Abstract
To reduce nitrogen (N) loss from vegetable fields with minimum labor, the effects of a single application of different types of controlled mineralizing acetaldehyde condensation urea (CM-CDU) fertilizer on crop yield and N uptake, nitrate leaching from the surface soil (0–20 cm) and N2O emission from the soil after two continuous croppings of a leafy vegetable (komatsuna, Brassica napa L.) were examined. The following four treatments were established in plastic containers filled with an Andisol: urea, CM-CDU-4, CM-CDU-10 at a rate of 30 g N m−2 and a control (no N fertilizer). The CM-CDU-4 and CM-CDU-10 treatments released 80% of N at 25°C in 30–60 and 90–140 days after fertilizer application, respectively. The yields for the two crops were 1.55, 1.55, 1.26 and 0.34 kg fresh weight m−2 in urea, CM-CDU-4, CM-CDU-10 and the control, respectively. The amount of total nitrate leaching was 3.4, 2.3, 1.2 and 0.3 g N m−2 from urea, CM-CDU-4, CM-CDU-10 and the control, respectively. The total amount of N2O emission from urea, CM-CDU-4, CM-CDU-10 and the control over two crops was 0.12, 0.06, 0.04 and 0.01 g N m−2, respectively. The emission factors were 0.7, 0.3 and 0.2 from the urea, CM-CDU-4 and CM-CDU-10 treatments, respectively. The N use efficiency of urea, CM-CDU-4 and CM-CDU-10 was 87, 85 and 59%, respectively. Among the examined fertilizers, we recommend CM-CDU-4 as best for komatsuna production after examining two continuous croppings with a single application.
Introduction
Nitrogen (N) fertilizer is an important growth factor for controlling the yield and quality of vegetable fields (CitationBooij et al. 1996). As most plants are able to utilize less than half of the N fertilizer applied by farmers, much of the remaining N fertilizer leaches into the groundwater and pollutes lakes, rivers, aquifers and oceans. In addition, significant portions of the unabsorbed N fertilizer volatizes in the form of N2O. This excessive N applied as fertilizer is harmful to the environment and the large quantities of nitrate accumulating in ecosystems are a cause of great concern, mainly from health and environmental viewpoints, because the nitrate affects groundwater quality through nitrate leaching and the global environment through nitrous oxide (N2O) emission (CitationNeeteson et al. 1999). Slow release fertilizers (SRF) have many advantages over conventional fertilizer, including a reduction in labor because they are applied as a single basal application and they have a higher N uptake efficiency by crops (CitationShoji et al. 1991, Citation2001). Slow release fertilizers are also environmentally friendly in terms of reducing fertilizer N losses caused by leaching and nitrification (CitationUeno and Yamamuro 1996).
Controlled mineralizing acetaldehyde condensation urea (CM-CDU) is a new biodegradable and controlled-release N fertilizer made of acetaldehyde condensation urea (CDU) granules (2-oxo-4-methly-6-ureidohexahydro-pyrimidine) with a water-repellent material (release-repressing material) and a phosphoric component (mineralization-promoting material) in the granule (CitationSakamoto et al. 2007). These fertilizers release some pattern as its granule size does not change and it is biodegradable and nitrifiable. CitationTakeshita et al. (1997) reported that some soil-borne diseases are repressed by an increase in CDU-degrading bacteria in soil. However, to date no studies on CM-CDU have examined its N efficiency or environmental impact by N leaching or N2O emission. In the present experiment, CM-CDU was examined in an effort to reduce N loss from vegetable fields.
In a previous paper, we reported that CM-CDU was less appropriate for growing komatsuna than some other crops because the amounts of N remaining in the soil after crop harvesting and the N2O emission were higher when CM-CDU was applied under a single crop compared with urea fertilizer (CitationAmkha et al. 2007). However, CM-CDU needs to be examined to consider its N use efficiency (NUE) for continuous cropping to save labor. A single application of CM-CDU for continuously growing leafy vegetable crops may allow a more efficient use of N and less labor than a single crop system with conventional N fertilizers through exploitation of residual fertility in the crop rotation. This may also reduce the contamination of groundwater and reduce the losses to the environment.
Therefore, the purpose of the present study was to determine the effects of a single application of different types of CM-CDU fertilizers for two croppings on: (1) leafy vegetable (komatsuna, Brassica napa L.) growth, (2) nitrate leaching from the soil surface (0–20 cm), (3) N2O emission from the soil.
