Abstract
Salinity stress is a major abiotic problem in arid land agriculture. In particular, Na stress severely limits crop production because it causes Na toxicity and disturbs the homeostasis of essential cations and microelements in crops. The purpose of the present study was to verify the validity of two indexes with regard to Na tolerance in plants: (1) cation balance (([K]+[Ca]+[Mg])/[Na]), (2) absorption ability of microelements. Salicornia bigelobii (highly salt tolerant), beet (tolerant), maize (moderately sensitive) and bean (sensitive) were grown in artificially prepared saline (SA), sodic (SO) and highly sodic (HSO) soils. Salicornia bigelobii showed the best growth in SO soil and accumulated Na in the growing part of the shoot. Beet showed the best growth in SA soil and also needed Na for satisfactory growth. Both species, therefore, can be classified as halophytes. There was no relationship between growth and cation balance in Salicornia bigelobii and beet. The salt treatments suppressed the growth of maize and bean, with more severe suppression in bean. The cation balance of maize was higher than that of bean. Cation balance can, therefore, be an index of Na tolerance in maize and bean, which are glycophytes. Salicornia bigelovii and beet actively absorbed microelements under high Na conditions. In maize and bean, the salt treatments lowered the uptake amount of microelements, more so in bean. Absorption ability of microelements can, therefore, be an index of Na tolerance, irrespective of whether the plants are halophytes or glycophytes.
INTRODUCTION
Salt-affected soils are widely distributed in dry lands (CitationFlowers and Yeo 1995; CitationGlenn and O’Leary 1985; CitationRhodes and Loveday 1990). Over 800 million hectares of land throughout the world are affected by salt, either by salinity (397 million ha) or the associated condition of sodicity (434 million ha) (CitationFood and Agriculture Organization 2005; CitationMunns 2005). Salt-affected soils occupy over 6% of the world’s total land area and most are formed naturally. However, human-induced wastelands have been increasing and 20% of irrigated land used for dry-land agriculture is affected by salt (CitationFood and Agriculture Organization 2005). The loss of farmable land as a result of salinization is in direct conflict with the needs of the world’s population, which is projected to increase by 1.5 billion over the next 20 years, and the challenge of maintaining the world’s food supplies (CitationYamaguchi and Blumwald 2005). Crop production in such soils is limited because salts directly and indirectly inhibit normal nutrition and metabolism of crops and reduce their dry matter production.
One problem caused by salts in soil is osmotic stress owing to the high electrical conductivity of the soil solution, which inhibits water absorption of crops, reduces stomatal conductance and decreases photosynthesis (CitationRivelli et al. 2002; CitationRomeroaranda et al. 2001). Cellular and metabolic inhibition is also involved in common to drought stress. According to CitationMunns (2002), osmotic stress is effective in the beginning (hours to days) of exposure to salt. The tolerance of many plant species to osmotic stress was classified by CitationMaas (1984).
After prolonged exposure to salt, nutritional stress becomes important in affecting plant growth (CitationMunns 2002). Nutritional stress is another salt problem induced by the ion toxicity and nutritional imbalance of essential elements in the plant body that reduces growth (CitationNiu et al. 1995; CitationSerrano et al. 1999; CitationZhu et al. 1998). It is well known that Na competitively inhibits the absorption of the essential cations, K, Ca and Mg, and induces their deficiencies. (CitationNiu et al. 1995; CitationRodriguez-Navarro 2000; CitationSong and Fujiyama 1996a,b). It is commonly recognized that salt tolerance, particularly Na tolerance, depends on a plant’s ability to exclude Na and to maintain nutritional homeostasis. Thus, Na tolerance is related to the ability to absorb essential nutrients, particularly K, Ca and Mg, against competitive inhibition by Na to maintain ionic balance. From this viewpoint, the cation balance, ([K]+[Ca]+[Mg])/[Na], in the plant body can be used as an index of nutritional homeostasis under high Na conditions.
