Abstract
The effects of saline and alkaline stresses (from 1:1 molar ratios of NaCl : Na2SO4 and NaHCO3 : Na2CO3, respectively) were compared on the germination, growth, osmotic adjustment and ionic balance of wheat seedlings to elucidate the mechanism of alkaline stress (high pH) damage to plants and their physiological adaptive mechanism to alkaline stress. The effects of salines on the activity and free concentrations of various ions in nutrient solutions were analyzed with the program GEOCHEM-PC. This analysis showed that alkaline stress caused a heavy precipitate of phosphate and metal ions, excluding Na+ and K+, which caused a sharp decrease in ionic activity and in the free concentrations of various ions. The inhibitory effects of saline stress on the growth and germination of wheat seeds were reduced compared with the effects of alkaline stress. Alkaline stress damaged root function, photosynthetic pigments and the membrane system and led to severe reductions in root system activity and in the content of photosynthetic pigments, and to a sharp increase in electrolyte leakage. Massive Na+ influx may be the main cause of damage from alkaline stress. The effect of alkaline stress on the accumulation of inorganic ions was stronger than that of saline stress. Under alkaline stress, Na+ sharply increased and NO−3 and H2PO4 − decreased in shoots, which caused a severe deficit in negative charge. Wheat might enhance organic acid synthesis to remedy the shortage of inorganic anions and maintain a stable intracellular pH, and accumulate betaine and soluble sugars to cope with the osmotic stress from the high Na+ concentration in the vacuoles.
Introduction
Agricultural productivity is severely affected by soil salinity. Natural salt-alkalinized soils are very complex, with Na+, Ca2+, Mg2+, K+, Cl−, SO2− 4, HCO− 3, CO2− 3 and NO3 − as the main ions (CitationLäuchli and Lüttge 2002). NaCl, Na2SO4, NaHCO3 and Na2CO3 are the main harmful salts in many inland areas, including China (CitationKawanabe and Zhu 1991). Previous studies have defined saline stress as the stress of neutral salines and alkaline stress as the stress of alkaline salts (CitationShi and Sheng 2005; CitationShi and Wang 2005; CitationShi and Yin 1993). When a salinized soil contains alkaline salts, thus raising the soil pH, there is damage to the plants from both saline and alkaline stresses. In some areas, soil alkalization as a result of NaHCO3 and Na2CO3 may be more severe than soil salinization from neutral salts such as NaCl and Na2SO4. For example, in north-east China, >70% of the land area is alkaline grassland (CitationKawanabe and Zhu 1991) and only a few alkali-tolerant halophytes can survive. To date, however, salinity stress research has focused on NaCl (CitationMunns and Tester 2008) and very little attention has been paid to alkaline stress (CitationGao et al. 2008; CitationShi and Sheng 2005; CitationShi and Wang 2005; CitationShi et al. 2002; CitationWang et al. 2007; CitationYang et al. 2008a,Citationb,Citationc, Citation2009a,Citationb).
Saline stress in soil generally involves osmotic stress and ion injury (CitationMunns 2002), whereas alkaline stress involves osmotic stress, ion injury and high-pH stress. The high pH environment surrounding the roots may severely affect the soil structure, interfere with ion uptake, break intracellular ion balances in plants (CitationYang et al. 2007) and inhibit growth (CitationShi and Yin 1993; CitationYang et al. 2007) and photosynthesis.
Wheat is an important crop, with some cultivars tolerant to saline stress. In north-east China, alkalinity (i.e. high pH) is an important factor limiting wheat productivity (CitationZheng and Li 1995). We compared the effects of saline stress (1:1 molar ratio of NaCl : Na2SO4) and alkaline stress (1:1 molar ratio of NaHCO3 : Na2CO3) on the germination, growth, osmotic adjustment and ionic balance of wheat seedlings to elucidate the mechanism of alkaline stress (high pH) damage to plants and the physiological adaptive mechanism of plants to alkaline stress.
Materials and methods
Experiment 1: Design of simulated saline and alkaline conditions
Two neutral salts, NaCl : Na2SO4, were mixed in a 1:1 molar ratio and applied to the saline stress group. Similarly two alkaline salts, NaHCO3 to Na2CO3, were mixed in a 1:1 molar ratio and applied to the alkaline stress group. In the saline stress group there were five concentrations: 30, 60, 90, 120 and 150 mmol L–1. Within Five concentrations were also used in the alkaline stress group: 15, 30, 45, 60 and 75 mmol L–1. These concentrations refer to the total salt concentrations of NaCl + Na2SO4 and NaHCO3 + Na2CO3. Therefore, in the 150 mmol L–1 saline stress solution, a mixture of 75 mmol L–1 NaCl and 75 mmol L–1 Na2SO4 resulted in a total ion concentration of 225 mmol L–1 Na+ + 75 mmol L–1 Cl− + 75 mmol L–1 SO2− 4.
Analysis of the activities and free concentrations of various ions
The effects of saline and alkaline stresses on the ionic activities (IA) and free concentrations (FC) of various ions in the nutrient solutions were analyzed with the program GEOCHEM-PC 2.0 (USA), derived from the multi-purpose chemical speciation program GEOCHEM, which has been widely used by soil and environmental chemists. The rationale for the use of this program has been described previously (CitationParker et al. 1987). In brief, the program calculates the IA and FC of ions by successive approximations that consider ion-pair formation and precipitation (CitationParker et al. 1987).
