Abstract
We compared the effects of saline stress (9:1 molar ratio of NaCl : Na2SO4, pH 6.44–6.65) and alkaline stress (9:1 molar ratio of NaHCO3 : Na2CO3, pH 8.71–8.89) on the germination, growth, photosynthesis, ionic balance and activity of anti-oxidant enzymes of Lathyrus quinquenervius to elucidate the physiological adaptive mechanism of plants to alkaline stress (high pH). The results showed that, at a low stress intensity, the effects of saline stress and alkaline stress on L. quinquenervius were similar. Compared with saline stress, high alkaline stress intensity clearly inhibited germination, growth, photosynthesis and root system activity, and led to a sharp increase in Na+ and an ion imbalance in the shoots, as well as enhanced H2O2 and malondialdehyde content, resulting in severe intracellular oxidative stress. The results indicated that the accumulation of organic acid was a central adaptive mechanism by which L. quinquenervius maintained intracellular ionic balance under alkaline stress. Lathyrus quinquenervius may enhance organic acid synthesis to remedy the shortage of negative charge resulting from the massive influx of Na+ and decreased inorganic anions. In addition, saline stress and low alkaline stress slightly enhanced the activities of superoxide dismutase (SOD) and ascorbate peroxidase (APX), but did not affect catalase (CAT) activity. However, strong alkaline stress significantly enhanced the activities of SOD and APX, and reduced CAT activity. We propose that enhancing the activities of SOD and APX may be a vital mechanism by which L. quinquenervius resists oxidative stress caused by alkaline stress.
Introduction
The existence of alkaline stress has been clearly demonstrated by a number of reports (CitationCampbell and Nishio 2000; CitationEl-Samad and Shaddad 1996; CitationHartung et al. 2002; CitationKawanabe and Zhu 1991; CitationLäuchli and Lüttge 2002; CitationRao et al. 2008), which have shown that alkaline stress is more severe than saline stress (CitationShi and Sheng 2005; CitationShi and Wang 2005; CitationYang et al. 2007, Citation2008a). In our previous studies, we suggested that saline stress should be defined as stress resulting from neutral salts and that alkaline stress is stress resulting from alkaline salts (CitationShi and Sheng 2005; CitationShi and Wang 2005; CitationShi and Yin 1993). In some areas alkalization of the soil as a result of NaHCO3 and Na2CO3 may be a more severe problem than soil salinization caused by neutral salts such as NaCl and Na2SO4. For example, in northeast China, more than 70% of the land area is alkaline grassland (CitationKawanabe and Zhu 1991), and only a few alkaline-tolerant halophytes can survive (CitationZheng and Li 1999). Stress resulting from soil salinity generally involves osmotic stress and ion-induced injury (CitationMunns 2002). A comparison of alkaline stress with saline stress revealed an added effect of alkaline stress as a result of high pH. The high-pH environment that surrounds the roots can cause metal ions and phosphorus to precipitate (CitationShi and Zhao 1997), can greatly affect the absorption of inorganic anions, and can disrupt the ionic balance and pH homeostasis in the tissues (CitationYang et al. 2007, Citation2008b). Thus, plants in alkaline soil must cope with both physiological drought and ion toxicity, and also maintain intracellular ionic balance.
Lathyrus quinquenervius is a naturally salt-tolerant leguminous forage plant commonly distributed in northern China; in Korea, Japan and the far east region of Russia this species shows localized, discontinuous distribution. This plant occurs naturally in high alkaline soil with a pH >9 and has a high protein content. As high-quality forage, it is of considerable economic value (CitationZheng and Li 1999) and is mostly used for hay, green forage, silage and grain.
In the present study, we compared the effects of saline stress (9:1 molar ratio of NaCl : Na2SO4, pH 6.44–6.65) and alkaline stress (9:1 molar ratio of NaHCO3 : Na2CO3, pH 8.71–8.89) on the germination, growth, photosynthesis, ionic balance and activity of anti-oxidant enzymes in L. quinquenervius seedlings to elucidate the mechanism of alkaline stress (high pH) damage to plants and the physiological adaptive mechanism of the plants to alkaline stress.
Materials and methods
Experiment 1: Seed germination
Seeds of L. quinquenervius were collected from alkaline grassland in western Jilin Province, China. Two neutral salts were mixed in a 9:1 molar ratio (NaCl : Na2SO4) and applied to the saline stress group. Two alkaline salts were mixed in a 9:1 molar ratio (NaHCO3 : Na2CO3) and applied to the alkaline stress group. Within each group, five concentrations were used: 50, 100, 150, 200 and 250 mmol L−1 (labeled as GS1–GS5 for the saline stress group and GA1–GA5 for the alkaline stress group). The pH ranges in the saline stress and alkaline stress groups were 6.40–6.64 and 8.83–8.98, respectively. Distilled water was used as a control. Before the germination assay, L. quinquenervius seeds were soaked in concentrated sulfuric acid for 25 min, removed and rinsed with sterile deionized water. Four replicates with 50 L. quinquenervius seeds each were used for each treatment. The seeds were placed on filter paper in a 9-cm tight-fitting Petri dish and submerged in 5 mL of each treatment solution. The dishes were placed in a growth cabinet at 20°C and exposed to 12 h light daily for 10 days. Germinated seeds were counted daily, with the emergence of the radicle considered as germination. Evaporated water was replenished with distilled water daily to avoid changes in salinity. The ungerminated seeds were transferred to distilled water for another 10 days to assess their capability of germination recovery.
Experiment 2: Seedlings
Plant materials
Seeds of L. quinquenervius were sown in 17-cm diameter plastic pots containing 2.5 kg of washed sand. The pots were sufficiently watered with Hoagland nutrient solution daily. All pots were placed outdoors and protected from the rain. Temperatures during the experimental period were 24–28°C during the day and 20–23°C at night.