Materials and methods
Experimental setting
The experiment was conducted at the soil science experimental field, Graduate School of Horticulture, Chiba University, Matsudo, Japan. Two crops of komatsuna were continuously cultivated in plastic containers (30 cm length × 40 cm width × 30 cm depth) filled with arable soil. The type of soil is described as Andisol, which is a volcanic soil and originally acidic; this soil covers 46.5% of the upland fields in Japan and had a total C content of 20.9 g kg−1 soil, a total N content of 2.1 g kg−1 soil, a cation exchange capacity (CEC) of 22.4 cmol(+) kg−1 soil, a bulk density of 0.80 Mg m−3 and a pH of 5.9 (CitationSakamoto and Hodono 2000). Four treatments were set up, three different N fertilizers, urea and two types of CM-CDU (CM-CDU-4 and CM-CDU-10) and a control (i.e. no N fertilizer). The fertilizers were mixed well with the soil (0–20 cm) in the containers. According to the CM-CDU product information from the Chissoasahi Fertilizer Company (Tokyo, Japan), CM-CDU-4 and CM-CDU-10 released 80% of N at 25°C in 30–60 and 90–140 days after fertilizer application (DAF) (CitationSakamoto et al. 2007). The CM-CDU designation indicates a type of fertilizer in which N is released in a linear form and has a 30% N content, light yellow color and a size of 2.8–4.1 mm. The urea fertilizer was applied twice at a rate of 15 g N m−2 (150 kg N ha−1) on the day of transplanting of each crop and mixed well with the soil; the CM-CDU fertilizer was only applied once at a rate of 30 g N m−2 (300 kg N ha−1) when the first crop of plants were transplanted. The total N of the applied fertilizer in the present experiment was 30 g N m−2 (300 kg N ha−1). In all treatments, commercial P-K fertilizer (0–13–13) at 10 g m−2 (100 kg of P2O5–K2O ha−1) was applied to the 0–20 cm soil layer uniformly as a basal application after transplanting the first and second crops. The first crop was sowed on 27 August and the second on 27 September 2005 into cell trays. Fourteen days after sowing, eight seedlings were transplanted into the containers. The plants were arranged with an inter-row spacing of 20 cm and intra-row spacing of 10 cm. We did not irrigate throughout the two croppings, but the containers received 120 mm rainfall over the two crop seasons. Each whole crop was harvested (shoot + root) 30 days after transplanting (DAT). The experimental design was completely randomized with four treatments and three replicates of each treatment.
Plant growth sampling
The weight of the fresh shoots was measured just after the harvest of each crop. The plant material was dried at 80°C for 5–7 days in a forced air-oven. The total N contents of the oven-dried, ground and sieved (0.5 mm) plant samples were determined using a Yanaco MT-700 CN corder (Yanagimoto, Kyoto, Japan). In addition, the following parameters were calculated: (1) yield (kg m−2), (2) N uptake (kg m−2), (3) NUE (%), as a percentage of the N uptake to the total N application rate.
Soil sampling
Soil samples (0–20 cm) were taken from the containers by a core sampler (internal diameter 5 cm) before transplanting and at crop harvest time. The soil samples were sieved and stored at 4–5°C until analysis. The soil samples were extracted with 1.0 mol L−1 KCl for analyses of the nitrate contents. The contents of nitrate in the soil extract were determined by hydrazine reduction (CitationHayashi et al. 1997).
Leachate sampling
The leachate samples collected on 13 and 26 September and 20 October 2005 were filtered (Advantec No. 6 filter paper, Tokyo, Japan) for the analyses of nitrate using the hydrazine reduction method (CitationHayashi et al. 1997).
Gas sampling
A closed chamber method was used to determine the N2O fluxes from each fertilizer treatment. A white plastic chamber with a cross-sectional area of 216 cm2 (16 cm × 13.5 cm) and a height of 10 cm was used. The chamber was settled in the center of the containers for air sampling at 1, 2, 4, 6, 9, 13, 20 and 30 DAT for each crop (CitationInubushi and Yagi 2007). Four samples of the chamber air were pulled into syringes at 0, 10, 20 and 30 min after the chamber was set up at each sampling time and the N2O concentration was analyzed using a gas chromatograph (GC) (Shimadzu GC 14B, Tokyo, Japan) equipped with an electron capture detector (ECD) at 280°C. The separating column (2 m length and 2 mm diameter) was packed with Porapak Q and the carrier gas (5% methane in argon).