The complete nutrient solution used in the present study contained 5.00 mmol L–1 Ca2+, 2.00 mmol L–1 Mg2+, 6.04 mmol L–1 K+, 2.22 × 10−2 mmol L–1 ethylenediaminetetraacetic acid (EDTA)-Fe2+, 6.72 × 10−3 mmol L–1 Mn2+, 3.16 × 10−4 mmol L–1 Cu2+, 7.65 × 10−4 mmol L–1 Zn2+, 2.10 mmol L–1 SO4 2−, 1.00 mmol L–1 H2PO− 4, 4.63 × 10−2 mmol L–1 H3BO3, 5.56 × 10−4 mmol L–1 H2MoO4 and 15.04 mmol L–1 NO− 3 (pH 6.56). The electrical conductivity (EC) and osmotic potential of the stress treatment solutions were measured using a conductivity meter (DDG-2080-S; Saike Anhui Environmental Protection Technology Co., Ltd., Anhui, China) and a water potential meter (Psypro Wescor Corporation in South Logan, USA), respectively.
Plant materials
Seeds of salt-tolerant Tai 15 were sown in 17-cm diameter plastic pots containing 2.5 kg of washed sand. Each pot contained 10 seedlings. Pots were watered daily with nutrient solution at 17.00–18.00 hours. All pots were kept in a greenhouse at 22.5 ± 1.5°C during the day and 18.5 ± 1.5°C at night. The plants grew at a uniform irradiance of photosynthetic photon flux density of 250 μmol m−2 s−1.
Stress treatments
When the seedlings were 2 weeks old, 60 pots with seedlings growing uniformly were selected and divided randomly into 12 sets, each with five pots. One set was used as the untreated control, a second set was used to determine the growth index at the beginning of treatment, and the remaining 10 sets were used for the stress treatments. Each pot was a single replicate, with five replicates per set. The pots were thoroughly watered daily with nutrient solution containing the appropriate stress saline at 17.00–18.00 hours. Control plants were watered with the nutrient solution. The stress treatments lasted 14 days.
Measurement of physiological indices
The relative growth rate (RGR) was determined from the dry weight (DW) as follows (CitationKingsbury et al. 1984): RGR = [(ln DW at the end of the stress treatment)−(ln DW at the start of the stress treatment)]/total treatment duration. The water content (WC; %) was calculated from the DW and the fresh weight (FW). Carotenoids (Car) and chlorophyll (Chl) a and b were extracted using acetone; and spectrophotometeric determination at 440, 645 and 663 nm for each sample was done three times. The calculations used the equations of CitationArnon (1949). Membrane permeability can be reflected by the electrolyte leakage rate (ELR), which was determined by the ameliorated method of CitationLutts et al. (1996). From each pot, 1 g of fresh leaf was taken and washed three times with deionized water to remove surface-adhered electrolytes. The leaves were placed in a closed cuvette containing 20 mL of deionized water. The cuvette was incubated at 25°C on a rotary shaker (Hinotek Technology Co., Ltd, Ningbo China) for 4 h and the EC of the solution (EC1) was determined with a conductivity gauge. After this the cuvette was autoclaved at 120°C for 20 min and the solution EC (EC2) was determined. The ELR was defined as follows: ELR (%) = (EC1/EC2) × 100. The activity of the root system was determined as described by CitationComas et al. (2000). Fresh roots were incubated for 60 min at 37°C in triphenyl tetrazolium chloride solution (0.04% in pH 7.0 phosphate buffer). The red product in the roots was extracted using ethyl acetate. Absorbances were determined by spectrophotometer at 485 nm and the activity of the root system was expressed relative to a control value of 100%.
Dry samples of plant material (100 mg) were treated with 20 mL of deionized water at 100°C for 2 h and the extract was used to determine the contents of free inorganic ions and organic acids (OAs) in the shoots. The OA content was measured by complexometry (CitationJing and Ding 1981). The NO3 −, Cl−, SO4 2− and H2PO4 − contents were determined by ion chromatography (DX-300 ion chromatographic system, AS4A-SC ion-exchange column, CD M-II electrical conductivity detector, mobile phase: Na2CO3/NaHCO3 = 1.7/1.8 mmol L–1; DIONEX, Sunnyvale, CA, USA). An atomic absorption spectrophotometer (TAS-990; Purkinje General, Beijing, China) was used to determine the contents of Na+, K+ and free Ca2+, Mg2+ and Fe2+. Proline was extracted using 3% sulfosalicylic acid for 30 min at 70°C and measured with ninhydrin (CitationZhu et al. 1983). The total soluble sugars (SS) were extracted using 70% alcohol for 30 min at 70°C and measured using anthrone (CitationBao 1981). Betaine was extracted with 80% methanol for 20 min at 70°C and determined using the method of CitationGrieve and Grattan (1983).