Design of the simulated salt and alkaline conditions
Two neutral salts were mixed in a 9:1 molar ratio (NaCl : Na2SO4) and applied to the saline stress group. For the alkaline stress group, two alkaline salts were mixed in a 9:1 molar ratio (NaHCO3 : Na2CO3). Within each group, three salt concentrations were used: 30, 60 and 90 mmol L−1. The saline stress groups were labeled S1–S3 and the alkaline stress groups were labeled A1–A3. Therefore, in the 90 mmol L−1 solution for saline stress, a mixture of 81 mmol L−1 NaCl and 9 mmol L−1 Na2SO4 resulted in ion concentrations of 99 mmol L−1 Na+, 81 mmol L−1 Cl− and 9 mmol L−1 SO4 2−. In the 90 mmol L−1 solution for alkaline stress, a mixture of 81 mmol L−1 NaHCO3 and 9 mmol L−1 Na2CO3 resulted in ion concentrations of 99 mmol L−1 Na+, 81 mmol L−1 HCO3 − and 9 mmol L−1 CO3 2−. In the saline stress and alkaline stress groups, the pH was 6.44–6.65 and 8.71–8.89, respectively.
Stress treatments
The stress treatments were applied when the seedlings were 8 weeks old. Thirty-two pots of uniformly growing seedlings were divided randomly into eight sets, with four pots per set. Each pot was considered to be a single replicate. One set was used as a control, a second set was used for growth index determination at the beginning of the treatment, and the remaining six sets were used for the various stress treatments. The pots were watered thoroughly daily at 17.00–18.00 hours with a nutrient solution that contained the appropriate stress salts. Control plants were maintained by watering with nutrient solution. The entire treatment duration was 10 days.
Measurement of growth indices
Leaves at the same positions were measured by a portable photosynthesis system (LI 6400 XT; Li-Cor, Lincoln, NE, USA) in their locations to determine the net photosynthetic rate (P N), stomatal conductance (g s) and transpiration rate (E) of the leaves. The relative growth rate (RGR) was determined according to CitationKingsbury et al. (1984) as follows: RGR = (ln W 2 − ln W 1)/t 2 − t 1, where W1 and W2 are the dry weights obtained from the start and end of the stress treatment, respectively, and t 2 − t 1 is the total treatment duration. Carotenoids (Car) and chlorophyll (Chl) a and b were extracted using acetone, and spectrophotometric determination at 440, 645 and 663 nm of each sample was carried out three times. The calculation was adopted from the equations of CitationArnon (1949). Membrane permeability can be reflected by the electrolyte leakage rate (ELR), which was determined using the modified method of CitationLutts et al. (1996). Fresh leaves (1 g) were taken from each pot and washed three times with deionized water to remove surface-adhered electrolytes. The leaves were placed in a closed cuvette containing 20 mL of deionized water. The cuvette was incubated at 25°C on a rotary shaker for 4 h, and the electrical conductivity of the solution (EC1) was determined with a conductivity gauge DDS-307; Shanghai precision & scientific instrument, Shanghai, China. The cuvette was then autoclaved at 120°C for 20 min and the electrical conductivity of the solution (EC2) was determined. The ELR can be defined as follows: ELR [%] = (EC1/EC2) × 100. The activity of the root system was determined as described by CitationComas et al. (2000). The fresh root was incubated for 60 min at 37°C in triphenyl tetrazolium chloride (TTC) solution (0.04% in pH 7.0 phosphate buffer). The red product in the root was extracted using ethyl acetate. The absorbances were determined by a spectrophotometer at 485 nm. The activity of the root system was expressed relative to a control value of 100%. To determine tissue sap pH, fresh shoots were thoroughly washed three times with neutral deionized water, followed by surface-drying with filter paper. The shoots were then crushed and the tissue sap was extruded and the pH measured with a digital pH meter PHS-3C; Shanghai precision & scientific instrument, Shanghai, China.
Measurement of ions
Dry samples of plant material (100 mg) were treated with 20 mL of deionized water at 100°C for 1 h and the resultant extract was used to determine the contents of inorganic ions and organic acids (OAs). The contents of NO3 −, Cl−, SO4 2−, H2PO4 − and oxalic acid were determined by ion chromatography using a DX-300 ion chromatographic system with an AS4A-SC ion-exchange column and a CDM-II electrical conductivity detector (mobile phase: Na2CO3/NaHCO3 = 1.7/1.8 mmol L−1; DIONEX, Sunnyvale, CA, USA). The levels of other OAs were also determined by ion chromatography using the DX-300 ion chromatographic system with an ICE-AS6 ion-exclusion column, CDM-II electrical conductivity detector and an AMMS-ICE II MicroMembrane suppressor (mobile phase: 0.4 mmol L−1 heptafluorobutyric acid; DIONEX). An atomic absorption spectrophotometer (TAS-990; Purkinje General, Beijing, China) was used to determine the levels of Na+, K+, Ca2+, Mg2+ and Fe.
Measurements of lipid peroxidation
The H2O2 content in the leaves was determined according to CitationWolff (1994). This assay is based on ferrous ion oxidation in the presence of the ferric ion indicator xylenol orange. Lipid peroxidation was determined as the amount of malondialdehyde (MDA), a product of lipid peroxidation, according to CitationDraper and Hadley (1990). The content of MDA in the leaves was measured according to the method of CitationDu and Bramlage (1992). In brief, 20% trichloroacetic acid containing 0.5% 2-thiobarbituric acid was added to 0.2 mL of the chloroplast suspension, and heated at 95°C for 25 min and then cooled in a cool water bath and centrifuged at 3,000 g for 5 min. The MDA content was measured at 532 nm and corrected by subtracting the absorbance at 600 nm.
