Abstract
The present study was carried out to evaluate the effect of permafrost thawing on methane (CH4) emission from different development stages of thermokarst formation in Central Yakutia, East Siberia. Four stages of thermokarst, “Bullar,”“Dyede,”“Tuumpu” and mature “Alas,” differing in age, depth of depression and water presence, were selected for examination. The emission of CH4 was measured at each thermokarst in forest, dry grassland, wet grassland and on the pond surface. The forest plots were CH4 sinks and uptake ranged from −1 to −34 μg C m−2 h−1. The CH4 fluxes from dry grasslands ranged from −5.4 to 0.9 μg C m−2 h−1, and in wet grasslands CH4 fluxes substantially increased, ranging from 3.6 to 285 μg C m−2 h−1. Pond surfaces had the highest CH4 emissions, ranging from 657 to 8.0 × 103 μg C m−2 h−1. The amount of CH4 stored in the permafrost (up to a depth of 15 m) was estimated to be approximately 0.259 g C m−2. This value was small compared with the current CH4 emission from pond surfaces (4.3–16 g C m−2 growing season−1) in thermokarst depressions. Accordingly, the release of CH4 trapped in the permafrost was not significant compared with the high CH4 emission from the pond surface in young thermokarst depressions. The formation of thermokarst involving permafrost thawing could influence the regional CH4 budget significantly by forming thermokarst wetlands that produce and emit CH4 as a result of current microbial processes under anaerobic conditions.
Key words:
Introduction
Methane (CH4) is a relatively potent greenhouse gas with a high global warming potential (i.e. 25-fold higher warming effect than carbon dioxide in mass over a 100-year time horizon; CitationIntergovernmental Panel on Climate Change 2007). CH4 is produced in soils under anaerobic conditions (e.g. wetlands) by methanogens, and is consumed in soils under aerobic conditions (e.g. forests) by methanotrophs (CitationLe Mer and Roger 2001). Regions with permafrost are considered to play a significant role in the global CH4 cycle by involving a large source of CH4, such as tundra wetlands and thermokarst lakes (CitationMorishita et al. 2003; CitationNakano et al. 2000; CitationTakakai et al. 2008; CitationWalter et al. 2006; CitationZimov et al. 1997). Permafrost regions occupy approximately 25% of the terrestrial surface in the Northern Hemisphere, and more than 60% of the permafrostis in Russia (CitationBrown and Grave 1981; CitationKudrjavtsev et al. 1978). Changes to the permafrost have important implications for natural ecosystems. The carbon (C) stock in the permafrost soils of Taiga forest and tundra ecosystems of Yakutia (1.6 × 106 km2) was estimated to be 17 Pg (Pg = 1015 g), accounting for approximately 25% of the total C stock in forest soils of Russia (CitationMaximov et al. 2005). This vast pool of soil C, protected by cold conditions, is believed to be highly susceptible to changes in temperature and permafrost thawing. Therefore, a huge C stock in the permafrost could become a large source of CO2 and CH4 to the atmosphere in a future global warming scenario.
CitationZimov et al. (1997) reported that a large proportion of the CH4 emission from thaw lakes in North Siberia was derived from Pleistocene-aged organic C stored in the permafrost, and the old C was supplied by erosion to the perimeters of the thaw lakes. CitationWalter et al. (2006) indicated that the CH4 emission from thaw lakes in North Siberia (3.8 Tg CH4 year−1 [Tg = 1012 g]) contributed significantly to the total CH4 emission from northern wetlands (<6–40 Tg CH4 year−1). These researchers also indicated that the recent expansion of thaw lakes over the past two decades increased the CH4 emission by 58%.
Very few studies have examined the CH4 content in permafrost. This lack of data makes estimations of CH4 emissions in response to global warming uncertain. A number of studies in Alaska and Siberia have suggested that frozen grounds might have been a large direct reservoir of CH4 trapped in the permafrost layer near the surface. The CH4 concentration in the permafrost is much larger than the present atmospheric concentration (1.8 p.p.m.v.) (CitationBrouchkov and Fukuda 2002). As this permafrost reservoir is located near the soil surface it is likely to be released quickly with permafrost thawing, owing to an increase in the surface temperature. The CH4 concentrations in the permafrost in the Prudho Bay area in Alaska varied over a wide range, 1,173–17,937 p.p.m.v. and 2,444–10,522 p.p.m.v. in the silt permafrost and ice wedge, respectively (CitationRasmussen et al. 1993). Their model predicted that CH4 release from permafrost is likely to reach 10 Tg CH4 year−1, accounting for 2% of the current global CH4 source under projected climate change in the future (CitationMoraes and Khalil 1993).
The loess soils found in the ice-rich permafrost region of north-eastern Siberia cover an area of approximately 1.0 × 106 km2, and are known as “Yedoma” in the Russian literature. These mineral soils have been formed syngenetically through loess deposition with an average thickness of 25 m (ranging from 10 to 60 m) and occupy up to 90% of the total ground volume (CitationRomanovsky 1993). These yedoma deposits on the Arctic coast of Siberia, Northern Yakutia, contain up to 10,000 p.p.m.v. of CH4 on average at different locations (CitationMoriizumi et al. 1995), although these high contents were observed only in the top 10-m layer of permafrost. In Central Yakutia, CitationBrouchkov and Fukuda (2002) conducted a similar study and reported that the CH4 concentrations in the frozen soil ranged from 1,000 to 6,000 p.p.m.v. and were higher than those in the ice wedges, ranging from 1.6 to 3,100 p.p.m.v.
Figure 1 Stages of thermokarst formation in Central Yakutia (after Bosikov 1991). The main feature of this region is the underground ice-wedge complex.