Experiment 2: Germination
Two neutral salts mixed at a 1:1 molar ratio (NaCl : Na2SO4) were applied to the saline stress group; similarly, two alkaline salts at 1:1 molar ratio (NaHCO3 : Na2CO3) were applied to the alkaline stress group. Within each group, there were five concentration treatments: 50, 100, 150, 200 and 250 mmol L–1. The pH ranges in the saline and alkaline stress groups were 6.66–7.01 and 9.83–10.03, respectively. Distilled water was used as a control. Five replicates with 50 wheat seeds each were used for each treatment. The seeds were sown onto filter paper in a 9-cm tight-fitting Petri dish and submerged in 5 mL of treatment solution (i.e. 5 mL was added to each dish). The dishes were placed in a growth cabinet and maintained at 20°C in darkness for 14 days. Germinated seeds were counted daily, with emergence of the radicle considered as germination. Evaporated water was replenished with distilled water daily to avoid changes in salinity. Non-germinated seeds were transferred to distilled water for another 10 days to assess their viability, from the germination recovery.
Statistical analysis
Statistical analyses (i.e. ANOVAs and correlations) were conducted using the statistical program SPSS 13.0 (SPSS Inc., Chicago, USA). All treatments were replicated five times and the means and calculated standard errors (SE) are reported. The level of statistical significance was P ≤ 0.05.
Results
Electrical conductivity and osmotic potential of the stress treatment solutions
The results in showed that with increased salinity, the EC increased, whereas the osmotic potential of the stress treatment solution decreased. The changes in the saline solution were greater than the changes in the alkaline solution.
The ionic activity and free concentrations of macronutrients in the nutrient solution
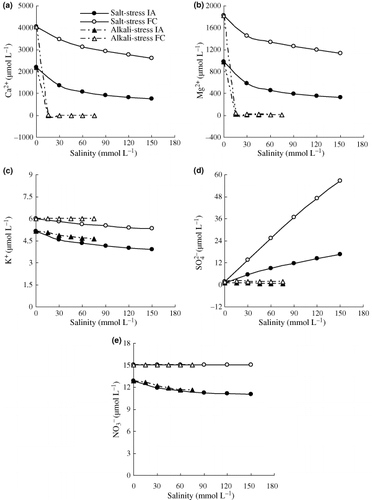
With increasing salinity, the IA and FC of Ca2+ and Mg2+ decreased, and the reduction for alkaline stress was far greater than the reduction for saline stress (). The FC of K+ decreased with increased saline stress, but increased slightly for alkaline stress; however, the IA of K+ decreased with increases of both stresses. At the same salinity, the IA and FC of K+ under saline stress were lower than those under alkaline stress (). Under alkaline stress, the IA and FC of SO2− 4 decreased with increasing salinity; however, the FC in all cases was higher than the controls, and the IA in all cases (except 15 mmol L–1) was lower than the controls (). For NO− 3 under both stresses, the FC was unaltered, but the IA decreased with increasing stress. Furthermore, the IA of NO− 3 was slightly lower for saline than for alkaline stress at the same degree of salinity ().
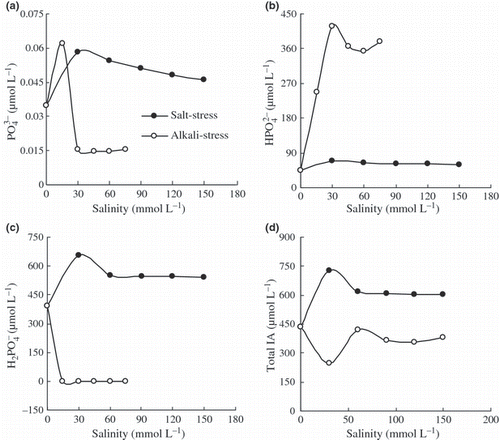
Phosphate was particularly susceptible to precipitation and ion-pair formation under saline and alkaline stresses (). In the controls, free phosphate was principally in the form of H2PO− 4. The total IA of free phosphate under alkaline stress was much lower than that under saline stress at the same degree of salinity (). Under saline stress, the IA values for PO3− 4, HPO2− 4 and H2PO− 4 were higher than the controls, and free phosphate was principally H2PO− 4 (∼ 90%) (). Under alkaline stress, the IA values of PO4 3− and HPO2− 4 were much higher than those under saline stress or in the controls. In contrast, the IA of H2PO4 − was much lower than that under saline stress or in the controls, and free phosphate was principally HPO2− 4 (∼ 99%) ().
Table 1 Electrical conductivity (EC) and osmotic potential of the stress treatment solutions
Growth
With increased saline concentrations both RGR and WC decreased and the reductions under alkaline stress were greater than under saline stress (; P < 0.01). A regression analysis between the RGR and the saline concentration was carried out, where Y Salt represented the RGR of the saline stress group, Y Alkali represented the RGR of the alkaline stress group and x was the salinity (). The RGR for shoots under saline stress decreased by 0.03 for each 100 mmol L–1 increase in salinity, and similarly decreased by 0.04 under increased alkaline stress from slopes of equations 1 and 2, respectively (). The RGR for roots under saline stress decreased by 0.04 for each 100 mmol L–1 increase in salinity, and similarly decreased by 0.10 under salinity from slopes of equations 3 and 4, respectively (). A comparison of the slopes of the two regression curves (RGR/salinity) showed that the inhibitory effects of alkaline stress on shoot and root growth, respectively, were approximately 1.33 and 2.5-fold that of saline stress. Saline stress caused only minor changes in ELR, pigment content and root activity (; P < 0.01). Alkaline stress led to a marked increase in ELR; and pigment content and root activity declined dramatically.
