Abstract
Manganese (Mn) is an essential micronutrient in all organisms, but may become toxic when present in excess. Four maize (Zea mays L.) varieties, Kneja 605, Kneja 434, Kneja 509 and Kneja 537, were studied with respect to their responses to excess Mn in hydroponic solution. In the varieties Kneja 605, Kneja 509 and Kneja 537, increasing Mn concentrations in the nutrient solution negatively affected biomass accumulation, photosynthetic rate, transpiration, stomatal conductance and chlorophyll content. In addition, these varieties showed increased electrolyte leakage and lipid peroxidation (malondialdehyde [MDA] content). Increased Mn leaf concentrations, higher contents of chlorophyll a and chlorophyll b, higher photosynthetic rate and transpiration, lower concentrations of MDA and insignificant changes in the electrolyte leakage in the leaves were found in var. Kneja 434 compared with the other maize varieties studied. This variety appeared to possess a stronger ability to cope with Mn phytotoxicity, suggesting high potential for Mn detoxification and var. Kneja 434 could be a good candidate for improving maize productivity on acid soils under non-tropical conditions.
Introduction
Maize (Zea mays L.) is one of the world’s most important cereal crops. As a result of increasing demand, a shift to maize production has been observed, especially in tropical and subtropical regions (CitationMorris 2001). Soil acidity can be an important constraint in these areas and the adaptation of maize to acid soils in the tropics is an important research issue (CitationThe et al. 2006a). Phosphorus efficiency and tolerance to aluminum or manganese (Mn) toxicity have been identified as relevant traits for plants to be productive on acid tropical soils (CitationFoy et al. 1978; CitationKochian et al. 2004). Different mechanisms are responsible for tolerance to these major constraints of acid soils and tolerance to these factors appears to be inherited in an independent way (CitationThe et al. 2006b).
The limitation of maize production as a result of soil acidity is not restricted to the tropics. In Bulgaria almost 40% of the arable land is dedicated to cereal production and corn production ranges second behind winter wheat. Maize productivity, however, is relatively low with an average of approximately 3 t ha−1 (CitationBalabanova 2004). The main constraint is drought as a result of insufficient rainfall or irrigation. In addition, soil-derived stress factors can play a role. Approximately 40% (4.3 million ha) of Bulgarian soils have a pH value below 5 and have been classified as non-resistant to acidification (CitationKolchakov et al. 2005). Soil acidification (pH <5.5) enhances the solubility of Mn, increasing its bioavailability and creates Mn toxicity in many natural and agricultural systems (CitationFoy 1988).
Manganese is an essential trace element throughout all stages of plant development. For optimal growth and development plants need to accumulate at least 30 mg Mn kg−1 dry weight in tissues, regardless of the plant species (CitationMarschner 1995). The most important role of Mn is associated with oxygen evolution in plants. Manganese participates in both oxygen radical production via the O2-evolving four-atom Mn cluster located on the luminal surface of the D1 protein in PSII and in oxygen radical detoxification by its role in superoxide dismutase activity in mitochondria (CitationShenker et al. 2004). Manganese is required as a co-factor in more than 30 enzymes (CitationBurnell 1988) or as an activator for an array of enzymes, such as carboxylases and phosphatases in the cytosol and glycosyl transferases in the Golgi apparatus (CitationMarschner 1995). The availability of Mn and its uptake by plants depend on soil properties. Plants exposed to excess Mn exhibit strong inhibition of chloroplast structure and functions (CitationDoncheva et al. 2009). It has been proposed that the accumulation of phenolic substances in the apoplast, an early symptom of Mn toxicity in legumes, could be triggered by Mn toxicity mechanisms in the symplast (CitationFührs et al. 2008).
Plant species and genotypes may differ in their tolerance to excess Mn (CitationHorst et al. 1999). The mechanisms of Mn tolerance are still not clearly established. In some species, resistance to Mn toxicity results from a tolerance of higher internal Mn concentrations in the leaf tissues (CitationHorst 1983; CitationMoroni et al. 2003). Investigations with Arabidopsis thaliana mtp11 mutants defective in a cation diffusive facilitator were found to be hypersensitive to excess Mn and to accumulate higher root and shoot Mn concentrations than wild-type plants (CitationPeiter et al. 2007). Manganese exclusion as a result of Golgi-mediated exocytosis was proposed as an operating mechanism in the more Mn-tolerant wild type. Internal detoxification as a result of storage in either or both a non-toxic form and in compartments with low metabolic activity, such as vacuoles or trichomes, can play a role.