Sodium raises soil pH and reduces the availability of the essential microelements Fe, Mn, Zn and Cu, and induces their deficiencies (CitationJumberi et al. 2001). Thus, the absorbing ability of such elements can be another index of the Na tolerance of plants.
The purpose of the present study was to verify the validity of both indexes of Na tolerance in plants. Four plant species with differing salt tolerance were grown in artificially prepared saline (electrical conductivity (EC) > 4, exchangeable sodium percentage (ESP) < 15, pH < 8.5) and sodic (EC < 4, ESP > 15, pH > 8.5) soils.
Salicornia bigelovii grows healthily in a high salt environment, such as coastal marshes and inland salt marshes, indicating that it is highly salt tolerant.
MATERIALS AND METHODS
Soil preparation
Saline soil (SA), sodic soil (SO) and highly sodic soil (HSO) were prepared from air-dried Tottori dune soil (CO) by applying solutions of CaCl2, MgSO4 and Na2CO3 (). Nitrogen, P and K were applied to the soils and Ca and Mg were applied to the CO soil as fertilizer (). The characteristics of the soils after fertilization are shown in . The exchangeable sodium percentage (ESP) was calculated using the following formula:
Table 1 Amount of Ca, Mg and Na added to the soil (g kg−1 soil)
Table 2 Amount of fertilizer applied (g kg−1 soil)
Table 3 Chemical properties of the soil after the addition of the salts and fertilizers
The pH was measured after 10 g of air-dried soil was stirred into 25 mL of distilled water. The EC was measured after 10 g of soil was stirred into 50 mL of distilled water.
Plant species and culture
The highly salt tolerant Salicornia biglovii, a salt tolerant beet (Beta vulgaris L. cv. Sugarmangold), a moderately salt sensitive maize (Zea mays L. cv. Whitedent) and a salt sensitive bean (Phaseolus vulgaris L. Nahl) (CitationMaas 1984) were used in the present study.
The beet, maize and bean were sown in small plastic pots. Two seedlings of each species without the plastic pot were transplanted to 4 L pots filled with either 4 kg of CO, SA, SO or HSO soil. Seeds of Salicornia were sown in vermiculite, grown for 1 month and then transplanted to small plastic pots. Plants were arbitrarily watered once per day. After 2.5 months, the plants were transplanted to 4 L pots.
All plants were grown for 1 month in a greenhouse at Tottori University and then harvested. Five replicate pots were prepared for each treatment.
Sampling of the plants and soils
One plant in each pot was used to determine the water deficit (WD). The shoots and roots of the Salicornia plants were separated into upper, middle and lower parts. Each part of the shoot was cut to the same length. The beet plants were separated into leaves, stems and roots. The maize plants were separated into leaf blades, leaf sheaths, stems and roots. The bean plants were separated into leaves, petioles, stems and roots. All parts were weighed immediately after harvest to obtain the fresh weight (FW) and dried at 70°C for 48 h to obtain the dry weight (DW). The WD was calculated from the FW and the DW:
Fresh leaves from another plant in each pot were collected for analysis of the Na concentration in the vacuole.
The soil in the pots was collected when the plants were sampled and well mixed and air-dried for analysis of the organic acids.
Contents of the elements in the shoots and roots
Dried materials were finely ground and digested by sulfuric acid and hydrogen peroxide to allow measurement of the minerals. The contents of K and Na in the shoots and roots were analyzed in dried samples using the flame photometric method. Calcium, Mg, Fe, Mn, Cu and Zn were analyzed using an atomic absorption spectrophotometric method (Polarization Zeeman Atomic Absorption Spectrophotometer Z-6000; Hitachi, Tokyo, Japan).
Cation balance
The cation balance was calculated using the following formula:
Organic acid in the soil
One hundred milliliters of distilled water was added to 30 g of soil. The solution was shaken at 25°C for 16 h and centrifuged at 33000 g for 40 min. The organic acids in the supernatant were analyzed by capillary electrophoresis (CAPI-3300; Otsuka Electronics, Osaka, Japan). Malic acid, citric acid, malonic acid, succinic acid and oxalic acid were used as standards.