Cations
With increasing stress, the Na+ content increased (P < 0.01), whereas the K+ content decreased (P < 0.01); with greater changes under alkaline than under saline stress (). Saline stress has no effect on the accumulation of free Fe2+ and Mg2+, but their content increased with increased alkaline stress (). The content of free Ca2+ was lowered by saline, but increased by alkaline stress (; P < 0.01)
Anions
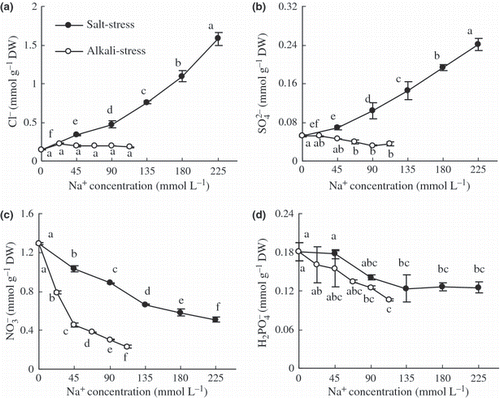
Under alkaline stress the contents of Cl− and SO2− 4 underwent a small change; with increased saline stress their contents increased (; P < 0.01), but the NO− 3 and H2PO− 4 contents decreased (; P < 0.01). However, the changes under alkaline stress were significantly greater than those under saline stress (P < 0.01).
Organic solutes
The impacts of saline stress on proline, SSs and betaine were similar, their contents significantly increased, with greater increases under alkaline than under saline stress (; P < 0.01). Under saline stress, there was no accumulation of OAs in the wheat shoots, but OAs under alkaline stress increased significantly (; P < 0.01).
Figure 3 Effects of salt and alkali stresses on (a) under ground and (b) above ground relative growth rate (RGR), (c) water content, (d) electrolyte leakage, (e) chlorophyll a, (f) chlorophyll b, (g) carotenoid content and (h) root system activity. Salt stress: NaCl : Na2SO4 = 1:1, pH 6.6–6.95; alkali stress: NaHCO3 : Na2CO3 = 1:1, pH 9.77–9.96. The values are the means of five replicates. Means followed by different letters in the same curve are significantly different at P ≤ 0.05 according to a least significant difference test. FW, fresh weight.
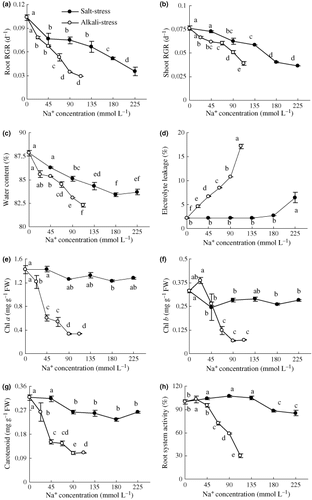
Table 2 Regression equation between the relative growth rate (RGR) and the salt concentration
Figure 4 Effects of salt and alkali stresses on the contents of (a) Na+, (b) K+, (c) free Fe2+, (d) free Mg2+ and (e) free Ca2+ in the wheat shoots. Salt stress: NaCl : Na2SO4 = 1:1, pH 6.6–6.95; alkali stress: NaHCO3 : Na2CO3 = 1:1, pH 9.77–9.96. The values are the means of five replicates. Means followed by different letters in the same curve are significantly different at P ≤ 0.05 according to a least significant difference test. DW, dry weight.
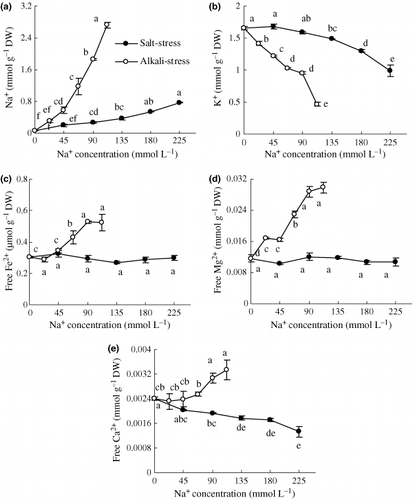
Figure 5 Effects of salt and alkali stresses on the contents of (a) Cl−, (b) SO42−, (c) NO3− and (d) H2PO4− in the wheat shoots. Salt stress: NaCl : Na2SO4 = 1:1, pH 6.6–6.95; alkali stress: NaHCO3 : Na2CO3 = 1:1, pH 9.77–9.96. The values are the means of five replicates. Means followed by different letters in the same curve are significantly different at P ≤ 0.05 according to least significant difference test. DW, dry weight.
Germination
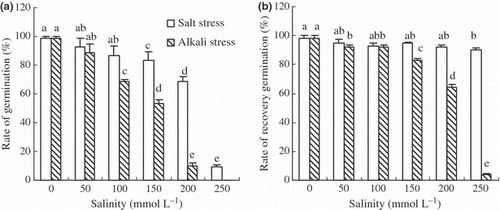
The trend in the changes in germination rate of wheat under the two stress conditions was similar; there was a decreased trend with increased saline and alkaline stress (P < 0.01), but the reductions under alkaline stress were greater than under saline stress (). There was no germination in the 250 mmol L–1 alkaline stress treatment. After the stress treatments, when the seeds were transferred to distilled water, most seeds that had been under saline stress recovered and germinated; but few recovered germination after high alkaline stress (). The recovery of germination of alkaline-stressed seeds decreased with increased alkaline stress; only 4% germinated after the highest alkaline stress.