The aim of the present study was to characterize the differences in tolerance to excess Mn in four Bulgarian varieties of maize (Zea mays L). A better understanding of the strategies that underlie Mn tolerance in this important crop species will help to improve maize productivity on acid soils in non-tropical regions.
Materials and methods
Plant materials
Seeds of maize (Zea mays L.) plants (var. Kneja 605, var. Kneja 434, var. Kneja 509 and var. Kneja 537) were germinated on wet filter paper. After 5 days the seedlings were transferred to aerated nutrient solution in a greenhouse under natural light. The basic nutrient solution contained (μmol L−1): 200 CaSO4.2H2O, 100 MgSO4.7H2O, 400 KNO3, 300 NH4NO3, 5 MnSO4.H2O, 0.38 ZnSO4.7H2O, 0.16 CuSO4.H2O, 10 Fe-ethylenediaminetetraacetic acid, 5 NaH2PO4.H2O, 8 H3BO3, 0.06 (NH4)Mo7O24.H2O. After 72 h the seedlings were transferred to a new fresh nutrient solution (two plants per beaker; beaker volume 1.2 L) with the same composition as above supplemented with 50, 200 and 500 μmol L−1 Mn as MnSO4. Control plants received the basic nutrient solution containing 5 μmol L−1 Mn throughout the experiment. The nutrient solution was changed twice per week. Plants were harvested for analyses 5 days after the Mn treatment. Twelve plants were used for each treatment and three independent experiments were carried out.
Plant growth parameters
Plant growth was determined as an increase in the dry weights of both root (RW) and shoot (SW) on day 5 after the onset of the Mn treatment.
Determination of leaf Mn content
Detached leaves were carefully washed under a continuous stream of deionized water and dried at 60°C for several days. Samples of dry leaves (0.1 g) were ashed at 550°C. The ash was dissolved and brought to a standard volume with 20% HCl and the concentration of Mn in the solution was determined using an inductively coupled-plasma atomic emission spectrometer ([ICP-AES] Liberty-II; Varian, Middelburg, The Netherlands).
Leaf pigments
Chlorophyll a, chlorophyll b and the total carotenoids were extracted from leaf disks with 80% acetone. The pigment content was determined spectrophotometrically according to CitationArnon (1949). The chlorophyll values were calculated following CitationWang et al. (2001) according to CitationPorra (1991).
Measurement of CO2 gas exchange rates
Measurements of the net photosynthetic rate (PN), stomatal conductance (g s ) and transpiration rate (E) were done with an infrared gas analyzer (Li 6400; Li Cor, Lincoln, NE, USA). Gas-exchange measurements were done under a saturating photosynthetic photon flux density (PPFD) of 500 μmol m−2 s−1, 380 μmol CO2 mol−1, and a leaf temperature of 34 ± 2°C. The data were statistically processed using Systat 7.0 (Chicago, IL, USA).
Lipid peroxidation
The peroxidation of lipids in the leaves was assessed by the thiobarbituric acid (TBA) reaction as described by CitationHeath and Packer (1968). Thiobarbituric acid (i.e. the reactive substance) was quantified as malondialdehyde (MDA), which is an end product of lipid peroxidation. The TBA reagent consisted of 20% (w/v) trichloroacetic acid (TCA) and 0.65% (w/v) TBA. The specific absorbance of the TBA-reactive substances was determined as a TBA–MDA complex at 532 nm. After subtracting the non-specific absorption at 600 nm, the MDA amount was calculated using its molar extinction coefficient (155 m[mol L−1]−1 cm−1) and the results are expressed as MDA g−1 fresh weight (FW).
Electrolyte leakage
The electrolyte leakage (EL) test was conducted according to CitationYamori et al. (2005) with modifications. One gram of tissue was cut into 2-cm segments, rinsed in deionised water to remove the contents of the cut cells and placed in test tubes containing 15 mL of bidistilled water for 36 h at 4°C. The conductivity of the supernatant (C 1) was measured with an electro-conductivity meter. The tubes were placed in a boiling-water bath for 60 min, and the electrical conductivity was obtained after attaining equilibrium at 25°C (C 2). The EL was calculated using the following equation: EL (%) = C1/C2 × 100.