Anti-oxidant enzyme assays
For the determination of anti-oxidant enzyme activities, 0.5 g of fresh leaves was homogenized in 4 mL of the respective extraction buffer in a pre-chilled mortar and pestle. The homogenate was filtered through four layers of cheesecloth and centrifuged at 15,000 g for 20 min at 4°C. The supernatant was centrifuged again at 15,000 g for 20 min at 4°C before determining the anti-oxidant enzyme activities. The soluble protein concentration was determined according to CitationBradford (1976). Activities of the anti-oxidant enzymes are expressed in units per gram of protein. The data on enzyme activities were expressed relative to the control value of 100%. Superoxide dismutase (SOD) activity was assayed by monitoring inhibition of the photochemical reduction of nitro blue tetrazolium (NBT) according to the method of CitationBeyer and Fridovich (1987). Leaves were homogenized in 1 mL of cold 100 mmol L−1 K-phosphate buffer (pH 7.8) containing 0.1 mmol L−1 ethylenediaminetetraacetic acid (EDTA), 1% (w/v) polyvinyl-pyrrolidone and 0.5% (v/v) Triton X-100. One unit of SOD activity was defined as the amount of enzyme required to cause 50% inhibition in the reduction of NBT as monitored at 560 nm. For the determination of ascorbate peroxidase (APX), leaves were homogenized in 100 mmol L−1 Na-phosphate buffer (pH 7.0) containing 5 mmol L−1 ascorbate, 10% glycerol and 1 mmol L−1 EDTA. The APX activity was determined in a 1 mL reaction mixture containing 50 mmol L−1 K-phosphate buffer (pH 7.0), 0.1 mmol L−1 Vitamin C (extinction coefficient, 2800 L−1 mol−1 cm−1) and 0.3 mmol L−1 H2O2. The decrease in absorbance was recorded at 290 nm for 3 min (CitationChen and Asada 1989). A blank was obtained by adding phosphate buffer instead of extracts to the reaction mixture. Catalase (CAT) activity was determined by recording the continuous decrease in hydrogen peroxide (H2O2) absorbance at 240 nm according to the method of CitationFerrari et al. (2009). The reaction was carried out in 3 mL sodium phosphate buffer (50 mmol L−1, pH 7.0) containing 25 mmol L−1 H2O2. The baseline absorbance remained constant and 20 μL of supernatant was added to initiate the catalyzed reaction.
Statistical data analysis
Measurements were obtained from four replicates. Data were analyzed by one-way ANOVA using the statistical software SPSS 13.0 (SPSS, Chicago, IL, USA). The mean values for the different treatments were compared by post-hoc least significant difference tests. Values of P < 0.05 were considered to indicate significance.
Results
Germination
Both stresses reduced the germination rate, but the extent of the reduction under alkaline stress was much greater than that under saline stress (). The seeds that did not germinate were placed in distilled water for recovery germination to examine their survival capacity. Most of these seeds resumed germination under all the saline stress and low alkaline stress conditions (30 and 60 mmol L−1), but under high alkaline stress only a few seeds were able to recover germination ().
Growth
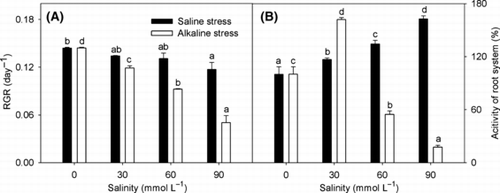
With increasing salinity, the RGR in shoots decreased; however, the reductions under alkaline stress were much greater than those under saline stress (). Saline stress and alkaline stress both increased the activity of the root system, but strong alkaline stress (>30 mmol L−1) caused a sharp decrease ().
Figure 1 Effects of saline and alkaline stress on the (A) rate of germination and (B) rate of recovery germination in Lathyrus quinquenervius seeds. The L. quinquenervius seeds were treated with saline stress (NaCl : Na2SO4 = 9:1; pH 6.44–6.65) and alkaline stress (NaHCO3 : Na2CO3 = 9:1; pH 8.71–8.89) for 10 days to assess the rate of germination, and then the ungerminated seeds were transferred to distilled water for another 10 days to assess their rate of recovery germination. In each column, the data markers identified with the same letters are not significantly different (P < 0.05) according to a least significant difference test. The error bars represent ± standard error (n = 4) of four replicates.
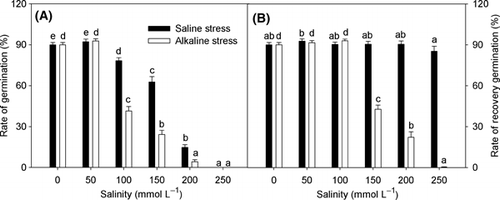
Figure 2 Effects of saline and alkaline stress on the (A) relative growth rate (RGR) and (B) activity of the root system in Lathyrus quinquenervius shoots. The 8-week-old L. quinquenervius seedlings were treated with saline stress (NaCl : Na2SO4 = 9:1; pH 6.44–6.65) and alkaline stress (NaHCO3 : Na2CO3 = 9:1; pH 8.71–8.89) for 10 days. In each column, the data markers identified with the same letters are not significantly different (P < 0.05) according to a least significant difference test. The error bars represent ± standard error (n = 4) of four replicates.
Photosynthesis
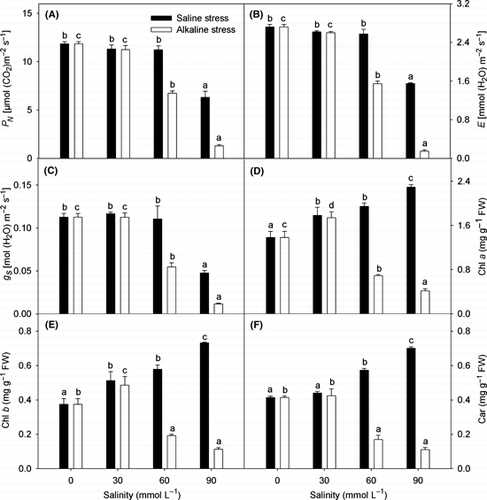
The effects of saline and alkaline stress on P N , g S and E were similar, neither stress affected the three parameters at low salinity intensities, but when the stresses were higher than 30 mmol L−1, the extent of the reductions under alkaline stress were greater than those under saline stress with increasing salinity (). The changes in Chl a, Chl b and Car were also similar, and were increased by saline stress and low alkaline stress () and were decreased by strong alkaline stress.
Anti-oxidant system
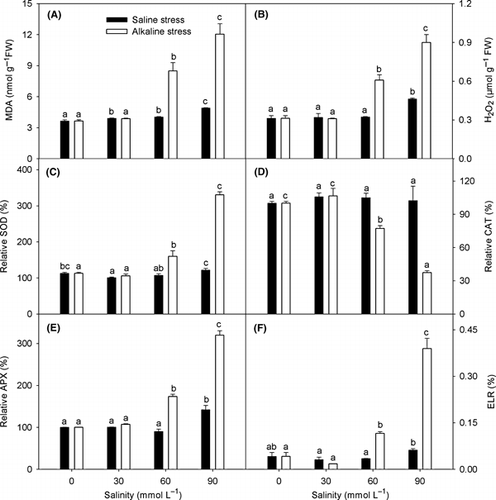
Saline stress slightly enhanced the contents of MDA and H2O2. The effects of low saline and alkaline stress (30 mmol L−1) on MDA and H2O2 were similar. However, when the alkaline stress was higher than 30 mmol L−1, the contents of MDA and H2O2 increased dramatically (), as did the ELR (). Saline stress did not affect CAT activity, and only high saline stress increased the activity of SOD and APX. Similar to the other parameters, there were comparable effects under low saline and alkaline stress, but high alkaline stress enhanced the activity of SOD and APX and reduced the activity of CAT.