Discussion
Effects of saline and alkaline stresses on the state of mineral elements in nutrient solutions
The activity and states of the ions in solution are related to Ca2+ activity at the plasmalemma and plant growth (CitationCramer et al. 1986). The GEOCHEM-PC program was used to calculate the IA and FC in a complete nutrient solution and showed that ion interactions in a complex solution were much more significant and complex than in a simple salt solution (CitationCramer et al. 1986). In addition, this indicated that the addition of any salt changes the IA and FC of the ions added, and could substantially alter the IA and FC of other ions in solution. This is particularly important when studying the effect of a specific ion in solution because the usual implicit assumption is that the IA and FC of other ions do not change. The IA and FC of some ions, such as Na+, K+ and NO− 3, lie principally in their activity coefficient, and the effects of saline and alkaline stresses on their IA and FC are slight. However, the analysis of the IA and FC for other ions is more complex and requires consideration of the formations of ion pairs and precipitates.
The decrease in the IA and FC caused by saline stress was limited, being merely the result of enhanced ion interaction caused by an increased degree of salinity (,). However, the change in phosphate IA caused by saline stress was different to the other ions. The total IA of phosphate under saline stress was higher than in the controls (). The GEOCHEM-P analysis showed that phosphate IA reduction in the controls resulted from complexing reactions or precipitation caused by Ca2+ and Mg2+. High Na+ concentration can inhibit these reactions by reducing the IA of Ca2+ and Mg2+. The effects of alkaline stress on ions (especially phosphate) in the nutrient solution were much more complex and greater than those of saline stress. Comparing both stresses, the effects of alkaline stress on the ions were related to ion interactions and to the effect of high pH caused by HCO3 − and CO3 2−. A high pH affects ion-pair formation and results in the precipitation of phosphorus and metal ions other than Na+ and K+, leading to sharp decreases in their IA and FC and to a serious lack of mineral nutrients surrounding the roots. However, phosphate is particularly susceptible to precipitation and to the formation of ion-pairs caused by the high pH of alkaline stress. The total IA of free phosphate under alkaline stress was much less than that under saline stress at the same degree of salinity (). Under saline stress, the free phosphate in the nutrient solution was principally H2PO− 4. Under alkaline stress, the phosphate is precipitated with metal ions and the free phosphate was principally HPO2− 4.
Figure 6 Effects of salt and alkali stresses on the contents of (a) proline, (b) soluble saccharides, (c) betaine and (d) organic acid in the wheat shoots. Salt stress: NaCl : Na2SO4 = 1:1, pH 6.6–6.95; alkali stress: NaHCO3 : Na2CO3 = 1:1, pH 9.77–9.96. The values are the means of five replicates. Means followed by different letters in the same curve are significantly different at P ≤ 0.05 according to least significant difference test. DW, dry weight.
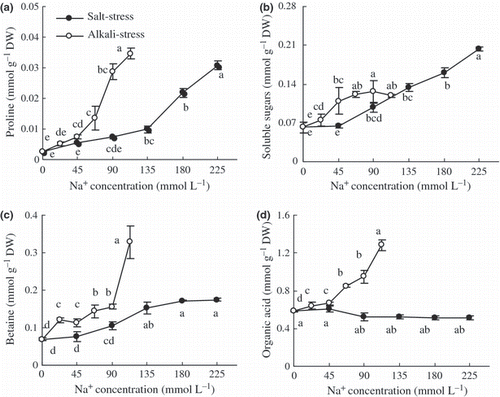
Figure 7 Effects of salt and alkali stresses on (a) the rate of germination and (b) the recovery germination of wheat. Salt stress: NaCl : Na2SO4 = 1:1, pH 6.66–7.01; alkali stress: NaHCO3 : Na2CO3 = 1:1, pH 9.83–10.03. The values are the means of five replicates. Means followed by different letters in the same column are significantly different at P ≤ 0.05 according to least significant difference test.
Growth
The RGR value reflects the life-sustaining activities of the plant and is considered to be an optimum index for the degree of stress and the response of plants to various stresses (CitationYang et al. 2007). The RGR decreased in wheat with increasing stress intensity for both saline and alkaline stresses (), and the decrease for alkaline stress was greater than the decrease observed under saline stress. The injurious effect of alkaline stress was greater than that of saline stress at the same stress intensity (i.e. Na+ concentration). Moreover, the regression analysis between RGR and salinity indicated that the injurious effect of alkaline stress was greater on roots than on shoots ().