Statistical analyses
The results are the means of three independent experiments. A comparison of the means was made using Fisher’s least significant difference test (P ≤ 0.05) after a multifactor anova analysis.
Results
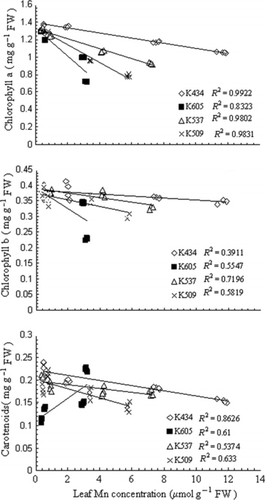
Manganese induced a reduction in both the dry shoot weight (SDW) and the dry root weight (RDW) in var. Kneja 605, var. Kneja 509 and var. Kneja 537 (). With increasing Mn concentrations in the nutrient solution, the SDW and RDW were significantly reduced in var. Kneja 605 compared with var. Kneja 509 and var. Kneja 537. The highest Mn concentration (500 μmol L−1) caused a reduction in SDW and RDW in var. Kneja 605, var. Kneja 509 and var. Kneja 537 by 44 and 52%, 28 and 34%, and 27 and 29%, respectively. In contrast, excess Mn did not cause a significant decrease in the shoot and root dry biomass in var. Kneja 434.
As the accumulation of Mn in the leaves could be responsible for Mn tolerance, Mn concentrations in the leaves were estimated. Exposure to excess Mn led to increased leaf Mn concentrations in all varieties studied. The Mn treatment caused higher Mn concentrations in the leaves of var. Kneja 434 compared with var. Kneja 509, var. Kneja 537 and var. Kneja 605 (). The differences in the Mn leaf concentrations were most pronounced at the highest Mn concentration (500 μmol L−1) in the nutrient solution. The net CO2 assimilation rate (PN), transpiration rate (E) and stomatal conductance (gs) in var. Kneja 509, var. Kneja 537 and var. Kneja 605 decreased with increasing leaf Mn content (). In contrast, increasing Mn leaf concentrations significantly increased the transpiration rate and stomatal conductance in var. Kneja 434, whereas the net photosynthetic rate was not affected. Pigment content showed changes in all varieties, but no correlation was found with increasing Mn leaf concentrations (). The chlorophyll content decreased to a higher extent in var. Kneja 605, var. Kneja 509 and var. Kneja 537 compared with var. Kneja 434. Our results showed a loss of chlorophyll a and b by 46 and 40%, respectively, in the leaves of var. Kneja 605, whereas in var. Kneja 434 the decrease in chlorophyll a and b was 22 and 17%, respectively. In addition, the total carotenoid levels in var. Kneja 605 were much higher than the levels in var. Kneja 434, var. Kneja 509 and var. Kneja 537 ().
Figure 1 Shoot dry weight and root dry weight of var. Kneja 434, var. Kneja 605, var. Kneja 509 and var. Kneja 537 grown in nutrient solutions with different Mn concentrations (μmol L−1): 5 (control), 50, 200 and 500. Values represent the means of three independent experiments. Different letters indicate significant differences assessed by Fisher’s least significant difference test (P < 0.05) using anova multifactor analysis.
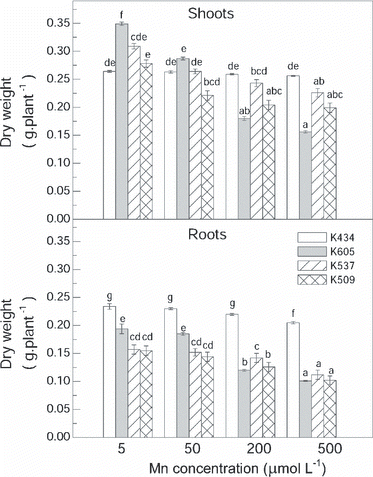
Figure 2 Manganese concentrations in the leaves of var. Kneja 434, var. Kneja 605, var. Kneja 509 and var. Kneja 537 after exposure to different Mn concentrations in nutrient solution. FW, fresh weight.