Figure 3 Effects of saline and alkaline stress on (A) net photosynthetic rate (PN), (B) transpiration rate (E), (C) stomatal conductance (gS), (D) chlorophyll (Chl) a, (E) chlorophyll (Chl) b and (F) carotenoids (Car) in Lathyrus quinquenervius leaves. The 8-week-old L. quinquenervius seedlings were treated with saline stress (NaCl : Na2SO4 = 9:1; pH 6.44–6.65) and alkaline stress (NaHCO3 : Na2CO3 = 9:1; pH 8.71–8.89) for 10 days. In each column, the data markers identified with the same letters are not significantly different (P < 0.05) according to a least significant difference test. The error bars represent ± standard error (n = 4) of four replicates. FW, fresh weight.
Cations
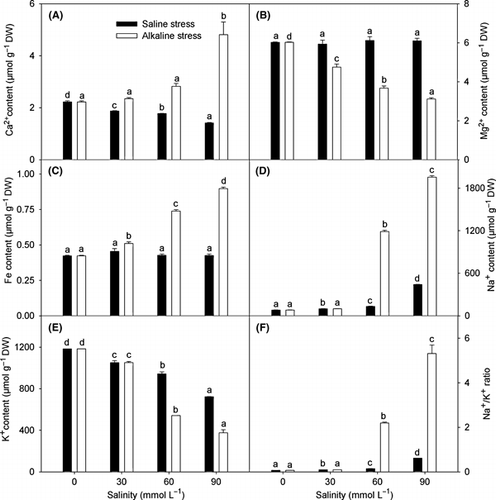
Saline stress caused a decrease in the Ca2+content, but did not affect the content of Mg2+and Fe, whereas alkaline stress increased the content of Ca2+and Fe and decreased the Mg2+content (). The effects of both stresses on Na+ and K+ were interesting. With increasing stress, both the saline and alkaline stresses resulted in an increased Na+ content and a decreased K+ content, and finally in an increased Na+/K+ ratio. When saline stress and alkaline stress were compared, there were no significant differences at low salinity. In contrast, when the salinity stress was higher than 30 mmol L−1, the extent of not only the increase in Na+, but also the reduction in K+ under alkaline stress was much greater than that under saline stress ().
Anions
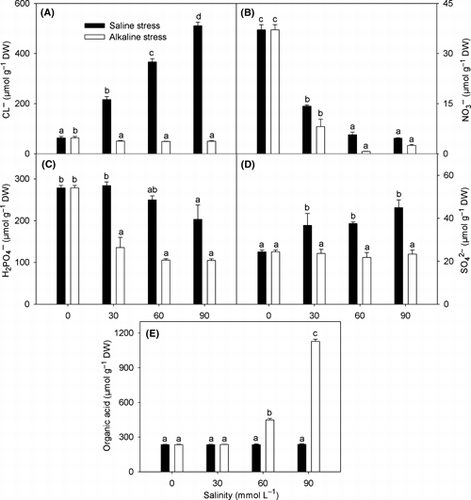
With increasing stress intensity, both Cl− and SO4 2− increased under saline stress, but remained unchanged under alkaline stress (). NO3 − and H2PO4 − decreased with increasing stress, but the extent of the reductions under alkaline stress was much greater than the reductions under saline stress (). Both saline and low alkaline stress did not affect the accumulation of OA. In contrast, OA increased sharply under high alkaline stress intensity (). Furthermore, when the tissue pH was >6, the OAs in the plant tissue were in the form of organic salts (CitationMa et al. 2001; CitationWang 2001)
Figure 4 Effects of saline and alkaline stress on the amount of (A) malondialdehyde (MDA), (B) H2O2, (C) superoxide dismutase (SOD), (D) catalase (CAT), (E) ascorbate peroxidase (APX) and (F) electrolyte leakage rate (ELR) in Lathyrus quinquenervius leaves. The 8-week-old L. quinquenervius seedlings were treated with saline stress (NaCl : Na2SO4 = 9:1; pH 6.44–6.65) and alkaline stress (NaHCO3 : Na2CO3 = 9:1; pH 8.71–8.89) for 10 days. In each column, the data markers identified with the same letters are not significantly different (P < 0.05) according to a least significant difference test. The error bars represent ± standard error (n = 4) of four replicates. SOD, CAT and APX were expressed relative to the control value of 100%. FW, fresh weight.
Tissue pH
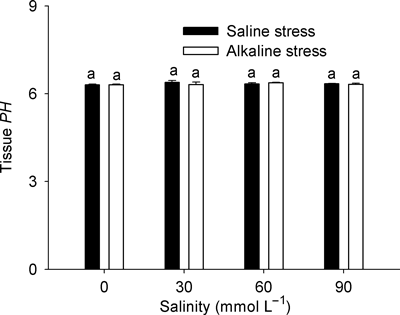
The tissue pH in L. quinquenervius shoots under both stresses was in close agreement with the control. The tissue pH was 6.31 in the control and the mean tissue pH at the different stress intensities was 6.36 under salt stress and 6.34 under alkaline stress (). The stress intensity did not affect the tissue pH.
Discussion
Germination
Germination is one of the most critical periods in the life cycle of plants. Under stress, a low water potential is a determining factor inhibiting seed germination (CitationDebez et al. 2004). However, our results indicated that high pH also significantly affects seed germination. Our results showed that the inhibiting action of alkaline stress on L. quinquenervius germination was greater than that of saline stress at the same salinity (). In , it is clear that after stress when the seeds were transferred to distilled water, most of the seeds under saline stress could recover germination, but only a few seeds recovered germination following high alkaline stress. According to these results, we propose that ungerminated L. quinquenervius seeds in saline soils may be in a state of dormancy to escape their rigorous environment; when rain falls and decreases soil salinity, then the seeds are able to germinate, and this may be a strategy for plants to survive in soils of high salinity. The effects of high pH on L. quinquenervius germination were much more complex than salinity. Germination was not inhibited by high pH at low salinity, but was inhibited at higher salinities, implying a function of pH adjustment in seeds that allowed germination at low salinity. At high salinity, high pH may decompose the seed structure and even result in seed death (). The reason for this may be complex and deserving of further research.