The statistical analyses were carried out using SPSS 10.0J software (SPSS, SPSS Japan Inc., Tokyo, Japan). Pearson correlation coefficients were calculated to assess the correlation between the different variables and the data were analyzed using one-way ANOVAs (F-tests).
RESULTS
Plant growth
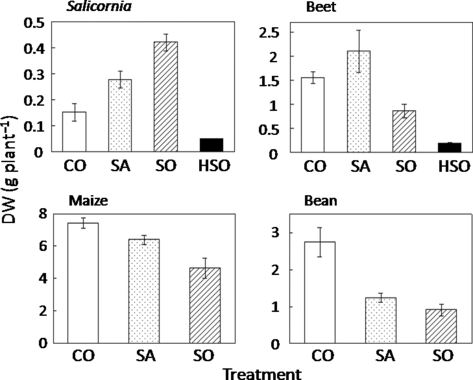
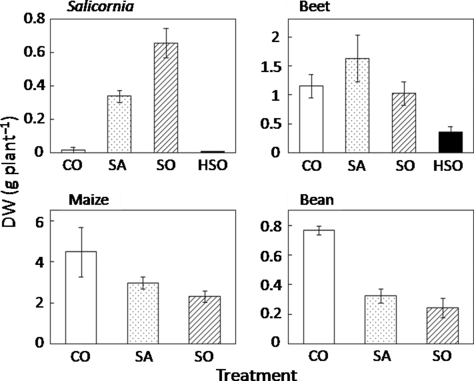
The dry shoot yield of Salicornia increased with increasing Na concentration in the soil and decreased in HSO (). Beet showed the best growth in SA. The growth of Salicornia and beet in HSO was very poor. An increased Na concentration brought gradual growth reduction in maize. The SA and SO treatments reduced bean growth substantially. Under HSO conditions, maize and bean could not survive. A similar tendency was found in the dry root yield in all species ().
Water deficit
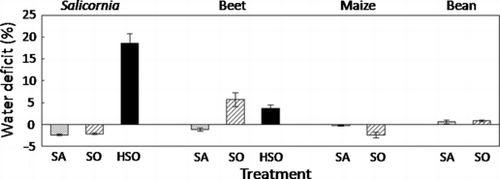
Salicornia did not suffer from water stress in SA or SO, but showed a severe water deficit in HSO (). Beet showed water stress in SO and HSO. Maize did not show water stress in any of the salt treatments. Bean showed relatively low water stress in the salt treatments.
Contents of the elements in the plants
In Salicornia, the Na content of the shoots increased with increasing Na concentration in the soil, but there was no significant difference between SA and SO. The contents of K, Ca and Mg decreased with increasing Na concentration except for K and Ca in HSO (). There was no significant difference in the Ca content between CO and SA and the Mg content of SA was higher than that of CO. The Mn, Cu and Zn contents of the shoots in the salt treatments tended to be lower than in the CO, except in HSO. There was no difference in the Na content of the roots except for HSO. In roots of SA and SO, the content of K and Ca was lower than that in CO, except for Ca in SA. The content of microelements in the roots tended to increase with increasing Na concentration in soil, except for Cu.
In beet, the Na content of the shoots increased and the contents of K, Ca and Mg decreased with increasing Na concentration in the soil (). The Cu content increased with increasing Na concentration. The content of Mn and Zn decreased with increasing Na concentration. The Na content of the roots in the salt treatments was higher than that of CO, but there was no difference between SA and SO. The content of Ca and Mg was higher in SA. The content of Fe and Zn in the roots in SA was higher than that in CO.
Figure 3 Water deficit of the shoots. Values are mean ± standard error (n = 5). CO, control; SA, saline soil; SO, sodic soil; HSO, highly sodic soil.