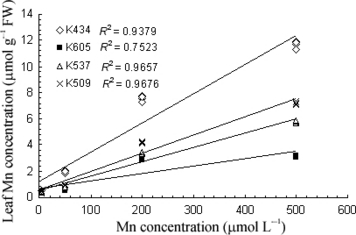
Figure 3 Effects of leaf Mn concentrations on (a) net CO2 assimilation (PN), (b) transpiration rate (E) and (c) stomatal conductance (gS) in the leaves of var. Kneja 434, var. Kneja 605, var. Kneja 509 and var. Kneja 537. Measurements were carried out at 500 μmol m−2 s−1 photosynthetically active radiation. FW, fresh weight.
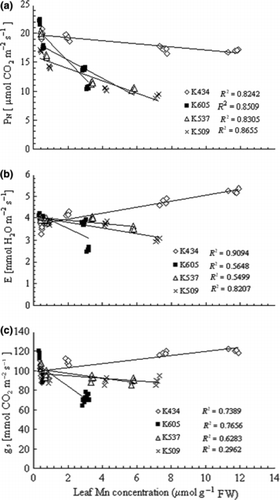
Figure 4 Effects of leaf Mn concentrations on chlorophyll a, chlorophyll b and carotenoids in the leaves of var. Kneja 434, var. Kneja 605, var. Kneja 509 and var. Kneja 537. FW, fresh weigt.
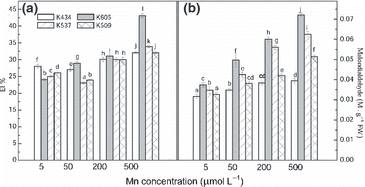
The membrane damage caused by Mn excess was assessed indirectly by the conductivity of solute leakage from the cells (). Data on EL indicated that the destruction of cell membranes in var. Kneja 605 plants was more severe than the destruction observed in the other varieties. On the contrary, the exposure of var. Kneja 434 plants to excess Mn did not change significantly the EL in the leaves, suggesting that the membrane integrity was not destroyed. Thus, var. Kneja 537 and var. Kneja 509 were less able to cope with excess Mn compared with var. Kneja 434. The MDA level as the final product of membrane lipid peroxidation in the leaves is given in . Significant lipid peroxidation was found in the leaves of var. Kneja 605, var. Kneja 537 and var. Kneja 509. In contrast, no significant changes were detected in the TBA-reactive substances in the leaves of var. Kneja 434.
Discussion
The present study reveals clear differences in Mn tolerance among maize varieties adapted to the non-tropical growth conditions in Bulgaria. In all tested varieties the Mn content in the leaves increased with increasing Mn supply (). Plants possess a range of potential internal tissue mechanisms that may be involved in detoxification of heavy metals, thus directly contributing to their metal tolerance capacity (CitationHall 2002). In our study, a Mn supply of 50 μmol L−1 or higher caused significantly higher Mn concentrations in the leaves of var. Kneja 434 compared with of the concentrations in var. Kneja 605, var. Kneja 509 and var. Kneja 537. However, despite the lower Mn accumulation in their leaves, var. Kneja 605, var. Kneja 509 and var. Kneja 537 exhibited a stronger Mn-induced growth reduction both in the shoots and roots than var. Kneja 434 (). These results confirm our previous observation about Mn-tolerant var. Kneja 434 based on an insignificant callose accumulation, slight alterations in the chloroplast structure, mainly in stomatal but not in granal thylacoids, unchanged maximum quantum efficiency of PSII photochemistry estimated by the ratio Fv/Fm and the actual efficiency of PSII electron transport, non-photochemical quenching qN of chlorophyll fluorescence, and a significant increase in the thickness of the epidermal layers, suggesting that non-photosynthetically active epidermal cells may be implicated in the sequestration of excess Mn (CitationDikova et al. 2005; CitationDoncheva et al. 2009; CitationStoyanova et al. 2008).
Figure 5 Changes in (a) electrolyte leakage (EL) and (b) the level of lipid peroxidation (MDA content) in the leaves of var. Kneja 434, var. Kneja 605, var. Kneja 509 and var. Kneja 537 grown in nutrient solutions with different Mn concentrations (μmol L−1): 5 (control), 50, 200 and 500. Values represent the means of three independent experiments. Different letters indicate significant differences assessed by Fisher’s least significant difference test (P < 0.05) using anova multifactor analysis. FW, fresh weight.