The N2O flux (mg of N2O-N m−2 h−1) was calculated from the temporal increase in the gas concentration inside the chamber per unit time as follows:
where k is a constant for the conversion from volume to weight (N2O = 1.250), V and A are the volume of effective space and the area of the bottom of the chamber, respectively, T is the air temperature inside the chamber (oC) and dc/dt is the increase in the gas concentration inside the chamber over the closure period (CitationInubushi and Yagi 2007).
The total N2O emission over the two cropping seasons (g of N2O-N m−2) was calculated by integrating the amount of emitted N2O with the duration of its measurement as follows:
The emission factor (EF) was calculated as the ratio of the total N2O emitted from the fertilized soil minus the total N2O emitted from the unfertilized soil to the total amount of N fertilizer applied to the soil.
Statistical analyses
Statistical analyses were carried out using the statistical package SPSS 11 (SPSS Inc., Chicago, USA). Differences between treatments and data variance were determined by ANOVA. Comparisons of the means among treatments were done using post-hoc Tukey’s tests at a significance level of P < 0.05.
Results and discussion
Effect of nitrogen fertilizers on plant growth
The fresh weight yield was highest with CM-CDU-4 followed by the urea, CM-CDU-10 and control treatments, but the yields were not significantly (P ≤ 0.05) different with CM-CDU 4 and urea fertilizer (). In SRFs, the yield in the CM-CDU-4 treatment was higher than that in the CM-CDU-10 treatment. Nitrogen uptake in all fertilizer treatments was significantly higher than the control (). Among the fertilizer treatments, the N uptake and NUE in the CM-CDU-4 and urea treatments were higher than in the CM-CDU-10 treatment, but there was no significant difference between the CM-CDU-4 and urea treatments. This could be explained by the N-release patterns of the different fertilizers. Nitrogen from CM-CDU-10 was also released as ammonium, but longer time periods were required.
Effect of nitrogen fertilizers on the nitrate remaining in the soil
The nitrate remaining in the soil was much higher in the CM-CDU-10 treatment than in the urea and CM-CDU-4 treatments after harvesting the second crop () because the cropping periods were shorter than the N release time from the CM-CDU-10 fertilizers. Komatsuna was cultivated after 60 days and the 80% release time for CM-CDU-4 and CM-CDU-10 is 30–60 and 90–140 days, respectively.
Table 1 Effect of the type of nitrogen fertilizer on the fresh weight, nitrogen uptake and nitrogen use efficiency (NUE) of komatsuna
Table 2 Effect of the type of nitrogen fertilizer on the nitrate remaining in the soil, nitrate leaching, N2O emission and the emission factor (EF)
Effect of nitrogen fertilizers on nitrate leaching
The leachate was collected at 3 and 16 DAT in the first crop, and at 10 DAT in the second crop. No significant differences in the amount of water leached from the fertilizer treatments were recorded (data not shown). The amount of total nitrate leachate from the control was significantly lower than the amounts from all fertilizer treatments. Among the fertilizer treatments, the highest amount of total nitrate leachate was in the urea treatment followed by the CM-CDU-4 and CM-CDU-10 treatments, respectively (). The total amount of nitrate leaching derived from the N fertilizer was equivalent to 10.3, 6.7 and 3.0% of the applied N fertilizer in the urea, CM-CDU-4 and CM-CDU-10 treatments, respectively. CitationTrindade et al. (1997) found that annual nitrate leaching losses ranged from 15.4 to 33.8 g N m−2, and these losses are equivalent to 5.1–11.3% of the applied N. A number of studies have shown that the amounts of N leached into groundwater in vegetable fields are >20 g N m−2 (CitationKraft and Stites 2003; CitationPrunty and Greenland 1997; CitationRamos et al. 2002; CitationSitites and Kraft 2001) and in some cases the amounts are even >50 g N m−2 (CitationZhu et al. 2005).