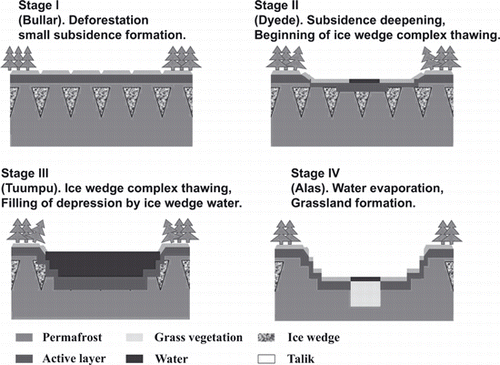
A massive ice complex, which is known as an “ice wedge”, is distributed widely in the upper permafrost in Central Yakutia lowland, East Siberia. Fluctuations in thermal conditions during the early Holocene led to thermokarst formation caused by degradation of the ice complex in this region (CitationBosikov 1991; CitationKatamura et al. 2006). This thermokarst formation process has four different stages (). The process is initiated with thawing of the ice-wedge complex as a result of soil heating after deforestation or forest fires (CitationDesyatkin 1993). The first stage, “Bullar” (stage I), is characterized by small polygonal subsidence of deforested areas. The other three stages, “Dyede” (stage II), “Tuumpu” (stage III) and the mature “Alas” (stage IV), are characterized by the presence of ponds, and the accumulation of pond sediments is the most important factor for Alas soil formation. Stage II (Dyede) is a well-depressed area with a hillocky bottom and slopes that may have a small pond. In stage III (Tuumpu), a deeper depression is fully filled with water supplied from the melted ice complex and run-off water from the surrounding forests. Stages II and III are considered to be young thermokarsts and have a flat bottom that is leveled by the pond sediments (CitationBosikov 1991). After an ice wedge under the depression thaws completely, the water inside the depression almost dries up, and a mature and stable thermokarst depression with a pond and steppe vegetation inside, “Alas” (stage IV) (CitationCzudek and Demek 1970; CitationFrench 1996; CitationSoloviev 1959), is formed. Approximately 16,000 Alases are located in lowland Central Yakutia, with a total area of 440,000 ha, which is approximately 17% of the total land area of the Central Yakutia lowland (CitationBosikov 1991). The size of an Alas (stage IV) can vary from 0.1 to 15 km in diameter and from 3 to 40 m in the depth of the depression depending on the ice complex. The Central Yakutia region is a well-known example of thermokarst terrain (CitationFrench 1996; CitationSoloviev 1959) as shown in . Pollen analysis has shown that formation of the thermokarst was initiated between 10,700–9,200 and 9,500–8,500 cal year bp in this region (CitationKatamura et al. 2006).
From a review of previous literature, it is considered that the thermokarst formation process involving permafrost thawing could influence CH4 budgets in the Central Yakutia lowland by releasing formerly trapped CH4 in the permafrost and CH4 production derived from organic matter in the thawed permafrost. The objectives of the present study were: (1) to evaluate current CH4 emission from different stages of thermokarst formation in Central Yakutia, (2) to evaluate the contribution of released CH4 from thawed permafrost by comparing the contribution with published data.
Materials and methods
Study site
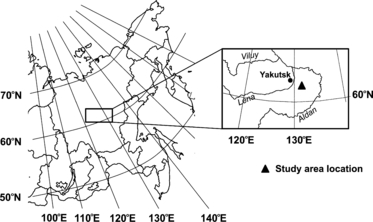
The studied thermokarst depressions with different ages are located at the most typical thermokarst terrain on the east bank of Lena River, approximately 50 km to the east of Yakutsk city, Russia (). The ice-wedge complex is considered to be an important factor for thermokarst formation. In this territory, thick syngenetic ice wedges occur at a depth of 2.0–2.5 m. The width of the upper part of the ice wedges varies from 1 to 3 m and the average depth of the ice wedges ranges from 15 to 30 m. The ice wedges are distributed regularly with several meter intervals (at most 5–6 m horizontally) (CitationIvanov 1984). Consequently, ice wedges could occupy 50% of the total upper permafrost volume in this territory. During the process of thermokarst formation, thawing of the ice-wedge complex led to the formation of various depressions differing in depth, depending on age, and different ground volumes were affected. The typical soil type in this territory is permafrost pale soils under the larch forest (Larix cajanderi), classified into Gelisols (Typic Psammoturbels) by USDA Soil Taxonomy. However, the soil type changes gradually with the formation of the thermokarst depressions. During the last three stages, accumulation of sediment occurs at the bottom of the lakes. After the lake dries up, these sediments reach above the water surface and become a substratum for the formation of Alas soils. Soils of both forest and mature Alas (Stage IV) biomes are clay-loam, but they have a drastic difference in humus and mineral horizons, and consequently in soil organic matter content. CitationMatsuura et al. (1994) reported that the total C storage in the active layer soils inside a mature Alas depression on the east bank of the Lena river ranged from 341 to 724 t C ha−1, and were 4–9-fold higher than that in zonal forest soil near the Alas (84.7 t C ha−1), owing to a large accumulation of soil organic matter.
Figure 3 Location map of the study region. The highlighted figure is the lowland region of Central Yakutia. The study sites are located between the Lena and Aldan rivers.
The site of thermokarst stage I is located at 62°54′N, 130°33′E. It is formed by polygons with a depth of subsidence of 60 cm on average (). The approximate age of this thermokarst depression is 100 years according to an interview with an inhabitant of the region.
Figure 4 Different stages of thermokarst: I, Bullar; II, Dyede; III, Tuumpu; IV, mature Alas. The observation plots are shown in the photographs with arrows.