Figure 5 Effects of saline and alkaline stress on (A) Ca2+, (B) Mg2+, (C) Fe2+, (D) Na+, (E) K+ and (F) Na+/K+ ratio in Lathyrus quinquenerviu shoots. The 8-week-old L. quinquenervius seedlings were treated with saline stress (NaCl : Na2SO4 = 9:1; pH 6.44–6.65) and alkaline stress (NaHCO3 : Na2CO3 = 9:1; pH 8.71–8.89) for 10 days. In each column, the data markers identified with the same letters are not significantly different (P < 0.05) according to a least significant difference test. The error bars represent ± SE (n = 4) of four replicates. DW, dry weight.
Growth
The RGR value reflects the life-sustaining activities of the plant and is considered to be an optimum index for degrees of stress and plant responses to stresses. The RGR decrease under alkaline stress was greater than the decrease under saline stress (). The injurious effect of alkaline stress was greater than that of saline stress at the same salinity, and this is consistent with previous reports (CitationShi and Yin 1993; CitationYang et al. 2007). The different injurious effects of the two stresses may result from their different mechanisms of action. The injurious effects of salinity are commonly thought to be a result of low water potentials and ion toxicities (CitationMunns 2002). Alkaline stress exerts the same stress factors as saline stress, but with the added influence of high-pH stress. The effects of saline stress on L. quinquenervius root system activity, membrane permeability and photosynthetic pigments were slight compared with alkaline stress (–). Alkaline stress induced severe reductions in root system activity and photosynthetic pigment contents, and a sharp increase in ELR (–). These results indicate that high pH from alkaline stress may damage root cell structure and functions such as the absorption of ions (,: Na+, K+, Cl− and NO3 −), damage photosynthetic pigments and the membrane system, and this may be why the RGR of L. quinquenervius under alkaline stress was less than the RGR under saline stress.
Figure 6 Effects of saline and alkaline stress on (A) Cl− , (B) NO3–, (C) H2PO4−, (D) SO42− and (E) organic acid in Lathyrus quinquenervius shoots. The 8-week-old L. quinquenervius seedlings were treated with saline stress (NaCl : Na2SO4 = 9:1; pH 6.44–6.65) and alkaline stress (NaHCO3 : Na2CO3 = 9:1; pH 8.71–8.89) for 10 days. In each column, the data markers identified with the same letters are not significantly different (P < 0.05) according to a least significant difference test. The error bars represent ± SE (n = 4) of four replicates. DW, dry weight.
Photosynthesis
The g s and E of many plants decreases with increasing saline stress intensity or alkaline stress (CitationYang et al. 2008c,Citationd), but the g s and E of L. quinquenervius decreased only when the saline stress was >60 mmol L−1 or when the alkaline stress was >30 mmol L−1. This demonstrated that the high pH caused by alkaline stress did not affect the gas exchange of L. quinquenervius at low salinity. The decrease in g S and E under both stresses () may be induced by the physical or chemical signal materials of the roots stimulated by low water potentials. The P N of a plant usually decreases with rising saline stress intensity (CitationYang et al. 2009a,Citationb), and it has been reported that alkaline stress, even at low salinity (15 mmol L−1), limited the photosynthesis of barley (CitationYang et al. 2009a) and wheat (CitationYang et al. 2008d). However, we found that the P N of L. quinquenervius did not decrease under moderate saline stress or alkaline stress (). Within the normal physiological adaptability of L. quinquenervius, its RGR decrease was a result of decreasing photosynthetic area, and was basically independent of its P N. CitationMarcelis and van Hooijdonk (1999) also reported that the reduction in plant growth at higher salinities mainly resulted from a reduction in photosynthetic area. For a plant growing in natural saline alkali conditions over a long period, photosynthesis tends to stabilize, which suggests that the effects of saline and alkaline stress on plant growth are mainly through a reduction in photosynthetic area rather than through a change in P N. Reduced plant P N under higher salinity probably results from a reduction in intracellular CO2 partial pressure caused by stomatal closure or of non-stomatal factors (CitationBethke and Drew 1992). The non-stomatal factors mainly depend on the cumulative effects of leaf water and osmotic potential, biochemical constituents, contents of photosynthetic pigments and ion toxicities in the cytosol (CitationYang et al. 2009a,Citationb). The results in the present study showed that the inhibitory effect of higher-alkaline stress on the P N of L. quinquenervius was greater than saline stress at the same salinity. The visible reduction in P N in L. quinquenervius under alkaline stress was related not only to a reduction in photosynthetic pigments and decreased g S (), but also to ion toxicities in the cytosol.
Figure 7 Effects of saline and alkaline stress on the tissue pH in Lathyrus quinquenervius shoots. The 8-week-old L. quinquenervius seedlings were treated with saline stress (NaCl : Na2SO4 = 9:1; pH 6.44–6.65) and alkaline stress (NaHCO3 : Na2CO3 = 9:1; pH 8.71–8.89) for 10 days. In each column, the data markers identified with the same letters are not significantly different (P < 0.05) according to a least significant difference test. The error bars represent ± SE (n = 4) of four replicates.