Effect of nitrogen fertilizers on N2O emission
N2O was quickly emitted after the application of urea and after rainfall the emission of N2O subsequently decreased to the same level as the control in both crops (). The emission of N2O just after the application of urea in the present study was similar to our previous report (CitationAmkha et al. 2007). Many researchers have reported a marked increase in the N2O emission rate just after the application of conventional N fertilizer because the nitrification process is active after NH4 + is applied to agricultural soils. CitationZhou et al. (2006) also found that when urea was added to a soil it was enzymatically hydrolyzed to NH4 + during its transport through the soil profile and this hydrolysis to NH4 + occurred within 1–2 h after application. Among the CM-CDU fertilizers, the peak value of N2O flux was highest in CM-CDU-4 by 37 DAF. The N2O flux in CM-CDU-10 tended to increase gradually from 34 DAF until harvest time for the second crop. The controlled-release fertilizer exhibited broad peaks of N2O flux with a longer duration of N2O emission.
Figure 1 Daily rainfall and the N2O flux emitted for the different nitrogen fertilizer types. Vertical bars indicate the standard deviation.
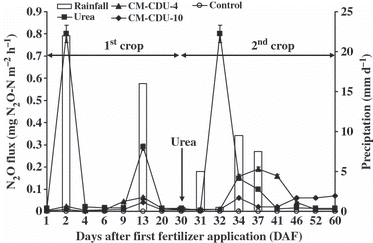
The total N2O emission was highest in the urea treatment followed by the CM-CDU-4, CM-CDU-10 and control treatments, respectively, for the two crops (). Previously reported values of total N2O emissions for fertilizer applied to Andisols range from 0.02 to 0.46 g N m−2 (CitationAkiyama and Tsuruta 2002, Citation2003; CitationHou and Tsuruta 2003; CitationKusa et al. 2006; CitationLi et al. 2002; CitationNoda 2001; CitationShoji et al. 2001; CitationWatanabe et al. 1997; CitationYan et al. 2001). CitationAkiyama et al. (2006) reported that the N2O emissions for a fertilized upland field were 0.10 g N m−2 for well-drained soil and 0.48 g N m−2 for poorly drained soil.
The EF was highest in the urea treatment, followed by the CM-CDU-4 and CM-CDU-10 fertilizer treatments, respectively (). Previously reported values of EF rates for various crops and vegetables range from 0.07 to 2.02% in Andisol (CitationHou and Tsuruta 2003; CitationLi et al. 2002; CitationNoda 2001; CitationShoji et al. 2001; CitationWatanabe et al. 1997; CitationYan et al. 2001), and these rates are similar to the rates recorded in the present study. In our experiment, the EF in CM-CDU-4 (0.3%) and the EF in CM-CDU-10 (0.2%) were lower than the default EF (1.25%) of the CitationIntergovernmental Panel on Climate Change (2001), the 1% of chemical fertilizer by the CitationNetherlands Environmental Assessment Agency (2006) and the 0.8% by the CitationFood and Agriculture Organization/International Fertilizer Industry Association (2001). CitationWebb and Harrison (2000) reported annual N2O losses of 0.5–2.7% from an arable field in the UK and CitationKaiser et al. (1998) reported values of N2O losses ranging from 0.7 to 4.1%. The N2O emission from agricultural land can vary from 0.03 to 2.7% (CitationEichner 1990) and can even reach as high as 5.8% (CitationDobbie et al. 1999) of the added N. CitationAkiyama et al. (2006) reported that the EF in a Japanese fertilized upland field was 0.62 ± 0.48%. In south Sulawesi, Indonesia, the EF was as high as 1.61% for urea and dropped to 1.42% for controlled released fertilizer (LP30) and 0.71% for inhibitor diacyandiamide (DCD) (CitationJumadi et al. 2008), whereas in south Kalimantan, it was 3.1% for urea, 0.15% for LP30 and almost zero for DCD plots (CitationHadi et al. 2008). Further studies are needed to clarify the factors controlling N2O emission and to determine mitigation options for N2O emission from various soils.
Conclusion
Among the fertilizers examined in the present study, we would recommend CM-CDU-4 as best for komatsuna production when using a single application for two continuous croppings. However, when growing three or more crops, CM-CDU-10 fertilizer may allow a more efficient utilization of N.
Acknowledgments
We are grateful for the financial support of the Ministry of Education, Culture, Sports, Science and Technology (MEXT) and the GHG-SSCP project, Global Environment Research, Ministry of Environment, Japan (B-S-2-3a). We thank Mr Oslan Jumadi and Mr Silvio Y. Ushiwata from the Laboratory of Soil Science, Faculty of Horticulture, Chiba University, for their assistance. We also thank Dr Agnes T. Padre, International Rice Research Institute, Philippines, for valuable suggestions.