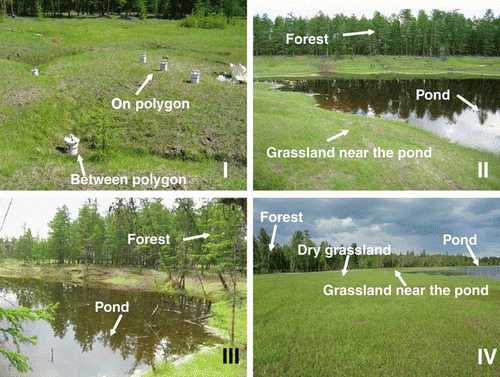
The site of stage II is located at 62°11′N, 130°33′E. This depression has a small pond filled with water from the thawed ice complex and run-off from the surrounding forest and grassland. The depth of the total depression is estimated to be 384 cm, including 242 cm from the surrounding forest level to the pond surface and 142 cm of pond depth at maximum thawing conditions. The depth of all thermokarst depressions was measured at the end of August 2007 using the theodolite leveling method (Institute for Biological Problems of Cryolithozone, unpubl. data, 2007).
The site of stage III is located at 62°11′N, 130°34′E. This deep depression is fully filled with water. The depth of the depression from the forest level to the pond surface was 178 cm. The maximum depth of the pond was 250 cm. The total depth of the thermokarst depression reached 428 cm.
When the ice complex completely melts, the final stage (IV) of thermokarst occurs, and most of the water is vaporized and grasslands are formed. Under the remaining pond, unfrozen soil called “Talik” is formed (CitationBosikov 1991). This is melted permafrost that results from the heating effect of high water temperatures, and this soil remains unfrozen for a long time (). The studied site of stage IV is located at 62°08′N, 130°30′E. The site was 63 ha in area and consisted of grassland and a pond in the center (CitationDesyatkin et al. 2007). The grassland area in the thermokarst depression is divided into four different belts of vegetation around the pond: pond vegetation, wet grassland, middle grassland and dry grassland. The depth of the depression from the surrounding forest level to the bottom of lake is 1,680 cm, and 180 cm of that is the depth of the pond. Under the depression of this site, the ice-wedge complex is completely absent. The approximate age of this depression is 8,500 years (CitationKatamura et al. 2006). The soil C storage in the topsoil (0–40 cm, including the organic layer) amounted to 49.7, 9.5 and 214 t C ha−1 in the surrounding forest, and dry and wet grasslands, respectively. Net primary production during the warm period (June–September) was 2.23 and 1.31 t C ha−1 in the dry and wet grasslands, respectively (CitationDesyatkin et al. 2007).
The forest surrounding all of the studied thermokarst is dominated by mature larch with mountain cranberry (Vaccinium vitis-idaea) on the forest floor. The dominant vegetation of the dry grassland belt in stages I and IV is Poa pratensis and Elytrigia repens, and the wet grassland belt in stages II and IV is dominated by Carex orthostachys (CitationNikolaeva and Desyatkin 2004).
The annual mean temperature in this region is −9.9°C, with average temperatures in January and July of −41.2 and 18.7°C, respectively. The annual mean precipitation over the past 100 years is 241 mm, of which 150 mm occurs in summer (May–September). In the study year (2007), precipitation in summer (191 mm) was higher than the long-term average. During the growing period, all months exceeded the long-term average precipitation, except for August when the rainfall was less than 2 mm. The total precipitation in 2007 was 289 mm. The average monthly air temperature during the summer (May–September) in the study year was 1.5–2°C, higher than the long-term average, except for July (16.7°C). Therefore, the summer of the study year was more humid and warmer than the long-term average (Yakutsk weather station).
Experimental setup and CH4 flux measurement
Three experimental plots were set up at each forest and pond surface in thermokarst stages II, III and IV. Five plots were set up in the grassland: two in stage I, on the polygon and between the polygon, one in stage II, near the pond, and two in stage IV, dry grassland and near the pond. An additional four plots were established for measuring CH4 emission from the whole ecosystem by including vegetation, of which two were in stage II, near the pond and on the pond surface, and the other two were in stage IV, near the pond and on the pond surface.
Measurement of the CH4 flux was carried out using the closed chamber method twice during the warm period, in June and in August 2007. Grass vegetation in the pond water was distributed with a high spatial variation during the observation period. In the spring (beginning of May), wet grasslands around the pond were flooded with expanded pond water as a result of the inflow of snow-melt water. Grass growth was inhibited in early summer because of the presence of water with a low temperature. Therefore, vegetation was not observed in the pond sites of stages II and IV during the first sampling (June). At these sites, grass and algae were observed only during the second sampling (August).
Six cylindrical stainless-steel chambers, 25 cm in height and 18.5–21.0 cm in diameter, with a detachable lid, were used to measure the CH4 flux from the soil and water surface (CitationMorishita et al. 2003; CitationTakakai et al. 2008). The chambers were installed to a depth of 3 cm in the soil after removing the vegetation, and were kept overnight to eliminate any fluctuations resulting from disturbance. On the next day, a 20-mL gas sample was taken into a 10-mL vacuum vial that was sealed with a butyl rubber stopper (SVF-10; Nichiden-Rika, Kobe, Japan) at time intervals of 0, 30 and 60 min after the chamber was covered by the lid. At the pond surface plots, the CH4 flux was measured using floating chambers equipped with floats made of polystyrene foam (CitationTakakai et al. 2008). The air temperature at a height of 1 m was measured with a thermistor thermometer (CT-410W; Custom, Tokyo, Japan) during the flux measurements. There were three replicates for each flux measurement.