Tolerance to high shoot or leaf Mn concentrations rather than restriction of uptake has also been described in other crop species, such as rice (CitationHoriguchi 1987), cotton (CitationFoy et al. 1995), rapeseed (CitationMoroni et al. 2003) and Douglas fir (CitationDucic and Polle 2007). The determination of the Mn leaf concentration in younger leaves could be useful in assessing soil Mn status (CitationKitao et al. 2001). The inherent tolerance mechanisms that give plants the ability to withstand high concentrations of Mn in photosynthetically active tissues are still not clearly established. Our results show that increased Mn leaf concentrations were positively correlated with transpiration rate, stomatal conductance and photosynthesis in var. Kneja 434 (). Higher Mn concentrations accompanied by an increase in tolerance and a lack of a decline in photosynthesis with Mn stress have been reported for deciduous broad-leaved plants in northern Japan (CitationKitao et al. 1997). In contrast, in var. Kneja 605, var. Kneja 509 and var. Kneja 537 excess Mn negatively affected photosynthesis and transpiration by closure of the stomata and inhibition of CO2 fixation. Manganese is considered to move towards the leaves by the transpiration stream in the xylem and it is mobile only in the xylem (CitationPage and Feller 2005; CitationXu et al. 2006). Transport in the xylem is directed from the roots to the above-ground plant parts in the transpiration stream (CitationMarschner 1995). Maintenance of high stomatal conductance and transpiration under high Mn exposure in the leaves of Mn-tolerant var. Kneja 434 may, at least in part, be responsible for its higher leaf Mn accumulation. An increase in the Mn concentration from the midrib to the marginal part was observed within a leaf of Phytolacca acinosa, suggesting that Mn was transported along the transpiration stream. Therefore, more Mn was accumulated at the end of the transpiration stream, indicating a transpiration-driven distribution pattern of Mn in the leaf (CitationXu et al. 2006). A similar distribution pattern was also observed for Cd (CitationCosio et al. 2005), Ni and Zn (CitationMizuno et al. 2003) in the leaves of Thlaspi caerulescens and Thlaspi japonicum, respectively.
It is well known that free radical induced peroxidation of membrane lipids is a reflection of stress-induced damage at the cellular level (CitationJain et al. 2001) and the level of MDA, produced during peroxidation of membrane lipids, is generally accepted as an indicator of severe oxidative stress (CitationKatsuhara et al. 2005). Our results indicated that the oxidative stress caused by the Mn treatment varied between the maize varieties. Among them, var. Kneja 434 showed the lowest level of MDA in the leaves, suggesting that the Mn leaf content did not contribute to the lipid peroxidation level. In agreement with the above result, the increased carotenoid concentrations in the Mn-sensitive varieties () could be considered to be a response to Mn-induced oxidative stress. Carotenoids are well known for their anti-oxidant activity within the chloroplasts, scavenging singlet oxygen and lipid peroxyradicals as well as preventing lipid peroxidation (CitationBurton and Ingold 1984; CitationWinston 1990). The fact that high light intensity increases the damaging effects of excess Mn on the photosynthetic apparatus (CitationClair and Lynch 2004) supports the view that Mn toxicity primarily affects chloroplast functions.
Although carotenoids were increased in var. Kneja 605 (), the photosynthetic apparatus was not efficiently protected against the oxidative stress induced by excess Mn in the sensitive varieties. As a result, chlorophyll levels decreased. In Mn-tolerant var. Kneja 434, chlorophyll a and b contents were maintained, despite the accumulation of high leaf Mn concentrations. Changes in chlorophyll levels are a good indicator of varietal differences in Mn tolerance in maize (), and support data reported previously for other crop species (CitationOhki 1985; CitationNable et al. 1988; CitationKitao et al. 1997). The photosynthetic activity and chlorophyll content of Mn-stressed plants have been used as suitable criteria for screening Mn tolerance in Triticum aestivum (CitationMacfie and Taylor 1992; CitationMoroni et al. 1991), Phaseolus vulgaris (CitationGonzález and Lynch 1997) and in deciduous broad-leaved tree seedlings (CitationKitao et al. 1998).