Notes
Present address: †Department of Soil Science, Faculty of Agriculture at Kamphaengsaen, Kasetsart University, Kamphaengsaen Campus, Nakhonpathom 73140, Thailand.
References
- Akiyama , H and Tsuruta , H . 2002 . Effect of chemical fertilizer form on N2O, NO and NO2fluxes from Andisol field . Nutr. Cycl. Agroecosystems. , 63 : 219 – 230 .
- Akiyama , H and Tsuruta , H . 2003 . Nitrous oxide, nitric oxide and N dioxide fluxes from soils after manure and urea application . J. Environ Qual. , 32 : 423 – 431 .
- Akiyama , H , Yan , XY and Yagi , K . 2006 . Estimations of emission factors for fertilizer-induced direct N2O emission from agricultural soils in Japan: summary of available data . Soil. Sci. Plant Nutr. , 52 : 774 – 787 .
- Amkha , S , Inubushi , K and Takagaki , M . 2007 . Effects of controlled-release N fertilizer application on N uptake of a leafy vegetable (Brassica campestrisL.), nitrate leaching and N2O emission . Jpn. J. Trop. Agric. , 51 : 152 – 159 .
- Booij , R , Willigen , P , Kreuzer , ADH , Smit , AL and Van der Werf , A . 1996 . N balances during growth of Brussels sprouts and leeks . Acta Hortic. , 428 : 31 – 43 .
- Dobbie , KE , McTaggart , LP and Smith , KA . 1999 . Nitrous oxide emission from intensive agricultural system: variations between crops and seasons, key driving variables, and mean emission factors . J. Geophys. Res. , 104 : 26891 – 26899 .
- Eichner , MJ . 1990 . Nitrous oxide emission from fertilized soil: summary of available data . J. Environ. Qual. , 19 : 272 – 280 .
- Food and Agriculture Organization/International Fertilizer Industry Association . 2001 . Global Estimate of Gaseous Emission of NH3, NO and N2O from Agricultural Land , Rome : FAO .
- Hadi , A , Jumadi , O , Inubushi , K and Yagi , K . 2008 . Mitigation options for N2O emission from a corn field in Kalimantan, Indonesia . Soil Sci.Plant Nutr. , 54 : 644 – 649 .
- Hayashi , A , Sakamoto , K and Yoshida , T . 1997 . A rapid method for determination of nitrate in soil by hydrazine reduction procedure . Jpn. J. Soil Sci. Plant Nutr. , 68 : 322 – 326 . (in Japanese with English summary)
- Hou , AX and Tsuruta , H . 2003 . Nitrous oxide and nitric oxide fluxes from an upland filed in Japan: effect of urea type, placement, and crop residues . Nutr. Cycl. Agroecosystems. , 65 : 191 – 200 .
- Intergovernmental Panel on Climate Change . 2001 . Climate Change 2001, Mitigation , Cambridge : Cambridge University Press .
- Inubushi , K and Yagi , K . 2007 . “ Greenhouses from soil ” . In Environmental Chemistry Experimental Chemistry , 5th , Edited by: Otani , M. 20 – 2 . 142 – 153 . Tokyo : Maruzen . in Japanese
- Jumadi , O , Hala , Y Muis , A . 2008 . Influences of chemical fertilizers and a nitrification inhibitor on greenhouse gas fluxes in a corn (Zea mays L.) field in Indonesia . Microbes Environ. , 23 : 29 – 34 .
- Kaiser , EA , Kohrs , K , Kucke , M , Schnug , E , Heinemeyer , O and Munch , JC . 1998 . Nitrous oxide release from arable soil: important of N-Fertilization, crops and temporal variation . Soil Biol. Biochem. , 30 : 1553 – 1563 .
- Kraft , GJ and Stites , W . 2003 . Nitrate impacts on groundwater from irrigated vegetable systems in humid north-central US sand and plain . Agric. Ecosyst. Environ. , 100 : 63 – 74 .
- Kusa , H , Hu , R , Sawamoto , T and Hatano , R . 2006 . Three years of nitrous oxide and nitric oxide emission from a Silandic Andosols cultivated with maize in Hokkaido, Japan . Soil Sci. Plant Nutr. , 52 : 103 – 113 .