In wet locations, such as the wet grasslands and the pond surface of stages II and IV, the CH4 flux from the whole ecosystem (including both soil and plants) was measured using transparent acryl chambers 30 cm × 30 cm × 60 cm (CitationTakakai et al. 2008). To measure the CH4 emission via the plants (CitationWhiting et al. 1991), all plants inside the chamber were retained during the measurement. The time intervals of sampling were 0, 10 and 20 min after inserting the chamber onto the collar and closing the top. A similar method to that used with the stainless-steel chambers was used to take out the gas samples. Using the difference between the results of the CH4 emission from the total ecosystem (transparent chamber) and the CH4 emission from the soil/water surface (stainless-steel chamber), the CH4 emission via the plants was estimated; this method has been used in a similar ecosystem (CitationTakakai et al. 2008).
Soil and water temperatures were measured with a digital thermometer at a depth of 5 cm. Volumetric soil water content (soil moisture) at the soil surface (0–5 cm) was measured by amplitude domain reflectometry (ADR) (DIK-311A; Daiki-rika, Saitama, Japan). Five replicate measurements were conducted at each chamber.
Analysis of the CH4 concentration and calculation of the CH4 flux
The CH4 concentration was analyzed using a gas chromatograph (GC-8A; Shimadzu, Kyoto, Japan) equipped with a flame ionization detector. The minimum detectable concentration of CH4 was ±0.1 p.p.m.v. The CH4 flux was calculated by a linear regression of three samples as follows:
Estimation of CH4 storage in the permafrost layer
Using the data of CH4 content in the permafrost layer in Central Yakutia reported by CitationBrouchkov and Fukuda (2002), estimation of CH4 storage in the permafrost (frozen soil and ice complex) was conducted. The reported data were divided into three horizons (active layer, upper permafrost and lower permafrost). The permafrost was divided into upper and lower parts by a difference in CH4 concentration.
The first horizon is an active layer, where soil freezing and thawing occur seasonally. For this territory, the depth of the maximum active layer varied from 1.0 m in the forest to 1.4 m in the grassland of adjacent mature Alas in 2007. The measured depth was averaged as 1.2 m for this estimation (Institute for Biological Problems of Cryolithozone, unpubl. data, 2007). The CH4 concentration in the soil air of the active layer of larch forest floor in this territory ranged from 2 to 10 p.p.m.v. (CitationMorishita et al. 2003). These values were low compared with those in the permafrost layers, and were ignored in the estimation of CH4 storage. The second horizon (1.2–3.0 m) is the upper permafrost, which includes ice wedges of 0.5–1.0 m in height. At undisturbed forest sites, ice wedges occurred at a depth of 2–2.5 m. The CH4 concentrations in air bubbles of this permafrost layer were averaged as 2,000 p.p.m.v. in frozen mineral soil blocks and 1,500 p.p.m.v. in the ice wedges (CitationBrouchkov and Fukuda 2002).
The average depth of the ice wedges ranged from 15 to 30 m in this territory (CitationIvanov 1984). The third horizon (3.0–15.0 m) covers all depths of the ice complex distributed at the studied Alas depression before the formation of thermokarst. The CH4 concentrations in air bubbles of this permafrost layer were averaged as 1,000 p.p.m.v. in the blocks of frozen mineral soil and 80 p.p.m.v. in the ice wedges (CitationBrouchkov and Fukuda 2002).
The volumetric content of the ice wedge in this territory occupies 50% of the total upper permafrost volume (CitationIvanov 1984). According to CitationBrouchkov and Fukuda (2002), volumetric air content in the permafrost could be averaged as 5% of the ground volume. Air pressure in the bubbles was assumed to be 1 atm. The total amount of stored CH4 in the permafrost was calculated for each chosen horizon by using these parameters and the density of CH4 (0.717 kg m−3) ().
Statistical analysis
The difference in CH4 fluxes between the measurement periods and among ecosystems of different thermokarst depressions were compared using a two-way ANOVA in SPSS 13.0 for Windows 2004 (SPSS Inc. Chicago, Illinois, USA).
Table 1 Parameters for estimation of CH4 storage in the permafrost layer
Results
Environmental variables
The soil temperature at a depth of 5 cm was lower in the shaded forest plots, ranging from 6.9 to 8.0°C in June and from 13.5 to 16.9°C in August (). At the grassland plots, the soil temperature ranged from 11.2 to 18.3°C in June and from 18.6 to 22.8°C in August. Pond water temperature was higher than in the dry grassland plots at both measurements, reaching 18.6 and 23.8°C on average in June and August, respectively ().
The soil moisture expressed as the volumetric soil water content (VSWC) was higher in June than in August at all plots (). In June, the lowest soil moisture was observed in the dry grassland of stage IV (0.21 m3 m−3). The highest soil moisture was observed at the plot between polygons of stage I (0.41 and 0.38 m3 m−3 in June and August, respectively), and in the grassland near the pond of stage II (0.39 and 0.39 m3 m−3) and near the pond of stage IV (0.41 and 0.41 m3 m−3). At the three forest locations, the soil moisture was higher in June (0.28–0.36 m3 m−3) than in August (0.047–0.15 m3 m−3). The soil moisture of the two grassland plots in stage IV decreased depending on the distance from the pond ().