Electrolyte leakage as a consequence of the loss of membrane integrity can also be used as a marker to measure cell membrane functional status under stress conditions (CitationAlmeselmani et al. 2006). Our results showed that the leakage of electrolytes was more strongly expressed in the the Mn-sensitive var. Kneja 605, var. Kneja 509 and var. Kneja 537 compared with var. Kneja 434 (). The formation of MDA can be used as a general indicator of the extent of lipid peroxidation resulting from oxidative stress (CitationSkórzyn′ska-Polit and Krupa 2006). In the present study, the MDA content increased significantly in the leaves of var. Kneja 605, var. Kneja 509 and var. Kneja 537, suggesting that Mn leads indirectly to the production of superoxide radicals, resulting in increased lipid peroxidative products. Thus, both the MDA content and EL represent further evidence of the high ability of var. Kneja 434 to tolerate high Mn concentrations. This result implies that Mn tolerance, which is under genetic control (CitationMeharg 1994) in maize, must be based on mechanisms of detoxification without decreasing Mn accumulation.
Conclusion
In conclusion, the results of the present study showed significant variations in the growth responses of four maize varieties to excess Mn. The varieties Kneja 605, Kneja 537 and Kneja 509 showed a stronger inhibition in biomass, photosynthesis, transpiration and loss of membrane integrity observed at the lower Mn leaf concentrations compared with var Kneja 434. The higher Mn tolerance of var. Kneja 434 may be accounted for by a stronger internal capacity for protection against the phytotoxicity of Mn and a higher potential for Mn detoxification. Maize variety Kneja 434 is a good candidate for improving maize productivity on acid soils under non-tropical conditions.
Acknowledgments
This work was supported by Grant No. B/1524/2006 of the National Science Fund, Ministry of Education and Science, Bulgaria, and the Spanish Government MEC ref. BFU2007-60332.
References
- Almeselmani , M , Deshmukh , PS , Sairam , RK , Kushwaha , SR and Singh , TP . 2006 . Protective role of antioxidant enzymes under high temperature stress . Plant Sci. , 171 : 382 – 388 .
- Arnon , PI . 1949 . Copper enzymes in isolated chloroplasts. Polyphenol oxidase in Beta vulgaris . Plant Physiol. , 24 : 1 – 15 .
- Balabanova , A . 2004 . Report from the State of Bulgaria. Interactive European network for industrial crops and their applicationsINFORRM-IENICA, 5th Framework Programme, DG XII . European Commission , : 29
- Burnell , JN . 1988 . “ The biochemistry of manganese in plants ” . In Manganese in Soils and Plants , Edited by: Graham , RD , Hannam , RJ and Uren , NC . 125 – 133 . Dordrecht, , The Netherlands : Klumer .
- Burton , GW and Ingold , KU . 1984 . Beta-carotene: an unusual type of lipid antioxidant . Science , 224 : 569 – 573 .
- Clair , SBS and Lynch , JP . 2004 . Photosynthetic and antioxidant enzyme responses of sugar maple and red maple seedlings to excess manganese in contrasting light environments . Funct. Plant Biol. , 31 : 1005 – 1014 .
- Cosio , C , De Santis , L , Frey , B , Diallo , S and Keller , C . 2005 . Distribution of cadmium in leaves of Thlaspi caerulescens . J. Exp. Bot. , 56 : 765 – 775 .
- Dikova , R , Stoyanova , Z and Doncheva , S . 2005 . Influence of silicon pretreatment on ascorbic acid content and redox status in Mn-treated maize plant . Compt. Rend. Acad. Bulg. Sci. , 58 : 45 – 50 .
- Doncheva , S , Poschenrieder , C , Stoyanova , Z , Georgieva , K , Velichkova , M and Barceló , J . 2009 . Silicon amelioration of manganese toxicity in Mn-sensitive and Mn tolerant maize varieties . Environ. Exp. Bot. , 65 : 189 – 197 .
- Ducic , T and Polle , A . 2007 . Manganese toxicity in two varieties of Douglas fir (Pseudotsuda menziesii var. viridisand glauca) seedlings as affected by phosphorus supply . Funct. Plant Biol. , 34 : 31 – 40 .
- Foy , CD . 1988 . Plant adaptation to acid, aluminum-toxic soils . Commun. Soil Plant Anal. , 19 : 959 – 987 .
- Foy , CD , Chaney , RL and White , MC . 1978 . The physiology of metal toxicity in plants . Annu. Rev. Plant Physiol. , 29 : 511 – 566 .