In maize, the Na treatment brought about an extreme increase in the Na content and a significant increase in the K content of the shoots (). The Ca and Mg contents decreased with increasing Na concentration in the soil, although there was no difference in Mg content between CO and SA. Sodium increased the Fe content and decreased the Mn and Zn contents in the shoots. The Na content of the roots increased with increasing Na concentration in the soil. Salt treatment decreased the content of Mg, but did not affect the Ca content of the roots. The content of Fe and Mn in the roots in SO was lower than that of CO, but the Cu content was not changed and the Zn content was higher in SO.
In bean, the salt treatments brought about an extreme increase in the Na content of the shoots (). The content of K and Mg in the shoots decreased with increasing Na concentration in the soil, although the Ca content in SA was higher than that of CO. The Fe and Cu contents in the shoots increased and the Mn content decreased with increasing Na concentration in the soil. The Na content of the bean roots increased with increasing Na concentration in the soil. Only the K content decreased in the salt treatments. The content of Cu in the roots tended to increase and the Mn content decreased with increasing Na concentration in the soil, but there was no definite pattern in Zn content.
Table 4 Contents of the elements in the shoots (above) and roots (below) of the examined plant species
Cation balance of the shoots
The cation balance of the shoots is shown in . In all species, the cation balance decreased with increasing Na concentration in the soil, with a milder decrease for Salicornia. The cation balance in CO was maize > bean > beet > Salicornia. This pattern was also found in SA and SO.
Difference in the Na concentration between plant parts
In Salicornia, Na accumulated at the growing upper part of the shoots (). The Na content of the roots was much lower than that of the shoots. In Salicornia grown in HSO, the shoot sample was too small to be divided into parts.
The Na content of the leaves and petioles in beet was higher than that of the tuberous roots and fibrous roots. In maize, the Na content of the leaf blades was lower than that in the leaf sheaths, stems and roots. In bean, the Na content of the stems was the highest and the content decreased in the order, petioles, roots and leaves.
Organic acids in the soil
The only organic acid detected in the salt-treated soils was oxalic acid. For Salicornia, oxalic acid was detected in SA, SO and HSO, with the highest concentration in SO (). For beet, oxalic acid was detected in the soil of HSO, and for maize, it was detected in SO.
DISCUSSION
The four plant species showed different growth responses to salt-affected soils. Salicornia had the best growth in SO; in SO the other species showed poor growth (,2). Beet had the best growth in SA. The salt treatments suppressed the growth of maize and bean, with more severe suppression in bean. These results indicate that Salicornia and beet are classified as halophytes and that maize and bean are classified as glycophytes.
Salicornia accumulated Na in the upper part of the shoots, which is a growing part (). A number of reports have shown that salt-tolerant plants use Na for osmotic adjustment by accumulating it in the vacuoles (CitationDracup and Greenway 1985; CitationFlowers and Yeo 1988; CitationTorello and Rice 1986). However, the specific function of Na in the metabolic processes of halophytes is still unknown. In the present study, we could not clarify the specific mechanisms of Na on growth stimulation. However, it is possible that Na is strongly related to the growth stimulation in Salicornia because Na accumulated in a growing part. The lower growth in SA compared with SO was not the result of osmotic stress (), but rather Na deficit. A high Na content in the shoots of the CO plants () suggests that Salicornia actively absorbs Na. The maintenance of a high cation balance in the plant shoots is related to the high absorption ability of essential elements (e.g. K, Ca, Mg) against the competitive inhibition by Na (CitationJumberi et al. 2002). However, Salicornia had a low cation balance () and this lower cation balance of Salicornia compared with the other species () means that Salicornia needs more Na than essential cations (K, Ca and Mg). There were no relationships between growth and cation balance. Even if Salicornia needs a quantity of Na for satisfactory growth, it needs to overcome the low availability of microelements in the high pH conditions of Na-rich soils.
Figure 4 Cation balance in the shoots of each plant species examined. CO, control; SA, saline soil; SO, sodic soil; HSO, highly sodic soil.