CH4 flux from the thermokarst ecosystems
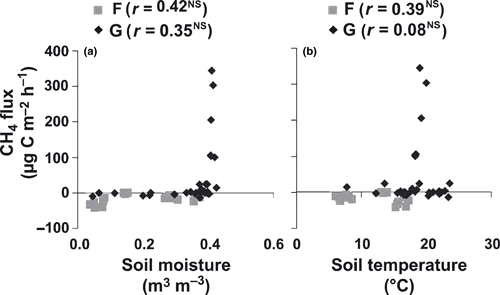
In the forest plots (near stages II, III and IV), CH4 uptake was observed with values ranging from −1.3 ± 1.5 to −34 ± 7.8 μg C m−2 h−1 (mean ± standard deviation [SD]; ). The CH4 uptake in stages II and IV forests increased from −12 ± 2.9 and −14 ± 6.2 μg C m−2 h−1 in June to −34 ± 7.8 and −26 ± 13 μg C m−2 h−1 in August, respectively. In contrast, the CH4 uptake in the forest plot of stage III decreased from −16 ± 6.5 in June to −1.3 ± 1.5 μg C m−2 h−1 in August. The CH4 fluxes in the forest plots were not correlated significantly with the soil moisture or soil temperature (P > 0.05; ). Among these plots, the CH4 uptake in stage II was significantly higher than that in stage III (P < 0.05). Moreover, the CH4 uptake did not differ significantly between June and August (P > 0.05).
The CH4 fluxes in the grassland plots (the plots on and between polygons in stage I, and the dry and wet grasslands in stages II and IV) showed high spatial variation. Slight CH4 fluxes (uptake and emission) were observed at the driest plots on the polygon in stage I and in the dry grassland in stage IV (). In June, when soil moisture was high, low CH4 emission was observed (0.9 ± 2.4 and 0.7 ± 4.4 μg C m−2 h−1 on the polygon in stage I and in the dry grassland in stage IV, respectively). In contrast, after a decrease in soil moisture in August, these soils became sinks of CH4 (−5.4 ± 7.1 and −9.1 ± 5.3 μg C m−2 h−1 on the polygon in stage I and in the dry grassland in stage IV, respectively). In the other grassland plots (i.e. the relatively wet plots), CH4 emission tended to increase in August, except for the plot between polygons in stage I (13 ± 14 and 11 ± 12 μg C m−2 h−1 in June and August, respectively). On the grassland near the pond in stage II, CH4 emission increased from 3.6 ± 6.0 μg C m−2 h−1 in June to 11 ± 12 μg C m−2 h−1 in August, and appeared to be affected by an increase in soil temperature rather than a decrease in soil moisture (). On the grassland near the pond of stage IV, CH4 emission increased from 104 ± 2.9 in June to 285 ± 71 μg C m−2 h−1 in August. In these five plots there was a significant difference in CH4 flux between the two measurement times (P < 0.05). Variation in CH4 flux among the measurement plots was very high. Although a significant linear correlation was not found between CH4 flux and soil moisture or temperature, high CH4 emissions were found under high soil moisture conditions, that is, where VSWC values were higher than 0.40 m3 m−3 ().
Table 2 Soil temperature (5 cm) and soil moisture (0–5 cm) at each measurement
Table 3 CH4 fluxes from different thermokarst stages and measurement times (June and August)
Figure 5 Relationship between CH4 flux and (a) soil moisture or volumetric soil water content and (b) soil temperature (°C) at the forest (F) and grassland (G) sites. NS, not significant.
High CH4 emissions were observed at the pond surface plots. Among these sites, CH4 emissions increased abruptly in August compared with June at younger thermokarst stages (II and III). The CH4 emissions were similar at the two sites in June (700 ± 194 and 657 ± 72 μg C m−2 h−1, respectively), whereas they were different at the two sites in August (increased up to 8.0 × 103 ± 4.3 × 103 μg C m−2 h−1 in stage II and up to 1.7 × 103 ± 1.3 × 103 μg C m−2 h−1 in stage III). The CH4 emission from the pond surface of stage IV increased from 3.3 × 103 ± 5.3 × 103 μg C m−2 h−1 in June to 4.1 × 103 ± 2.5 × 103 μg C m−2 h−1 in August. Although this difference was not significant, CH4 emissions from the pond surface in stage III were lower than those in the other thermokarsts. In addition, increasing rates of CH4 emission between June and August in younger thermokarsts (1,049% and 156% for stages II and III, respectively) were much higher than that in stage IV (22%). In these three plots, the increase in CH4 emission corresponded well with the increase in water temperature in August (,). There were no significant differences in CH4 emissions among these locations or between the measurement times (P > 0.05; ).
The CH4 emissions via plants at the wet locations are given in . In June, CH4 emissions via plants were observed at grasslands near the pond only (50 ± 31 and 203 ± 31 μg C m−2 h−1 for stages II and IV, respectively), whereas they were not observed at the pond locations. These values amounted to 93% and 66% of the CH4 emissions from the whole ecosystem for the grassland near the pond in stages II and IV, respectively. In contrast, CH4 emission via plants increased abruptly at the pond water plots in August. The values reached 3.5 × 104 ± 1.7 × 104 μg C m−2 h−1 and 1.3 × 104 ± 5.6 × 103 μg C m−2 h−1 for the pond surface plots in stages II and IV, respectively. The contribution of CH4 emission via plants also increased from 0% in June to 81% and 76% in August. CH4 emissions via plants at the grassland near the pond increased from June (50 ± 31 μg C m−2 h−1 and 203 ± 31 μg C m−2 h−1) to August (230 ± 131 μg C m−2 h−1 and 1.7 × 103 ± 119 μg C m−2 h−1) in stages II and IV, respectively, and were lower than those in the pond surface plots. However, CH4 emission via plants still contributed largely (85–95%) to the total CH4 emission from the whole ecosystem.
Amount of CH4 storage in the permafrost layer
An approximate estimation of CH4 storage in the permafrost layer in this territory is presented in . In the upper permafrost layer (1.2–3.0 m in depth), frozen soil contained CH4 of 48.4 mg C m−2. The ice-wedge complex in the layer contained less CH4 (36.3 mg C m−2). The lower permafrost layer, which had a thickness of 12 m (3.0–15.0 m depth), had higher CH4 storage (161 mg C m−2) under frozen conditions compared with the upper permafrost layer (1.2 m in thickness), despite relatively low CH4 concentrations. In contrast, the CH4 concentration in the ice wedges in this layer was low and CH4 storage in this section was estimated to be as low as 12.9 mg C m−2. Summarizing the values of the three horizons, CH4 storage in permafrost with an ice-wedge complex was estimated to be 259 mg C m−2.