- Foy , CD , Wei , RR and Coradetti , CA . 1995 . Differential manganese tolerances in cotton genotypes in nutrient solution . J. Plant Nutr. , 18 : 685 – 706 .
- Führs , H , Hartwig , M Buitrago Molina , LE . 2008 . Early manganese-toxicity response in Vigna unguiculata(L.) – a proteomic and transcriptomic study . Proteomics , 8 : 149 – 159 .
- González , A and Lynch , JP . 1997 . Effects of manganese toxicity on leaf assimilation of contrasting common bean genotypes . Physiol. Plant. , 101 : 872 – 880 .
- Hall , L . 2002 . Cellular mechanism for heavy metal detoxification and tolerance . J. Exp. Bot. , 53 : 1 – 11 .
- Heath , RL and Packer , L . 1968 . Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation . Arch. Biochem. Biophys. , 125 : 189 – 198 .
- Horiguchi , T . 1987 . Mechanism of manganese toxicity and tolerance of plants. II. Deposition of oxidized manganese in plant tissue . Soil Sci. Plant Nutr. , 34 : 595 – 606 .
- Horst , WJ . 1983 . Factors responsible for genotypic manganese tolerance in cowpea (Vigna unguiculata) . Plant Soil , 72 : 213 – 218 .
- Horst , WJ , Fesht-Christoffers , M , Naumann , A , Wissemeier , AH and Maier , P . 1999 . Physiology of manganese toxicity and tolerance in Vigna unguiculata(L.) Walp . J. Plant Nutr. Soil. Sci. , 162 : 263 – 274 .
- Jain , M , Mathur , G , Koul , S and Sarin , NB . 2001 . Ameliorative effects of proline on salt stressed lipid peroxidation in cell lines of groundnut (Arachis hypogeaL.) . Plant Cell Rep. , 20 : 463 – 468 .
- Katsuhara , M , Otsuka , T and Ezaki , B . 2005 . Salt stress-induced lipid peroxidation is reduced by glutathione S-transferase, but this reduction of lipid peroxides is not enough for a recovery of root growth in Arabidopsis . Plant Sci. , 169 : 369 – 373 .
- Kitao , M , Lei , TT and Koike , T . 1997 . Effects of manganese toxicity on photosynthesis of white birch (Betula platyphyllavar. japonica) seedlings . Physiol. Plant. , 101 : 249 – 256 .
- Kitao , M , Lei , TT and Koike , T . 1998 . Application of chlorophyll fluorescence to evaluate Mn tolerance of deciduous broad-leaved tree seedlings native to northern Japan . Tree Physiol. , 18 : 135 – 140 .
- Kitao , M , Lei , TT , Nakamura , T and Koike , T . 2001 . Manganese toxicity as indicated by visible foliar symptoms of Japanese white birch (Betula platyphyllavar. japonica) . Environ. Pollut. , 111 : 89 – 94 .
- Kochian , LV , Hoekenga , OA and Piñeros , MA . 2004 . How do crop plants tolerate acid soils? – mechanisms of aluminium tolerance and phosphorous efficiency . Annu. Rev. Plant Biol. , 55 : 459 – 493 .
- Kolchakov , I , Rousseva , S , Georgiev , B and Stoychev , D . 2005 . “ Soil survey and soil mapping in Bulgaria ” . In Soil Resources of Europe , 2nd edn , European Soil Bureau research Report No 9 Edited by: Jones , RJA , Houšková , B , Bullock , P and Montanarella , L . 83 – 87 . Luxembourgh : EUR 20559 EN Office for Official Publications of the European Communities .
- Macfie , SM and Taylor , GJ . 1992 . The effects of excess manganese on photosynthetic rate and concentration of chlorophyll in Triticum aestivumgrown in solution culture . Physiol. Plant. , 85 : 467 – 475 .
- Marschner , H . 1995 . Mineral Nutrition of Higher Plants , 889 London, , UK : Academic Press .
- Meharg , AA . 1994 . Integrated tolerance mechanisms: constitutive and adaptive plant responses to elevated metal concentrations in the environment . Plant Cell Environ. , 17 : 989 – 993 .