The injurious effect of alkaline stress as greater than that of saline stress was consistent with previous reports (CitationShi and Yin 1993; CitationYang et al. 2007). The different injuriousness of the two stresses may result from their different mechanisms of action. The injurious effects of salinity are commonly thought to be from low water potentials and ion toxicities (CitationMunns 2002). Alkaline stress, however, involves osmotic stress and ion injury as well as high-pH stress. Saline stress did not affect wheat root activity (), whereas alkaline stress led to a sharp decrease and even to the death of root cells. Moreover, alkaline stress resulted in a lack of mineral nutrients surrounding the roots (,) and severe nutritional stress. Nutrient stress and root damage may be the main reasons why alkaline stress is more harmful to wheat than saline stress. The effects of saline stress on the WC (), membrane permeability () and photosynthetic pigments () were slight compared with alkaline stress; however, alkaline stress induced severe reductions in WC () and photosynthetic pigment contents, and sharply increased ELR. These results indicate that the high pH resulting from alkaline stress might damage root functions, such as the absorption of water and ions. These may be the main reasons why the wheat RGR was lower for alkaline than saline stress.
Osmoregulation
Low Na+ and high K+ in the cytoplasm are essential for the maintenance of many enzymatic processes. Plants in saline conditions usually accumulate high concentrations of Na+ in the vacuoles to decrease the cell water potential (CitationMunns and Tester 2008). The Na+ enters the plant cells through the high-affinity K+ transporter and non-selective cation channels (CitationZhu 2003). The similarity of the hydrated ionic radii of Na+ and K+ makes them difficult to discriminate, and this is the basis of Na+ toxicity (CitationBlumwald 2000). Under saline stress, Na+ competes with K+ for uptake into roots (CitationMunns and Tester 2008). The results of the present study showed that with increasing stress intensity the Na+ content in the shoot increased, whereas the K+ content decreased, with greater change under alkaline than saline stress (). This indicates that the high pH of alkaline stress might interfere with the control of Na+ uptake in roots and increase intracellular Na+ to a toxic level, which may cause most of the damage under higher alkaline stress (). We propose that the increased Na+ accumulation caused by alkaline stress was not a response to osmotic stress, but rather a specific response to high pH. Alkaline stress might have weakened the controls on the absorption or transport of Na+, leading to the sharp increase in the Na+ content in the shoots () and disrupting the ionic balance or pH homeostasis in the tissues. The increased Na+ in the shoots under alkaline stress might also be related to decreased Na+ exclusion. It is well known that wheat plants have a Na+ exclusion mechanism depending on a Na+/H+ antiport, such as salt overly sensitive 1 (SOS1), which exchanges cytoplasmic Na+ with external H+ (CitationMunns and Tester 2008; CitationZhu 2003). The exchange activity relies on the transmembrane proton gradient achieved by H+-ATPase (CitationZhu 2003). Under high alkaline stress, the lack of external protons might weaken the exchange activity of the Na+/H+ antiport on the root plasma membrane (CitationMunns and Tester 2008), reducing exclusion of Na+ into the rhizosphere and enhancing in vivo accumulation.
There were interesting responses of free Ca2+ and Mg2+ and Fe2+ to both stresses (). This might be a strain-specific response of wheat to alkaline stress, or simply a specific adaptative response, which should be further investigated. Although Ca2+ and Mg2+ and Fe2+ increased under alkaline stress, their contributions to osmotic adjustment were small because their ratios to total cations were very low. Betaine, proline and SS are three organic osmolytes distributed principally in the protoplasm. Our results show that their accumulations in wheat may be a response to Na+ influx (,), and they may distribute in the cytoplasm to balance the osmotic pressure from vacuoles and protect biomacromolecules. Under saline or alkaline stress, the OAs accumulated in plants are usually in vacuoles to neutralize the cations (CitationShi et al. 2002; CitationYang et al. 2007). Betaine (0.9–4.0% of DW) and SS (1.4–3.0% of DW) are clearly primary osmolytes under both stresses, and the proportions contributing to osmotic adjustment in the protoplasm were significantly greater than proline (). For both stresses, the key feature of wheat pertaining to osmotic adjustment was its ability to accumulate inorganic ions and OAs in vacuoles and to accumulate a large amount of betaine and SS in the protoplasm. The difference between the two stresses was that the contribution of Cl− was much greater under saline than under alkaline stress, but the contribution of OAs was much greater under alkaline than saline stress.
Table 3 Percentage of each solute molarity to the total molarity
Ion balance
An ion imbalance in plants is mainly caused by the influx of superfluous Na+ (CitationBlumwald 2000; CitationYang et al. 2007, Citation2008b). Plants usually accumulate inorganic anions such as Cl−, NO3 − and SO4 2− or synthesized organic anions to maintain ion balance (CitationYang et al. 2007). The pH homeostasis of the internal environment is related to all free ions and also to all solutes with charge, and is a result of ion balance that includes organic and inorganic ions (CitationYang et al. 2007). Under saline stress, wheat also accumulated OAs and inorganic anions to balance the massive influx of Na+. However, the concentrations of inorganic anions were significantly lower under alkaline stress than under saline stress of the same intensity, suggesting that the high pH of alkaline stress might inhibit the uptake of anions such as NO3 − and H2PO4 − (). Wheat might enhance OA synthesis to remedy the shortage of inorganic anions and to maintain a stable intracellular pH. The material and energy demands of OA synthesis are much greater than the demands required for the absorption of inorganic ions (CitationMunns 2002), and possibly a reason for the lower RGR under alkaline stress. The synthesized OA might also be transported to the roots for pH regulation. The process of pH regulation may occur outside the root, in the root apoplast or both. Therefore, epidermal, cortical or xylem parenchymatous cells might participate in pH regulation of the roots. The means of pH regulation in the roots may be the exudation of buffer compounds, such as H+, OAs and amino acids, and CO2 produced by root respiration. The accumulation of OAs may be a central adaptive mechanism by which wheat can maintain a stable intracellular pH under alkaline stress. The accumulation of OAs in shoots may not be a simple passive response to a negative charge deficit, but rather a result of active metabolic regulation after sensing signals of alkaline stress. The metabolic regulation of OAs under alkaline stress may involve one or more key enzymes that may be known to participate in basal metabolic pathways, such as the tricarboxylic acid cycle, glyoxylate cycle or glycolysis. The regulation of enzyme activities may occur at synthesis (transcription, translation and new polypeptide modification) or after synthesis by regulation or inhibition of the activator. If the pattern of OA response can be drawn clearly, the key genes of plant alkaline stresstolerance would be easily identified and cloned. This should be further investigated.
