionic strength on soybean root elongation in solution culture . Soil Sci , 136 : 197 – 207 .
- Eswaran , H , Kimble , J , Cook , T and Beinroth , FH . 1992 . “ Soil diversity in the tropics: Implications for agricultural development ” . In Myths and Science of Soils of the Tropics. SSSA Special Publication Number 29 , Edited by: Lal , R and Sanchez , PA . 1 – 16 . Madison : American Society of Agronomy .
- Fisher , RA . 1958 . Statistical Methods for Research Workers , Edinburgh : Oliver & Boyd . 13th edn
- Foy , CD and Brown , JC . 1964 . Toxic factors in acid soils. II. Differential aluminum tolerance of plant sciences . Soil Sci. Soc. Am. Proc , 28 : 27 – 32 .
- Hãussler , K , Rao , IM , Schultzekraft , R and Marschner , H . 2006 . Shoot and root growth of two tropical grasses, Bracharia ruziziensis andB. dictyoneura, as influenced by aluminum toxicity and phosphorus deficiency in a sandy loam Oxisol of the eastern plains of Colombia . Tropical Grassland , 40 : 213 – 221 .
- Ishikawa , S , Wagatsuma , T , Takano , T , Tawarays , K and Oomata , K . 2001 . The plasma membrane intactness of root-tip cells is a primary factor for Al-tolerance in cultivars of five species . Soil Sci. Plant Nutr , 47 : 489 – 501 .
- Jackson , ML . 1958 . “ Phosphorus determinations for soils ” . In Soil Chemical Analysis , 134 – 182 . Englewood Cliffs : Prentice-Hall .
- Khan , MSH , Tawaraya , K Sekimoto , H . 2008 . Relative abundance of Δ5-sterols in plasma membrane lipids of root- tip cells correlates with aluminum tolerance of rice . Physiol. Plant , 135 : 73 – 83 .
- Kobayashi , Y , Yamamoto , Y and Matsumoto , H . 2004 . Studies on the mechanism of aluminum tolerance in pea (Pisum sativum L.) using aluminum-tolerant cultivar ‘Alaska’ and aluminum-sensitive cultivar ‘Hyogo’ . Soil Sci. Plant Nutr , 50 : 197 – 204 .
- Ma , JF , Shen , R , Zhao , Z , Wissuwa , M , Takeuchi , Y , Ebitani , T and Yano , M . 2002 . Response of rice to Al stress and identification of quantitative trait loci for Al tolerance . Plant Cell Physiol , 43 : 652 – 659 .
- Mariano , ED and Keltjens , W . 2005 . Long-term effects of aluminum exposure on nutrient uptake by maize genotypes differing in aluminum resistance . J. Plant Nutr , 28 : 323 – 333 .
- Ofei-Manu , P , Wagatsuma , T , Ishikawa , S and Tawaraya , K . 2001 . The plasma membrane strength of the root-tip cells and root phenolic compounds are correlated with Al tolerance in several common woody plants . Soil Sci. Plant Nutr , 47 : 359 – 375 .
- Okada , K and Fischer , AJ . 2001 . “ Adaptation mechanism of upland rice genotypes of highly weathered acid soils of South American Savannas ” . In Plant Nutrient Acquisition: New Perspectives , NIAES Series 4 Edited by: Ae , N , Arihara , J , Okada , K and Srinivasan , A . 185 – 200 . Tokyo : Springer .
- Pavan , MA , Bingham , FT and Pratt , PF . 1982 . Toxicity of aluminum to coffee in Ultisols and Oxisols amended with CaCO3, MgCO3and CaSO4·2H2O . Soil Sci. Soc. Am. J , 46 : 1201 – 1207 .
- Piñeros , MA , Shaff , JE , Manslank , HS , Alves , VMC and Kochian , LV . 2005 . Aluminum resistance in maize cannot be solely explained by root organic acid exudation. A comparative physiological study . Plant Physiol , 137 : 231 – 241 .
- Pintro , J , Inoue , TT and Tescaro , MD . 1999 . Influence of the ionic strength of nutrient solutions and tropical acid soil solutions on aluminum activity . J. Plant Nutr , 22 : 1211 – 1221 .
- Pintro , JC and Taylor , GJ . 2004 . Effects of aluminum toxicity on wheat plants cultivated under conditions of varying ionic strength . J. Plant Nutr , 27 : 907 – 919 .
- Rao , IM . Adapting tropical forages to low-fertility soils . Proc. of the XIX International Grassland Congress . February 10–21 , 2001. pp. 247 – 254 . Brazil : São Pedro .
- Rao , IM , Zeeigler , RS , Vera , R and Sarkarung , S . 1993 . Selection and breeding for acid soil tolerance in crops. Upland rice and tropical forages as case studies . BioScience , 43 : 454 – 465 .
- SAS Institute . 1988 . SAS/STATTM User's Guide , Cary : SAS Institute . release 6.03 edition
- Sere , C and Estrada , RD . 1987 . “ Potential role of grain sorghum in the agricultural systems of regions with acid soils in tropical Latin America ” . In Sorghum for Acid Soils , Edited by: Gourley , LM and Salinas , JG . 145 – 169 . Cali : International Center for Tropical Agriculture .
- Wada , S-I and Seki , H . 1994 . A compact computer code for ion speciation in aqueous solutions based on a robust algorithm . Soil Sci. Plant Nutr , 40 : 165 – 172 .
- Wagatsuma , T , Ishikawa , S , Obata , H , Tawaraya , K and Katohda , S . 1995 . Plasma membrane of younger and outer cells is the primary specific site for aluminum toxicity in roots . Plant Soil , 171 : 105 – 112 .
- Watanabe , T and Okada , K . 2005 . Interactive effects of Al, Ca and other cations on root elongation of rice cultivars under low pH . Ann. Bot , 95 : 379 – 385 .
- Wenzl , P , Mancilla , LI , Mayer , JE , Albert , R and Rao , IM . 2003 . Simulating infertile acid soils with nutrient solutions: the effect on Brachiaria species . Soil. Sci. Soc. Am. J , 67 : 1457 – 1469 .
- Wenzl , P , Patiño , GM , Chaves , AL , Mayer , JE and Rao , IM . 2001 . The high level of aluminum resistance in signalgrass is not associated with known mechanisms of external aluminum detoxification in root apices . Plant Physiol , 125 : 1473 – 1484 .
- Wheeler , DM and Edmeads , DC . 1995 . “ Effect of ionic strength on wheat yield in the presence and absence of aluminum ” . In Plant–Soil Interactions at Low pH , Edited by: Date , RA , Grundon , NJ , Rayment , GE and Probert , ME . 623 – 626 . Dordrecht : Kluwer Academic Publishers .
- Wright , RJ , Baligar , VC and Ahlrichs , JL . 1989 . The influence of extractable and soil solution aluminum on root-growth of wheat seedlings . Soil Sci , 148 : 293 – 302 .
- You , JF , He , YF , Yang , JL and Zheng , SJ . 2005 . A comparison of aluminum resistance among Polygonum species originating on strongly acidic and neutral soils . Plant Soil , 276 : 143 – 151 .
- Wagatsuma , T , Khan , MSH , Rao , IM , Wenzl , P , Tawaraya , K , Yamamoto , T , Kawamura , T , Hosogoe , K and Ishikawa , S . 2005 . Methylene blue stainability of root-tip protoplasts as an indicator of aluminum tolerance in a wide range of plant species, cultivars and lines . Soil Sci. Plant Nutr , 51 : 991 – 998 .