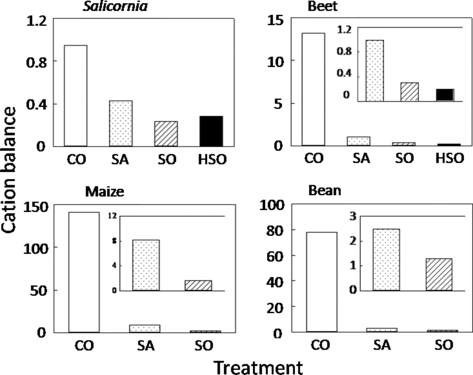
Figure 5 Sodium distribution in the plant organs. Each beet, maize and bean plant was divided into four parts; Salicornia was divided into three parts. Salicornia was divided into upper, middle and lower parts of shoots and roots. Beet was divided into leaf, petiole, tuberous root and fibrous root. Maize was divided into leaf blade, leaf sheath, stem and root. Bean was divided into leaf, petiole, stem and root. Values are mean ± standard error (n = 5). CO, control (□); DW, dry weight; SA, saline soil (□); SO, sodic soil (□); HSO, highly sodic soil (□).
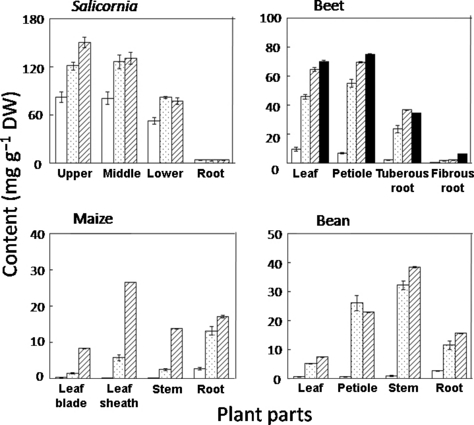
Table 5 Concentration of oxalic acid in the soil extracts (mg L−1)
The secretion of oxalic acid by Salicornia was detected, particularly when Salicornia was grown on sodic soil (). Iron as well as Cu, Mn and Zn in the soil might become available by chelation with phytosiderophores such as mugineic acid (CitationRömheld and Awad 2000; CitationSchaaf et al. 2004; CitationTolay et al. 2001; CitationTreeby et al. 1989). In addition, a number of reports have shown that the chemical components of root exudates (e.g. phenolics) solubilize microelements from unavailable sources for uptake by plants (CitationD’Arcy 1982; CitationD’Arcy-Lameta 1986; CitationDakora and Phillips 2002; CitationWhite and Broadley 2009). There might be a possibility that oxalic acid also has the same function for plants. The soil in the pots was mixed before analysis, and this might be the reason why other organic acids were not detected. It might be necessary to analyze the soil near the roots. The Fe and Zn contents of the roots of Salicornia tended to increase with increasing Na concentration in the soil, which indicates that oxalic acid lowered the soil pH and made the elements available (). The SO soil had the highest concentration of oxalic acid, suggesting that the Na concentration or the pH of the soil regulates the secretion of oxalic acid by Salicornia. A lower concentration of oxalic acid in HSO is due to poor growth of Salicornia against high Na condition.
Beet had the best growth in the SA soil () and the Na content in SA was eightfold higher than the content in CO and the content of K, Ca and Mg in SA was significantly lower than the contents in CO (); these results suggest that beet also needs Na for satisfactory growth. In beet the cation balance cannot be an index of Na stress, as it is in Salicornia. The Na content in the leaves and petioles was higher than that in the tuberous roots and fibrous roots in all salt treatments (), which means that absorbed Na was actively transported to the shoots, as in Salicornia. The Na content in the shoots in SO was higher than that in CO and SA (), suggesting that the Na in the shoots of SO was too high and was transported to the vacuole. These observations mean that beet cannot regulate Na absorption under excessively high Na conditions. It is interesting to note that beet did not suffer from water stress in SA, but did suffer in SO and HSO, soils in which the EC was lower (; ). It is possible that high Na damages beet roots, affecting their ability to absorb water. Although the contents of microelements in the shoots and roots of beet in SA was not always highest among the treatments (), the uptake amount (DW × content) in the whole plant was highest in SA.