Germination
Germination is one of the most critical periods in the life cycle of plants. Under saline stress, a low water potential is a determining factor inhibiting seed germination (CitationDebez et al. 2004). However, our results indicated that alkaline stress (high pH) also significantly affected seed germination (). The inhibiting action of alkaline stress on wheat germination was greater than the inhibiting action of saline stress at the same salinity (). Ungerminated seeds may be in a state of dormancy to escape from the rigorous environment (CitationDebez et al. 2004). When rain falls and decreases soil salinity, then wheat seeds would be able to germinate again, and this might be a strategy for living in a soil with high salinity. The effects of high pH on wheat germination were much more complex than the effects of salinity. Germination was not inhibited by high pH at low salinity, but was inhibited at higher salinity, implying a function of pH adjustment in the seeds that allowed germination at low salinity. Under higher salinity, high pH may decompose the seed structure and even result in death (), a possibly complex response that deserves further research.
Conclusion
The present study indicated that saline and alkaline stresses are two different stresses, the harmful effect of alkaline stress on the growth and germination of wheat was significantly greater than that of saline stress, which might result from the negative effects of high pH on plants.
First, high pH caused phosphate and metal ions to precipitate and caused their availability to decline, making the supply of mineral nutrient ions near the root seriously imbalanced. This may destroy the physiological structure of the roots, reducing root activity, and finally causing function reduction or even loss.
Second, high pH strongly influenced the accumulation of inorganic ions in the wheat shoot; the Na+ content sharply increased, and NO− 3, H2PO− 4 and K+ contents obviously decreased. The main reason for the negative impact of high pH on ion accumulation was that high pH caused a lack of protons outside the root, which impeded the establishment of a transmembrane proton gradient. Further study of high pH is needed because high pH may also directly affect the related activities of ion absorption channels or carriers.
Third, under alkaline stress a large Na+ inflow may cause wheat cell stress responses, directly leading to decreased photosynthetic pigments, increased membrane permeability, and to the destruction of the photosynthetic system (CitationYang et al. 2008c). The accumulation of compatible solutes and OAs may be an adaptive response to the substantial influx of Na+ and may be a function to deal, respectively, with the cell osmotic stress and to maintain the intracellular ion balance
Alkaline stress is a very severe abiotic stress that has caused very serious problems in China and some other areas. It is one of the main causes of grassland degradation and plant physiologists and government departments should pay more attention to the harm caused by alkaline stress. It is expected that the present study will provide a scientific theoretical foundation for wheat growers and specialists for the cultivation and research of saline and alkaline stresses of wheat.
Acknowledgments
This work was supported by grants from the Project of The National Key Basic Research Special Foundation (No. 2007CB106801), The National Science and Technology Supporting Program (No. 2006BAD16B06) and The Natural Science Foundation of China (No. 30470272 and No. 30770397). We thank Dr ChunWu Yang from Northeast Normal University for assistance with the experiments and International Science Editing for editing the English.
References
- Arnon , DI . 1949 . Copper enzymes in isolated chloroplasts. Polyphenooxidase in Beta vulgaris . Plant Physiol. , 24 : 1 – 15 .
- Bao , SD . 1981 . “ Determine of soluble sugar in plant ” . In SD Analytic Methods for Soil and Agriculture Chemistry , Edited by: Li , Sijing . 150 – 160 . Beijing : China Agriculture Press .
- Blumwald , E . 2000 . Sodium transport and salt tolerance in plants . Curr. Opin. Cell Biol. , 12 : 431 – 434 .
- Comas , LH , Eissenstat , DM and Lakso , AN . 2000 . Assessing root death and root system dynamics in a study of grape canopy pruning . New Phytol. , 147 : 171 – 178 .
- Cramer , GR , Laüchli , AE and Epstein , E . 1986 . Effects of NaCl and CaCl2on ion activities in complex nutrient solutions and root growth of cotton . Plant Physiol. , 81 : 792 – 797 .
- Debez , A , Hamed , BK , Grignon , C and Abdelly , C . 2004 . Salinity effects on germination, growth, and seed production of the halophyte Cakile maritime . Plant Soil , 262 : 179 – 189 .