- Li , X , Inubushi , K and Sakamoto , K . 2002 . Nitrous oxide concentrations in an Andisol profile and emission to the atmosphere as influenced by the application of N fertilizers and manure . Biol. Fertil. Soils , 35 : 108 – 113 .
- Neeteson , JJ , Booij , R and Whitmore , AP . 1999 . A review on sustainable N management in intensive vegetable production systems . Acta Hortic. , 506 : 17 – 26 .
- Netherlands Environmental Assessment Agency . 2006 . Greenhouse Gas Emission in the Netherlands 1990–2004: National Inventory Report 2006 , Bilthoven : Netherlands Environmental Assessment Agency .
- Noda , S . 2001 . Application and use of fertilizers for the reduction of nitrous oxide emission from uplands soils . Jpn. J. Soil. Sci. Plant Nutr. , 72 : 575 – 581 . (in Japanese)
- Prunty , L and Greenland , R . 1997 . Nitrate leaching using two potato-corn N-fertilizer plans on sandy soil . Agric. Ecosyst. Environ. , 65 : 1 – 13 .
- Ramos , C , Agutt , A and Lidon , AL . 2002 . Nitrate leaching in important crops of the Valencian Community region (Spain) . Environ. Pollut. , 118 : 215 – 223 .
- Sakamoto , S , Hanawa , H and Tachibana , M . 2007 . Methods for mineralization control of acetaldehyde condensation urea by additives . Jpn. J. Soil Sci. Plant Nutr. , 78 : 303 – 307 . (in Japanese)
- Sakamoto , K and Hodono , N . 2000 . Turnover time of microbial biomass carbon in Japanese upland soils with different textures . Soil Sci. Plant Nutr. , 46 : 483 – 490 .
- Shoji , S , Delgado , J , Mosier , A and Miura , Y . 2001 . Use of controlled release fertilizer and nitrification inhibitors to increase N use efficiency and to converse air and water quality . Commun. Soil. Sci. Plant Anal , 32 : 1051 – 1070 .
- Shoji , S , Gandez , AT and Kimura , K . 1991 . Stimulation of crop response to polyolefin-coated urea II. N uptake by dent corn . Soil Sci. Soc. Am. J. , 55 : 1468 – 1473 .
- Sitites , W and Kraft , GJ . 2001 . Nitrate and chloride loading to groundwater from irrigated north-central US sand and plain vegetable fields . J. Environ. Qual. , 30 : 1176 – 1184 .
- Takeshita , S , Kato , K and Suzuki , T . 1997 . Microbiological study on soil sickness due to continuous cropping in greenhouse culture . Soil Microorganisms. , 19 : 19 – 28 .
- Trindade , H , Coutinho , J , Beusichem Van , ML , Scholefield , D and Moreiva , N . 1997 . Nitrate leaching from sandy loam soil under a double-cropping forage system estimated from suction-probe measurement . Plant Soil , 195 : 247 – 256 .
- Ueno , H and Yamamuro , S . 1996 . N dynamics and plant uptake in paddy field amended with slow release coat urea . Proceedings of the International Symposium on Maximizing Sustainable Rice Yields through Improve Soil and Environment Management , 2 : 857 – 865 .
- Watanabe , T , Osada , T , Yoh , M and Tsuruta , H . 1997 . N2O and NO emission from grassland soils after the application of cattle and swine excreta . Nutr. Cycl. Agroecosystems , 49 : 35 – 39 .
- Webb , J and Harrison , R . 2000 . N fluxes in three arable soils in the UK . Eur J Agronomy , 13 : 207 – 223 .
- Yan , X , Hosen , Y and Yagi , K . 2001 . Nitrous oxide and nitric oxide emissions from maize field plots as affected by N fertilizer type and application method . Biol. Fertil. Soils , 35 : 297 – 303 .
- Zhou , JB , Xi , JG , Chen , ZJ and Li , SX . 2006 . Leaching and transformation of N fertilizers in soil after application of N with irrigation: A soil column method . Pedosphere , 16 : 245 – 252 .
- Zhu , JH , Li , XL , Christie , P and Li , JL . 2005 . Environmental implications of low N use efficiency in excessively fertilized hot pepper cropping systems . Agric. Ecosyst. Environ. , 111 : 70 – 80 .
- Present address: †Department of Soil Science, Faculty of Agriculture at Kamphaengsaen, Kasetsart University, Kamphaengsaen Campus, Nakhonpathom 73140, Thailand.