Maize and bean showed the best growth in CO (). The contents of K and Mg in bean decreased with increasing Na concentration in the soil (). In maize, the content of Ca and Mg in the shoots also decreased with increasing Na concentration in the soil, but the K content of the shoots increased. In maize, the Ca, Mg and K contents all decreased with an increase in Na concentration in the soil. This enhancement of the K content in the shoots under higher Na conditions may explain the reduced growth suppression in maize compared with bean with increasing Na concentration in the soil (). In both species, the patterns of growth () and cation balance () were the same, and the cation balance of maize was higher than that of bean. In bean, the K, Ca and Mg contents were higher than the contents in maize, but the Na content was also higher in bean. It appears that excessive Na disturbs the ionic balance, and maintenance of the ionic balance is more important for glycophytes to enhance salt tolerance. Thus, cation balance can be used as an index of Na hazard for glycophytes only. Bean showed water stress in SA and SO soils. In contrast, maize maintained an adequate water status under salt conditions, which partially contributes to salt tolerance in maize.
In conclusion, cation balance can be an index of Na tolerance in glycophytes, but not in halophytes. The cation balance values differed greatly depending on the species and these values may be used to define glycophytes or halophytes.
Acknowledgment
The authors gratefully thank the Japan Society for the Promotion of Science for supporting the study through the “Global Center of Excellence for Dryland Science”.
REFERENCES
- Dakora , FD and Phillips , DA . 2002 . Root exudates as mediators of mineral acquisition in low-nutrient environments . Plant Soil , 245 : 35 – 47 .
- D’Arcy , AL . 1982 . Study of soja and lentil exudates. I. Kinetics of exudation of phenolic compounds, amino acids and sugars in the first days of plant growth . Plant Soil , 68 : 399 – 403 .
- D’Arcy-Lameta , A . 1986 . Study of soybean and lentil root exudates. II. Identification of some polyphenolic compounds, relation with plantlet physiology . Plant Soil , 92 : 113 – 123 .
- Dracup , MNH and Greenway , H . 1985 . A procedure for isolating vacuoles from leaves of the halophyte Suaeda maritime . Plant Cell Environ. , 8 : 149 – 154 .
- Flowers , TJ and Yeo , AR . 1988 . “ Ion relations of salt tolerance ” . In Salute Transport in Plant Cells and Tissues , Edited by: Baker , DA and Hall , JL . 392 – 416 . New York : Longman .
- Flowers , TJ and Yeo , AR . 1995 . Breeding for salinity resistance in crop plants: where next? . Aust. J. Plant Physiol. , 22 : 875 – 884 .
- Food and Agriculture Organization . 2005 . Global Network on Integrated Soil Management for Sustainable Use of Salt-affected Soils , Rome, Italy : FAO Land and Plant Nutrition Management Service .
- Glenn , EP and O’Leary , JW . 1985 . Productivity and irrigation requirements of halophytes grown with seawater in the Sonora Desert . J. Arid Environ. , 9 : 81 – 91 .
- Jumberi , A , Oka , M and Fujiyama , H . 2002 . Response of vegetable crops to salinity and sodicity in relation to ionic balance and ability to absorption microelements . Soil Sci. Plant Nutr. , 48 ( 2 ) : 203 – 209 .
- Jumberi , A , Yamada , M , Yamada , S and Fujiyama , H . 2001 . Salt tolerance of grain crops in relation to ionic balance and ability to absorb microelements . Soil Sci. Plant Nutr. , 47 : 657 – 664 .