- Gao , C , Wang , Y , Liu , G , Yang , C , Jiang , J and Li , H . 2008 . Expression profiling of salinity–alkali stress responses by large-scale expressed sequence tag analysis in Tamarix hispid . Plant Mol. Biol. , 66 : 245 – 258 .
- Grieve , CM and Grattan , SR . 1983 . Rapid assay for determination of water-soluble quaternary-amino compounds . Plant Soil , 70 : 303 – 307 .
- Jing , JH and Ding , ZR . 1981 . “ Determining organic acid content ” . In Analysis Method of Plant Biochemistry , Edited by: Boqinnoke , XH . 264 – 267 . Beijing : Science Press . (in Chinese)
- Kawanabe , S and Zhu , TC . 1991 . Degeneration and conservation of Aneurolepidium chinensegrassland in Northern China . J. Jpn. Grassland Sci. , 37 : 91 – 99 .
- Kingsbury , RW , Epstein , E and Pearcy , RW . 1984 . Physiological responses to salinity in selected lines of wheat . Plant Physiol. , 74 : 417 – 423 .
- Läuchli , A and Lüttge , U . 2002 . Salinity: Environment – Plants – Molecules , 552 Dordrecht Boston : Springer .
- Lutts , S , Kine , JM and Bouharmont , J . 1996 . NaCl-induced senescence in leaves of rice (Oryza sativaL.) cultivars differing in salinity resistance . Ann. Bot. , 78 : 389 – 398 .
- Munns , R . 2002 . Comparative physiology of salt and water stress . Plant Cell Environ. , 25 : 239 – 250 .
- Munns , R and Tester , M . 2008 . Mechanisms of salinity tolerance . Annu. Rev. Plant Biol. , 59 : 651 – 681 .
- Parker , DR , Zelazny , LW and Kinraide , TB . 1987 . Improvements to the program Geochem . Soil Sci. Soc. Am. J. , 51 : 488 – 491 .
- Shi , DC and Sheng , Y . 2005 . Effect of various salt–alkaline mixed stress conditions on sunflower seedlings and analysis of their stress factors . Environ. Exp. Bot. , 54 : 8 – 21 .
- Shi , DC and Wang , D . 2005 . Effects of various salt–alkali mixed stresses on Aneurolepidium chinense(Trin.) Kitag . Plant Soil , 271 : 15 – 26 .
- Shi , DC and Yin , LJ . 1993 . Difference between salt (NaCl) and alkaline (Na2CO3) stresses on Puccinellia tenuiflora(Griseb.) Scribn et Merr. plants . Acta Bot. Sin. , 3 : 144 – 149 .
- Shi , DC , Yin , SJ , Yang , GH and Zhao , KF . 2002 . Citric acid accumulation in an alkali-tolerant plant Puccinellia tenuifloraunder alkaline stress . Acta Bot. Sin. , 44 : 537 – 540 .
- Wang , Y , Yang , C , Liu , G and Jiang , J . 2007 . Development of a cDNA microarray to identify gene expression of Puccinellia tenuifloraunder saline–alkali stress . Plant Physiol. Biochem. , 45 : 567 – 576 .
- Yang , C , Chong , J , Kim , C , Li , C , Shi , D and Wang , D . 2007 . Osmotic adjustment and ion balance traits of an alkali resistant halophyte Kochia sieversianaduring adaptation to salt and alkali conditions . Plant Soil , 294 : 263 – 276 .
- Yang , C , Jianaer , A , Li , C , Shi , C and Wang , D . 2008a . Comparison of the effects of salt-stress and alkali-stress on the photosynthetic production and energy storage of an alkali-resistant halophyte Chloris virgata . Photosynthetica , 46 : 273 – 278 .
- Yang , C , Shi , D and Wang , D . 2008b . Comparative effects of salt stress and alkali stress on growth, osmotic adjustment and ionic balance of an alkali resistant halophyte Suaeda glauca(Bge.) . Plant Growth Regul. , 56 : 179 – 190 .
- Yang , C , Wang , P , Li , C , Shi , DC and Wang , D . 2008c . Comparison of effects of salt and alkali stresses on the growth and photosynthesis of wheat . Photosynthetica , 46 : 107 – 114 .
- Yang , C , Xu , HH , Wang , L , Liu , J , Shi , DC and Wang , D . 2009b . Comparative effects of salt-stress and alkali-stress on the growth, photosynthesis, solute accumulation, and ion balance of barley plants . Photosynthetica , 47 : 79 – 86 .
- Yang , C , Zhang , M , Liu , J , Shi , DC and Wang , D . 2009a . Effects of buffer capacity on growth, photosynthesis, and solute accumulation of a glycophyte (wheat) and a halophyte (Chloris virgata) . Photosynthetica , 47 ( 1 ) : 55 – 60 .
- Zheng , HY and Li , JD . 1995 . A preliminary study on the formation of saline-alkaline plant communities in the Songnen Plain . Acta phytoecologica Sinica , 19 : 1 – 12 .
- Zhu , JK . 2003 . Regulation of ion homeostasis under salt stress . Curr. Opin. Cell Biol. , 6 : 441 – 445 .
- Zhu , GL , Deng , XW and Zuo , WN . 1983 . Determination of free proline in plants . Plant Physiol. Commun. , 1 : 35 – 37 .