Discussion
Controlling factor for CH4 flux
Soils can act either as a sink or a source of CH4. The CH4 flux from soil represents the net production and consumption (uptake) of CH4 in the soil (CitationConrad 1995). In general, CH4 flux tends to correlate with soil moisture and temperature because microbiological processes regulate the production and consumption of CH4 (CitationDunfield et al. 1993; CitationLe Mer and Roger 2001). In the present study, forest and grassland soils with low moisture content showed steady CH4 consumption, whereas large CH4 emissions were observed from the grasslands near the pond and from the pond surface (). It was apparent that the CH4 flux in stage I, where there was the smallest difference in relief affecting soil moisture and temperature conditions, changed from uptake on the polygon to emission between polygons (). Inside the thermokarst depression, the soil moisture was lowest in the grasslands located at relatively elevated areas (i.e. on the polygon in stage I and in the dry grassland in stage IV), owing to the draining of soil water towards the lower parts. Consequently, CH4 consumption exceeded CH4 production under aerobic conditions in these dry grasslands.
Table 4 CH4 emission via plants and its contribution to CH4 emission from the whole ecosystem at wet locations (wet grassland near the pond and pond water surface)
Flooding of pond water in thermokarst depressions is an important factor for CH4 production (CitationTakakai et al. 2008). Higher CH4 emissions from the wet grasslands and from the pond surface were observed compared with the emissions from the forest and dry grasslands ().
CH4 transportation via plants from the wet grassland vegetation was apparently observed only in August (). This is because the vegetation inside the pond did not grow well in June as a result of oppressed conditions after the flooding of snow-melt water. In August, after enough grass growth, significant transportation of CH4 via vascular plants was observed at both thermokarst stages II and IV.
Among the studied thermokarst depressions, stage III had the deepest pond compared with stages II and IV. The lowest CH4 emission from the pond surface in stage III compared with the other stages suggests that deep pond water might suppress the exchange of CH4 between water and the atmosphere, and this result is consistent with the result reported by CitationNakagawa et al. (2002).
Comparison with previous studies
There are several studies on CH4 emissions from boreal regions, especially tundra peatlands, but very few studies have examined different thermokarst stages and grasslands. There are two previous studies (CitationMorishita et al. 2003; CitationTakakai et al. 2008) that were carried out in the same region as the present study (). The boreal forest (>50°N) soils presented in are sinks of CH4 with a low fluctuation. The CH4 uptake rates at larch forest soils in the present study (−17 to −3.4 μg C m−2 h−1) were relatively low compared with the reported boreal forest soils and similar to the rates at larch forest soils in this region (CitationMorishita et al. 2003; CitationTakakai et al. 2008) and to a spruce forest in Sweden (Citationvon Arnold et al. 2005).
Dry grasslands developed at this latitude (approximately 60°N) are unique landscapes. Therefore, our work can only be compared with previous work carried out in this region (CitationMorishita et al. 2003; CitationTakakai et al. 2008). Dry grasslands in thermokarst depressions (i.e. the plots on the polygon in stage I and the dry grassland in stage IV) were generally slight sinks of CH4 (−5.4 to 0.9 μg C m−2 h−1), and the values were similar to the values recorded in previous studies conducted in dry grasslands of stage IV thermokarst (mature Alas) in this region (−28 to 4.8 μg C m−2 h−1).
Wet grasslands had a significant variation in CH4 flux (). The CH4 emissions from the wet grassland soil between the polygons in stages I and II in the present study (11–12 μg C m−2 h−1) were lower than those from the grassland soil on tussock of Alaskan tundra (45–325 μg C m−2 h−1; CitationWhalen and Reeburgh 1992). The whole-ecosystem CH4 emissions from the wet grasslands near the pond in stages II and IV in the present study (3.6–285 μg C m−2 h−1) were lower than those from a similar location (edge of the pond) of stage IV thermokarst (mature Alas) in this region (4.3 × 103 to 4.1 × 104 μg C m−2 h−1; CitationTakakai et al. 2008) and wet grassland soils at the edge of a pond of Alaskan tundra (87–2.7 × 104 μg C m−2 h−1; CitationWhalen and Reeburgh 1992). Ponds in the studied thermokarst depressions expanded with snow-melt water from the surrounding forest and grassland in May 2007. The measured wet grassland plots at the edge of the pond began to flood at that time, resulting in a reduction status not sufficient for CH4 production in the soil under the pond water. The CH4 emissions from the studied wet grasslands were lower than the reported values and lower than those in the pond surface plots with prolonged flooding in the present study.
Contribution of permafrost thawing to CH4 emission from thermokarst ecosystems
At the first measurement in June, the bottom sediment of the ponds in the thermokarst depressions was still frozen. Therefore, CH4 emissions from the pond surface of the thermokarsts were assumed to not be affected by permafrost thawing, even in younger thermokarsts that are supposed to be thawing further. In August, further permafrost thawing could progress in younger thermokarsts and might affect CH4 emissions from the pond surface. This phenomenon did not occur in the stable ecosystem of stage IV where the ice complex had already melted. Consequently, the higher rate of increase in CH4 emissions from the pond surface in younger thermokarst depressions (1,049% and 156% for stages II and III, respectively) compared with old thermokarst depressions (22% for stage IV) from June to August might be affected by permafrost thawing in younger thermokarst depressions. There was a possibility that the CH4 emissions from the pond surface in young thermokarst depressions could have involved an additional source of CH4 from permafrost thawing, such as direct release of CH4 trapped in the upper permafrost and CH4 production fueled by released labile organic C from the melted permafrost (CitationZimov et al. 1997). However, as estimated in , only as little as 0.259 g C m−2 CH4 had been stored in the permafrost under the intact forest in this region, and it is assumed that this CH4 is released during the whole process of thermokarst formation leading to stage IV. According to CitationKatamura et al. (2006), since the beginning of thermokarst formation in the early Holocene, complete thawing of the ice complex might have taken approximately 1,200–2,000 years in this permafrost region.