Ion toxicity and ion imbalance
Plants in saline soils usually accumulate high concentrations of Na+ in vacuoles to decrease cell water potential (CitationMunns and Tester 2008). The Na+ enters plant cells through the high-affinity K+ transporter (HKT) and non-selective cation channels (CitationZhu 2003). The similarity in the hydrated ionic radii of Na+ and K+ make them difficult to discriminate between, and this is the basis of Na+ toxicity (CitationBlumwald 2000). Under saline stress, Na+ competes with K+ for uptake into the roots (CitationMunns 2002; CitationMunns and Tester 2008). At lower stress intensity, the effects of both stresses on Na+ and K+ accumulation in L. quinquenervius were similar (). However, when the salinity was increased to >30 mmol L−1, the Na+ content increased slowly under saline stress, but increased sharply under alkaline stress (). The responses of Na+ and K+ in L. quinquenervius to low alkaline stress were significantly different from other plants, such as barley (CitationYang et al. 2009a), wheat (CitationYang et al. 2008d) and Suaeda glauca (CitationYang et al. 2008b), all of which had higher Na+ and lower K+ under low alkaline stress than under low saline stress, indicating that a specific transport mechanism of Na+ and K+ may exist in L. quinquenervius. We propose that the increased Na+ caused by alkaline stresses was not a response to osmotic stress, but rather a specific response to high-pH stress. Under low alkaline stress, the harmful effect of high pH was resisted by a pH adjustment outside the roots, and consequently the intracellular environment was not affected. However, when the stress intensity exceeded the capacity of the root adjustment (>30 mmol L−1), the alkaline stress might have weakened the controls on the absorption or transport of Na+ and K+, leading to a sharp increase in the Na+ content and to a sharp decrease in the K+ in shoots (), which disrupted the ionic balance in the tissues and increased intracellular Na+ to a toxic level. This may explain some of the damage that emerged under higher alkaline stress (–). The increased Na+ in the shoots under alkaline stress might also be related to a possible decrease in Na+ exclusion. It is well known that many plant species have a Na+ exclusion mechanism depending on a Na+/H+ antiport, such as salt overly sensitive 1 (SOS1), which exchanges cytoplasmic Na+ with external H+ (CitationMunns and Tester 2008; CitationZhu 2003). The exchange activity relies on the transmembrane proton gradient achieved by H+-ATPase (CitationZhu 2003). Under high alkaline stress, a lack of external protons might weaken the exchange activity of the Na+/H+ antiport on the root plasma membrane (CitationMunns and Tester 2008), possibly reducing the exclusion of Na+ into the rhizosphere and enhancing in vivo accumulation of Na+. In addition, because the ratios of Ca2+, Mg2+and Fe to total cations were very low, their contributions to osmotic adjustment and ion balance were small.
Ion balance
A stable tissue pH, as a result of intracellular ion balance, is necessary for plants to maintain normal metabolism (CitationYang et al. 2007). In a living plant, as long as the plant can adapt to the environment, the pH value in its tissues should be stable, regardless of how the environmental pH value changes. The observations that the tissue pH in L. quinquenervius shoots under both stresses were similar to the control and that stress intensity did not have any affect on tissue pH suggested that L. quinquenervius was able to maintain an ionic balance and stable pH in cells (), not only under saline stress, but also under alkaline stress even at pH >8.8. Ionic imbalance in plants is mainly caused by the influx of superfluous Na+ (CitationMunns and Tester 2008; CitationYang et al. 2007). Plants usually accumulate inorganic anions, such as Cl−, NO3 − and SO4 2−, or synthesize organic anions to maintain ionic balance (CitationYang et al. 2007). Under saline stress, L. quinquenervius accumulated OAs and inorganic anions to balance the massive influx of Na+ (). The concentrations of inorganic anions under alkaline stress were significantly lower than the concentrations under saline stress of the same intensity (), suggesting that the high pH caused by alkaline stress might inhibit the uptake of anions such as NO3 −, SO4 2− and H2PO4 − (). Under alkaline stress, even low alkaline stress, OA was the dominant factor in maintaining an ionic equilibrium in stressed L. quinquenervius (). In addition, different inorganic anions differed in their contributions to the ion balance under different stress intensities (). Under alkaline stress, the contribution of H2PO4 − was much higher than that of the other inorganic anions, and this differed significantly from other plants, such as barley (CitationYang et al. 2009a), Suaeda glauca (CitationYang et al. 2008b) and Kochia sieversiana (CitationYang et al. 2007). In these species, NO3 − played a role in maintaining the ion balance and its contribution to the overall negative charge was much higher than H2PO4 − and the other inorganic anions. Although we did not measure the other physiological parameters involved in the nitrogen metabolism of L. quinquenervius, the changes in the NO3 − content clearly indicated that alkaline stress can interfere with the uptake and/or metabolism of NO3 −, which should be investigated further.
Table 1 Percentage of the contribution of various anions to the total negative charge (ion balance) in Lathyrus quinquenervius shoots under saline and alkaline stresses
At low alkaline stress, the harmful effect of high pH was resisted by a pH adjustment outside the roots, consequently the intracellular environment was not affected. We observed that L. quinquenervius could maintain low Na+, high K+ and a high K+/Na+ ratio () as well as well growth () under 30 mmol L−1 alkaline stress,, which ensured that photosynthesis was not affected (). also showed that 30 mmol L−1 alkaline stress did not induce the accumulation of OA in the shoots. This OA result was significantly different from barley (CitationYang et al. 2009a), wheat (CitationYang et al. 2008a), S. glauca (CitationYang et al. 2008b) and K. sieversiana (CitationYang et al. 2007). These plants, under both low alkaline stress and strong saline stress, accumulate OAs in the shoots. These data indicated that OA metabolism adjustment may play different roles in different plant species, and also that there might be different alkaline-resistant mechanisms. At low alkaline stress, L. quinquenervius was able to maintain an ion balance in the shoot. However, when the stress intensity exceeded the capacity for root adjustment (>30 mmol L−1), the massive influx of Na+ () and the reduced amounts of inorganic anions () resulted in a severe ion imbalance. Lathyrus quinquenervius might enhance OA synthesis to remedy the shortage of inorganic anions and maintain a stable intracellular pH.
In summary, OA accumulation may be a central adaptive mechanism by which L. quinquenervius maintains intracellular ion balance under alkaline stress. The accumulation of OAs in the shoots may not be a simple passive response to a negative charge deficit, but rather the result of active metabolic regulation after sensing signals of alkaline stress. The metabolic regulation of OAs under alkaline stress may involve known enzymes that participate in basal metabolic pathways, such as the tricarboxylic acid cycle, the glyoxylate cycle, glycolysis or other pathways. This requires further investigation.