- Mizuno , N , Nosaka , S , Mizuno , T , Horie , K and Obata , H . 2003 . Distribution of Ni and Zn in the leaves of Thlaspi japonicumgrowing on ultramafic soil . Soil Sci. Plant Nutr. , 49 : 93 – 97 .
- Moroni , JS , Briggs , KG and Taylor , GJ . 1991 . Chlorophyll content and elongation rate in wheat seedlings as a measure of manganese tolerance . Plant Soil , 136 : 1 – 9 .
- Moroni , JS , Scott , BJ and Wratten , N . 2003 . Differential tolerance of high manganese among rapeseed genotypes . Plant Soil , 253 : 509 – 519 .
- Morris , ML . 2001 . “ Assessing the benefits of international maize breeding research. Overview on the global maize impacts study ” . In CIMMYT 1999–2000. World Maize Facts and Trends. Meeting Worlds Maize needs: technological opportunities and priorities for the public sector , Edited by: Pingali , PL . 60 Mexico, DF : CIMMYT .
- Nable , RO , Houtz , RL and Cheniae , GM . 1988 . Early inhibition of photosynthesis during development of Mn toxicity in tobacco . Plant Physiol. , 86 : 1136 – 1142 .
- Ohki , K . 1985 . Manganese deficiency and toxicity effects on photosynthesis, chlorophyll and transpiration in wheat . Crop Sci. , 25 : 187 – 191 .
- Page , V and Feller , U . 2005 . Selective transport of zinc, manganese, nickel, cobalt and cadmium in the root system and transfer to the leaves in young wheat plants . Ann. Bot. , 96 : 425 – 434 .
- Peiter , E , Montanini , B Gobert , A . 2007 . A secretory pathway-localized cation diffusion facilitator confers plant manganese tolerance . Proc. Natl Acad. Sci. USA , 104 : 8532 – 8537 .
- Porra , RJ . 1991 . “ Recent advances and re-assessments in chlorophyll extraction and assay procedures for terrestrial, aquatic, and marine organisms, including recalcitrant algae ” . In Chlorophyll , Edited by: Scheer , H . 31 – 57 . Boca Raton : CRC Press .
- Shenker , M , Plessner , OE and Tel-Or , E . 2004 . Manganese nutrition effects on tomato growth, chlorophyll concentration, and superoxide dismutase activity . J. Plant Physiol. , 161 : 197 – 202 .
- Skórzyn′ska-Polit , E and Krupa , Z . 2006 . Lipid peroxidation in cadmium-treated Phaseolus coccineusplants . Arch. Environ. Contam. Toxicol. , 50 : 482 – 487 .
- Stoyanova , Z , Zozikova , E , Poschenrieder , C , Barcelo , J and Doncheva , S . 2008 . The effect of silicon on the symptoms of manganese toxicity in maize plants . Acta Biol. Hung. , 59 : 479 – 487 .
- The , C , Calba , H Zonkeng , C . 2006a . Responses of maize grain yield to changes in acid soil characteristics after soil amendments . Plant Soil , 28 : 45 – 57 .
- The , C , Mafouasson , HA Calba , H . 2006b . Identification of heterotic groups for the tolerance of maize (Zea maysL.) for tropical acid soils . Cah. Agri. , 15 : 337 – 346 .
- Wang , W , Xiufeng , Y , Fuchen , S , Yuangang , Z and Shaoquan , N . 2001 . A trial to accelerate afforestation of Korean pine forests using a strip-cutting method for deciduous b road-leaved secondary forest in northeastern China – an ecophysiological approach . Eur. J. For. Res. , 3 : 27 – 48 .
- Winston , GW . 1990 . “ Physicochemical basis for free radical formation in cells. Production and defences ” . In Stress Responses in Plants Adaptation and Acclimation Mechanisms v. 12 Plant Biology , Edited by: Alscher , RG and Gumming , JR . 57 – 86 . New York : Willey-Liss .
- Xu , X , Shi , J , Chen , Y , Chen , X , Wang , H and Perera , A . 2006 . Distribution and mobility of manganese in the hyperaccumulator plant Phytolacca acinosaRoxb (Phytolaccaceae) . Plant Soil , 285 : 323 – 331 .
- Yamori , W , Kogami , H and Masuzawa , T . 2005 . Freezing tolerance in alpine plants as assessed by the FDA-staining method . Polar Biosci. , 18 : 73 – 81 .