To compare the storage of CH4 in permafrost, approximate CH4 emissions from the thermokarst pond during the growing season (May–September, 5 months) were estimated by assuming that the average CH4 emission from two measurements in June and August was the average for the whole growing season. The estimated CH4 emissions from the pond surface were 16, 4.3 and 13 g C m−2 growing season−1 for stages II, III and IV, respectively, and were relatively lower than the emissions at the pond surface in stage IV thermokarst (mature Alas) in this region (21–24 g C m−2 growing season−1; CitationTakakai et al. 2008). Consequently, the storage was too small compared with the present CH4 emission from the thermokarst pond, suggesting that the release of CH4 trapped in the permafrost should not be significant compared with the high CH4 emission from the pond surface in young thermokarst depressions. Therefore, current CH4 emission from the thermokarst depressions could have been derived mainly from microbial CH4 production. In addition, CH4 flux fueled by the labile organic C supplied from the melted permafrost (e.g. CitationZimov et al. 1997) might be a significant additional source of high CH4 emission from the pond surface in young thermokarst depressions. Consequently, thawing of the permafrost leading to the formation of thermokarst in the future under the projected warming in this region could contribute to the global CH4 cycle not by releasing prevalent CH4 trapped in the permafrost, but rather by the formation of thermokarst wetlands that produce and emit massive amounts of CH4 by current microbial processes.
Table 5 CH4 flux from different boreal ecosystems
Conclusions
The results of the present study have shown that the younger thermokarst stage with a shallow pond had higher CH4 emissions from the pond surface in warm conditions. Storage of CH4 in the permafrost was estimated to be small compared with the present CH4 emission from the thermokarst ponds. Therefore, current microbial CH4 production could be the main source of increased CH4 emissions in this region. The results of our study also indicated that the amount of CH4 trapped in the upper permafrost could be insignificant to greatly increase CH4 emissions under current projections of temperature increase in boreal regions.
Acknowledgments
We are deeply thankful to the staff members of the Institute for Biological Problems of Cryolithozone, Siberian Branch, and the Russian Academy of Science for their support during the field research. This study was funded by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Science and Technology of Japan (No. 18255014)
References
- von Arnold , K , Weslien , P , Nilsson , M , Svensson , BH and Klemedtsson , L . 2005 . Fluxes of CO2, CH4and N2O from drained coniferous forests on organic soils . For. Ecol. Manage. , 210 : 239 – 254 .
- Bosikov , NP . 1991 . Evolution of Alases in Central Yakutia , Yakutsk : Permafrost Institute press . (in Russian)
- Bradford , MA , Ineson , P , Wookey , PA and Lappin-Scott , HM . 2000 . Soil CH4oxidation: response to clearcutting and thinning . Soil Biol. Biogeochem. , 32 : 1035 – 1038 .
- Brouchkov , A and Fukuda , M . 2002 . Preliminary measurements on methane content in permafrost, Central Yakutia, and some experimental data . Permafrost Periglac. Process. , 13 : 187 – 197 .
- Brown , J and Grave , NA . 1981 . Disturbance of Surface and its Protection during Urbanization of North , Novosibirsk : Nauka . (in Russian)
- Conrad , R . 1995 . Soil microbial process involved in production and consumption of atmospheric traces gases . Adv. Microb. Ecol. , 14 : 207 – 238 .
- Corradi , C , Kolle , O , Walter , K , Zimov , SA and Shulze , ED . 2005 . Carbon dioxide and methane exchange of a north-east Siberian tussock tundra . Glob. Chang. Biol. , 11 : 1910 – 1925 .
- Czudek , T and Demek , J . 1970 . Thermokarst in Siberia and its influence on the development of lowland relief . Quat. Res. , 1 : 103 – 120 .
- Desyatkin , RV . 1993 . Syngenetic soil salinization during thermokarst Alas formation . Eurasian Soil Sci. , 25 : 38 – 46 .
- Desyatkin , AR , Takakai , F , Fedorov , Desyatkin , RV and Hatano , R . 2007 . Effect of human activity on carbon balance in meadows in a thermokarst depression in Siberia . Eurasian J. For. Res. , 10 : 89 – 96 .
- Dunfield , P , Knowles , R , Dunmont , R and Moore , TR . 1993 . CH4production and consumption in temperate and subarctic peat soils: response to temperature and pH . Soil Biol. Biochem. , 25 : 321 – 326 .
- French , HM . 1996 . The Periglacial Environment , 2nd edn , Harlow : Addison Wesley Longman .
- Intergovernmental Panel on Climate Change . 2007 . “ Climate Change 2007: The Physical Science Basis ” . In Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change , Edited by: Solomon , S , Qin , D Manning , M . 212 Cambridge : Cambridge University Press .
- Ivanov , MS . 1984 . Cryogenic Composition of Quaternary Deposits of Lena-Aldan Depression , Novosibirsk : Nauka . (in Russian)
- Katamura , F , Fukuda , M , Bosikov , NP , Desyatkin , RV , Nakamura , T and Moriizumi , J . 2006 . Thermokarst formation and vegetation dynamics inferred from a palynological study in central Yakutia, eastern Siberia . Arct. Antarct. Alp. Res. , 38 : 561 – 570 .