- Maas , EV . 1984 . “ Salt tolerance of plants ” . In Handbook of Plant Science in Agriculture , Edited by: Christie , BR . Vol. 2 , 57 – 75 . Cleveland : CRC Press Inc. .
- Munns , R . 2002 . Comparative physiology of salt and water stress . Plant Cell Environ. , 25 : 239 – 250 .
- Munns , R . 2005 . Genes and salt tolerance: bringing them together . New Phytol. , 167 : 645 – 663 .
- Niu , X , Bressan , RA , Hasegawa , PM and Pardo , JM . 1995 . Ion homeostasis in NaCl stress environments . Plant Physiol. , 109 : 735 – 742 .
- Rhodes , JD and Loveday , J . 1990 . “ Salinity in irrigated agriculture ” . Agronomy Monograph No. 30 Edited by: Stewart , BA and Nielsen , DR . 1089 – 1142 . Madison : ASA Publishers .
- Rivelli , AR , Lovelli , S and Perniola , M . 2002 . Effects of salinity on gas exchange, water relations and growth of sunflower (Helianthus annuus) . Funct. Plant Biol. , 29 : 1405 – 1414 .
- Rodriguez-Navarro , A . 2000 . Potassium transport in fungi and plants . Biochim. Biophys. Acta , 1469 : 1 – 30 .
- Romeroaranda , R , Soria , T and Cuartero , J . 2001 . Tomato plant water uptake and plant water relationships under saline growth conditions . Plant Sci. , 160 : 265 – 272 .
- Römheld , V and Awad , F . 2000 . Significance of root exudates in acquisition of heavy metals from a contaminated calcareous soil by graminaceous species . J. Plant Nutr. , 23 : 1857 – 1866 .
- Schaaf , G , Ludewig , U , Erenoglu , BE , Mori , S , Kitahara , T and von Wiren , N . 2004 . ZmYS1 functions as a proton-coupled symporter for phytosiderophore and nicotianamine-chelated metals . J. Biol. Chem. , 279 : 9091 – 9096 .
- Serrano , R , Mulet , JM Rios , G . 1999 . A glimpse of the mechanism of ion homeostasis during salt stress . J. Exp. Bot. , 50 : 1023 – 1036 .
- Song , JQ and Fujiyama , H . 1996a . Ameliorative effect of potassium on rice and tomato subjected to sodium salinization . Soil Sci. Plant Nutr. , 42 : 493 – 501 .
- Song , JQ and Fujiyama , H . 1996b . Difference in response of rice and tomato subjected to sodium salinization to the addition of calcium . Soil Sci. Plant Nutr. , 42 : 503 – 510 .
- Tolay , I , Erenoglu , B , Römheld , V , Braun , HJ and Cakmak , I . 2001 . Phytosiderophore release in Aegilops tauschiiand Triticumspecies under zinc and iron deficiencies . J. Exp. Bot. , 52 : 1093 – 1099 .
- Torello , WA and Rice , LA . 1986 . Effects of NaCl stress on proline and cation accumulation in salt sensitive and tolerant turfgrass . Plant Soil , 93 : 241 – 247 .
- Treeby , M , Marschner , H and Römheld , V . 1989 . Mobilization of iron and other micronutrient cations from a calcareous soil by plant-borne microbial, and synthetic metal chelators . Plant Soil , 114 : 217 – 226 .
- White , PJ and Broadley , MR . 2009 . Biofortification of crops with seven mineral elements often lacking in human diets-iron, zinc, copper, calcium, magnesium, selenium and iodine . New Phytol. , 182 : 49 – 84 .
- Yamaguchi , T and Blumwald , E . 2005 . Developing salt-tolerant crop plants: challenges and opportunities . Trends Plant Sci. , 10 ( 12 ) : 615 – 620 .
- Zhu , JK , Liu , J and Xiong , L . 1998 . Genetic analysis of salt tolerance in Arabidopsis. Evidence for a critical role of potassium nutrition . Plant Cell. , 10 : 1181 – 1191 .