Oxidative stress and the anti-oxidant system
Under normal growth conditions, the production of reactive oxygen species (ROS) in cells is low, and saline stress can disrupt the cellular homeostasis of ROS and enhance the production of ROS, resulting in intracellular oxidative stress (CitationMittler 2002). In most plants, the superoxide anion radical (O2 −) is efficiently converted to H2O2 by the action of SOD, whereas H2O2 is destroyed predominantly by APX and CAT (CitationMittler 2002). Saline stress usually enhances the H2O2 content and the activities of the anti-oxidative enzymes, such as CAT, APX, glutathione reductase (GR) and SOD. Our results show that high pH stress also significantly affected the anti-oxidant system. Saline stress and low alkaline stress had little effect on the lipid peroxidation (MDA content) and anti-oxidative enzyme activities in L. quinquenervius. However, strong alkaline stress clearly enhanced the content of MDA and H2O2, resulting in severe intracellular oxidative stress (), which may be the main reason why the harmful action of alkaline stress on L. quinquenervius is stronger than that of saline stress. Saline stress and low-alkali stress slightly enhanced the activities of SOD and APX, but did not affect CAT activity. However, strong-alkali stress significantly enhanced the activities of SOD and APX, and reduced the activity of CAT. By significantly enhancing the activities of SOD and APX, this may be a vital mechanism by which L. quinquenervius resists the oxidative stress caused by alkaline stress. The lipid peroxidation caused by alkaline stress might result from excess Na+ () or from an ion imbalance, and Na+ might participate in the signal transduction of ROS synthesis during the response to alkaline stress, which should be further investigated. During the response to alkaline stress, the roles of different anti-oxidative enzymes differed during ROS metabolic adjustment. The metabolic regulation of ROS under alkaline stress may involve many enzymes, and this regulation is very complex. These enzymes may be known enzymes. Enzyme activity can be regulated at the level of synthesis (transcription, translation and modification of new polypeptides) or after synthesis by the action of activators and inhibitors, which requires further investigation.
Conclusion
The present study indicated that saline and alkaline stresses are two different types of stress. The harmful effect of alkaline stress on the seedling and seed germination of L. quinquenervius was significantly greater than that of saline stress; this harmful effect might have resulted from the negative effects of high pH on the plant. High pH caused strong alkaline stress to severely affect ion accumulation and to destroy the structure of the root cells and even lead to cell death. The main reason for the negative impact of high pH on ion accumulation was that high pH caused a lack of protons outside the root, which impeded the transmembrane proton gradient. At low stress intensity, the harmful effect of high pH was resisted by pH adjustment outside the roots and consequently the intracellular environment was not affected, and the stress effects of saline stress and alkaline stress on L. quinquenervius were similar. However, when the stress intensity exceeded the capacity for root adjustment (>30 mmol L−1), compared with saline stress, alkaline stress clearly inhibited germination, growth, photosynthesis and root system activity, and led to a sharp increase in Na+ and to an ion imbalance in the shoots, as well as to enhanced H2O2 and MDA content, leading to severe intracellular oxidative stress, which may be the main reason why the harmful action of alkaline stress on L. quinquenervius was stronger than that of saline stress. Our results indicated that OA accumulation was a central adaptive mechanism by which L. quinquenervius maintained intracellular ionic balance under alkaline stress, and that enhancing the activities of SOD and APX may be a vital mechanism by which L. quinquenervius resists the oxidative stress caused by alkaline stress. Our results also indicated that the metabolic adjustment of OA and Na–K in L. quinquenervius might be different to that observed in other plant species. There might also be different alkali-resistant mechanisms.
In summary, alkaline stress is a very severe abiotic stress that has caused very serious problems in China and in some other areas. It is one of the main causes of grassland degradation in China. Researchers involved in plant physiology and governmental departments should pay attention to the harmful effects of alkaline stress. It is expected that the present study may provide a scientific theoretical foundation for the cultivation and research of alkali-tolerant crops.
Acknowledgments
Financial support was provided by a collaborative grant from the National Science Foundation (30471231), the Project Foundation from the Education Ministry of China (106063), the Specialized Research Fund For the Doctoral Program of Higher Education (20050200006), the National TCM Main Project in 11th Five-Year-Period (2008BADB3B09) and the Science and Technology Project of Jilin Province (20080563).
References
- Arnon , DI . 1949 . Copper enzymes in isolated chloroplasts. Polyphenooxidase in Beta vulgaris . Plant Physiol. , 24 : 1 – 15 .
- Bethke , PC and Drew , MC . 1992 . Stomatal and non-stomatal components to inhibition of photosynthesis in leaves of Capsicum annuumduring progressive exposure to NaCl salinity . Plant Physiol. , 99 : 219 – 226 .
- Beyer , WF and Fridovich , I . 1987 . Assaying for superoxide dismutase activity: some large consequences of minor changes in condition . Anal. Biochem. , 161 : 559 – 566 .
- Blumwald , E . 2000 . Sodium transport and salt tolerance in plants . Curr. Opin. Cell Biol. , 12 : 431 – 434 .
- Bradford , MM . 1976 . A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dye binding . Anal. Biochem. , 72 : 248 – 254 .
- Campbell , SA and Nishio , JN . 2000 . Iron deficiency studies of sugar beet using an improved sodium bicarbonate-buffered hydroponics growth system . J. Plant Nutr. , 23 : 741 – 757 .
- Chen , GX and Asada , K . 1989 . Ascorbate peroxidase in tea leaves: occurrence of two isozymes and the differences in their enzymatic and molecular properties . Plant Cell Physiol. , 30 : 987 – 998 .
- Comas , LH , Eissenstat , DM and Lakso , AN . 2000 . Assessing root death and root system dynamics in a study of grape canopy pruning . New Phytol. , 147 : 171 – 178 .
- Debez , A , Ben Hamed , K , Grignon , C and Abdelly , C . 2004 . Salinity effects on germination, growth, and seed production of the halophyte Cakile maritime . Plant Soil , 262 : 179 – 189 .
- Draper , HH and Hadley , M . 1990 . Malondialdehyde determination as index of lipid peroxidation . Methods Enzymol. , 186 : 421 – 431 .
- Du , Z and Bramlage , WJ . 1992 . Modified thiobarbituric acid assay for measuring lipid oxidation in sugar-rich plant tissue extracts . J. Agric. Food. Chem. , 40 : 1566 – 1570 .
- El-Samad , HMA and Shaddad , MAK . 1996 . Comparative effect of sodium carbonate, sodium sulphate, and sodium chloride on the growth and related metabolic activities of pea plants . J. Plant Nutr. , 19 : 717 – 728 .