- Kudrjavtsev , VA , Kondratjeva , KA and Romanovskii , NN . 1978 . “ The zonal and regional laws of formation of cryolithozone in the USSR ” . In Works of the III International Conference on Permafrost Studies , Vol. 1 , 419 – 426 . Albert : Ottawa Edmonton .
- Le Mer , J and Roger , P . 2001 . Production, oxidation, emission and consumption of methane by soils: a review . Eur. J. Soil Biol. , 37 : 25 – 50 .
- Matsuura , Y , Ohta , S , Sanada , M and Desyatkin , RV . Carbon and nitrogen storage in soil developed on two different toposequences of the Lena River terrain . Proceedings of the Second Symposium on the Joint Siberian Permafrost Studies between Japan and Russia in 1993, 13–14 January 1994 . Tsukuba, Isebu. Edited by: Inoue , G . pp. 177 – 182 .
- Maximov , TC , Dolman , AJ , Moors , EJ , Ohta , T , Sugimoto , A and Ivanov , BI . 2005 . Parameters of carbon and water cycles in the forest ecosystems of the cryolithozone . Earth Science Reports , 405 : 684 – 686 . (in Russian; English translation is available from Pleiades Publishing, Inc. Doklady Earth Sciences ISSN 1028-334X
- Moraes , F and Khalil , MAK . 1993 . Permafrost methane content: 2. Modeling theory and results . Chemosphere , 26 : 591 – 594 .
- Moriizumi , J , Iida , T and Fukuda , M . Radiocarbon dating of methane obtained from air in the ice complex (Edoma), in Arctic coast area of east Siberia . Proceedings of the Third Symposium on the Joint Siberian Permafrost Studies between Japan and Russia in 1994 . pp. 14 – 21 . Sapporo : Institute of Low Temperature Science, Hokkaido University .
- Morishita , T , Hatano , R and Desyatkin , RV . 2003 . CH4flux in an Alas ecosystem formed by forest disturbance near Yakutsk, eastern Siberia, Russia . Soil Sci. Plant Nutr. , 49 : 369 – 377 .
- Nakagawa , F , Yoshida , N , Yukihiro , N and Makarov , VN . 2002 . Production of methane from alases in eastern Siberia: implications from its 14C and stable isotopic composition . Global Biogeochem. Cycles , 16 : 14-1 – 14-15 .
- Nakano , T , Kuniyoshi , S and Fukuda , M . 2000 . Temporal variation in methane emission from tundra wetlands in a permafrost area, northeastern Siberia . Atmos. Environ. , 34 : 1205 – 1213 .
- Nakano , T , Takeuchi , W , Inoue , G , Fukuda , M and Yasuoka , Y . 2006 . Temporal variations in soil-atmosphere methane exchange after fire in a peat swamp forest in West Siberia . Soil Sci. Plant Nutr. , 52 : 77 – 88 .
- Nikolaeva , MC and Desyatkin , AR . Change of spatial structure of Alas vegetative cover as a parameter of soil conditions change/Permafrost soils: ecology and protection . Materials of the All-Russia Scientific Conference Devoted to 4th Congress of Dokuchaev Society of Soil Scientists and the 100-Anniversary of Professor Vasily Georgievich Zolnikov, June 28–30 . Edited by: Desyatkin , RV . pp. 114 – 119 . Yakutsk : Publishing house of Permafrost Institute SB RAS .
- Nykänen , H , Alm , J , Lang , K , Silvola , J and Martikainen , PJ . 1995 . Emissions of CH4, N2O and CO2from a virgin fen and a fen drained for grassland in Finland . J. Biogeogr. , 22 : 351 – 357 .
- Rasmussen , RA , Khalil , MAK and Moraes , F . 1993 . Permafrost methane content: 1. Experimental data from sites in Northern Alaska . Chemosphere , 26 : 591 – 594 .
- Repo , ME , Huttunen , JT Naumov , AV . 2007 . Release of CO2and CH4from small wetland lakes in western Siberia . Tellus , 59B : 788 – 796 .
- Romanovsky , NN . 1993 . Fundamentals of the Cryogenesis of the Lithosphere , Moscow : University Press . (in Russian)
- Soloviev , PA . 1959 . Permafrost in Northern Lena-Amga Interfluves , Moscow : AN USSR Press . (in Russian)
- Takakai , F , Desyatkin , AR , Lopez , CML , Fedorov , AN , Desyatkin , RV and Hatano , R . 2008 . CH4and N2O emissions from a forest-alas ecosystem in the permafrost taiga forest region, eastern Siberia, Russia . J. Geophys. Res. – Biogeosci. , 113 : G02002 doi
- Walter , KM , Zimov , SA , Chanton , JP , Verbyla , D and Chapin , FS III . 2006 . Methane bubbling from Siberian thaw lakes as a positive feedback to climate warming . Nature , 443 : 71 – 75 .
- Whalen , SC and Reeburgh , WS . 1992 . Interannual variation in tundra methane emission: a 4-year time series at fixed sites . Global Biogechem. Cycles , 6 : 139 – 159 .
- Whiting , GJ , Chanton , JP , Bartlett , DS and Happell , JD . 1991 . Relationship between CH4emission, biomass, and CO2exchange in a subtropical grassland . J. Geophys. Res. , 96 : 13067 – 13071 .
- Zimov , SA , Voropaev , YV Semiletov , IP . 1997 . North Siberian lakes: a methane source fuelled by Pleistocene carbon . Science , 277 : 800 – 802 .