- Ferrari , A , Lascano , CI , Anguiano , OL , Pechen de D’Angelo , AM and Venturino , A . 2009 . Antioxidant responses to azinphos methyl and carbaryl during the embryonic development of the toad Rhinella(Bufo) arenarumHensel . Aquat. Toxicol. , 93 : 37 – 44 .
- Hartung , W , Leport , L , Ratcliffe , RG , Sauter , A , Duda , R and Turner , NC . 2002 . Abscisic acid concentration, root pH and anatomy do not explain growth differences of chickpea (Cicer arietinumL.) and lupin (Lupinus angustifoliusL.) on acid and alkaline soils . Plant Soil , 240 : 191 – 199 .
- Kawanabe , S and Zhu , TC . 1991 . Degeneration and conservation of Aneurolepidium chinensegrassland in Northern China . J. Jpn. Grassland. Sci. , 37 : 91 – 99 .
- Kingsbury , RW , Epstein , E and Peary , RW . 1984 . Physiological responses to salinity in selected lines of wheat . Plant Physiol. , 74 : 417 – 423 .
- Läuchli , A and Lüttge , U . 2002 . “ Salinity in the soil environment ” . In Salinity:Environment-Plants-Molecules , Edited by: Tanji , KK . 21 – 23 . Boston, MA, , USA : Boston Kluwer Academic Publishers .
- Lutts , S , Kiner , JM and Bouharmont , J . 1996 . NaCl-induced senescence in leaves of rice (Oryza sativaL.) cultivars differing in salinity resistance . Ann. Bot. , 78 : 389 – 398 .
- Ma , JF , Ryan , PR and Delhaize , E . 2001 . Aluminium tolerance in plants and the complexing role of organic acids . Trends Plant Sci. , 6 : 273 – 278 .
- Marcelis , LFM and van Hooijdonk , J . 1999 . Effect of salinity on growth, water use and nutrient use in radish (Raphanus sativusL.) . Plant Soil , 215 : 57 – 64 .
- Mittler , R . 2002 . Oxidative stress, antioxidants and stress tolerance . Trends Plant Sci. , 7 : 405 – 410 .
- Munns , R . 2002 . Comparative physiology of salt and water stress . Plant Cell Environ. , 25 : 239 – 250 .
- Munns , R and Tester , M . 2008 . Mechanisms of salinity tolerance . Annu. Rev. Plant Biol. , 59 : 651 – 681 .
- Rao , PS , Mishar , B , Gupta , SR and Rathore , A . 2008 . Reproductive stage tolerance to salinity and alkalinity stresses in rice genotypes . Plant Breed. , 127 : 256 – 261 .
- Shi , DC and Sheng , YM . 2005 . Effect of various salt–alkaline mixed stress conditions on sunflower seedlings and analysis of their stress factors . Environ. Exp. Bot. , 54 : 8 – 21 .
- Shi , DC and Wang , DL . 2005 . Effects of various salt–alkali mixed stresses on Aneurolepidium chinense(Trin.) Kitag . Plant Soil , 271 : 15 – 26 .
- Shi , DC and Yin , LJ . 1993 . Difference between salt (NaCl) and alkaline (Na2CO3) stresses on Puccinellia tenuiflora(Griseb.) Scribn et Merr. plants . Acta Bot. Sin. , 3 : 144 – 149 .
- Shi , DC and Zhao , KF . 1997 . Effects of NaCl and Na2CO3on growth of Puccinellia tenuifloraand on present state of mineral elements in nutrient solution . Acta pratacu. sin. , 6 : 51 – 61 .
- Wang , XL . 2001 . “ Carboxylic acid ” . In Organic chemistry , Edited by: Wang , XL . 149 – 150 . Beijing : Higher Education Press .
- Wolff , SP . 1994 . Ferrous ion oxidation in presence of ferric ion indicator xylenol orange for measurement of hydroperoxides . Methods Enzymol. , 233 : 182 – 189 .
- Yang , CW , Chong , JN , Kim , CM , Li , CY , Shi , DC and Wang , DL . 2007 . Osmotic adjustment and ion balance traits of an alkali resistant halophyte Kochia sieversianaduring adaptation to salt and alkali conditions . Plant Soil , 294 : 263 – 276 .
- Yang , CW , Jianaer , A , Li , CY , Shi , DC and Wang , DL . 2008c . Comparison of the effects of salt-stress and alkali-stress on the photosynthetic production and energy storage of an alkali-resistant halophyte Chloris virgata . Photosynthetica , 46 : 273 – 278 .
- Yang , C , Li , C , Zhang , M , Liu , J , Ju , M and Shi , D . 2008a . Ion and pH homeostasis in the shoot of wheat-wheatgrass (Triticum aestivumL. Agropyron intermedium) under salt and alkali stresses . Chin. J. Appl. Ecol. , 19 : 1000 – 1005 . (in chinese)
- Yang , C , Shi , D and Wang , D . 2008b . Comparative effects of salt stress and alkali stress on growth, osmotic adjustment and ionic balance of an alkali-resistant halophyte Suaeda glauca(Bge.) . Plant Growth Regul. , 56 : 179 – 190 .
- Yang , CW , Wang , P , Li , CY , Shi , C and Wang , DL . 2008d . Comparison of effects of salt and alkali stresses on the growth and photosynthesis of wheat . Photosynthetica , 46 : 107 – 114 .
- Yang , CW , Xu , HH , Wang , LL , Liu , J , Shi , DC and Wang , DL . 2009a . Comparative effects of salt-stress and alkali-stress on the growth, photosynthesis, solute accumulation, and ion balance of barley plants . Photosynthetica , 47 : 79 – 86 .
- Yang , CW , Zhang , ML , Liu , J , Shi , DC and Wang , DL . 2009b . Effects of buffer capacity on growth, photosynthesis, and solute accumulation of a glycophyte (wheat) and a halophyte (Chloris virgata) . Photosynthetica , 47 : 55 – 60 .
- Zheng , HY and Li , JD . 1999 . “ Form and dynamic trait of halophyte community ” . In Saline Plants in Songnen Plain and Restoration of Alkaline-saline Gras , Edited by: Zheng , HY and Li , JD . 137 – 138 . Beijing : Science Press .
- Zhu , JK . 2003 . Regulation of ion homeostasis under salt stress . Curr. Opin. Plant Biol. , 6 : 441 – 445 .