Abstract
Zinc (Zn) nutrition and plant genotype are two factors that may affect the tolerance of wheat to root-rot diseases. The aim of the present study was to determine the effect of Zn on shoot yield, root permeability and infection by Fusarium solani in six wheat genotypes with different Zn efficiency. A greenhouse (solution culture) experiment was carried out in which five bread wheat genotypes (Triticum aestivum L. cvs Rushan, Kavir, Cross, Pishtaz and Falat) and one durum wheat genotype (Triticum durum L. cv. Yav79), which are common in Zn-deficient soils of Iran and were exposed to two levels of Zn (0 and 1 μmol L–1 Zn kg−1, as ZnSO4.7H2O) and two F. solani infection levels (0 and 106 spore mL−1). Zinc deficiency significantly decreased shoot dry matter in five of the genotypes (Yav79, Kavir, Rushan, Cross and Falat), but had no effect on shoot growth in Pishtaz. Infection with F. solani significantly decreased the shoot dry matter in Yav79, but did not affect the shoot dry weight of the other wheat genotypes. Root membrane permeability was lower in the Zn treatments than in the Zn-free treatments. Zinc deficiency caused a decrease in root reactive sulfhydryl (SH) groups, particularly in the Cross genotype. Root sulfhydryl groups decreased with Fusarium infection. Zinc application sharply increased the Zn content and decreased the Mn content of the shoots. Application of Zn had a positive effect on the tolerance of wheat to F. solani root rot. The relationship between Zn nutrition and disease tolerance suggests that Zn deficiency should be treated before evaluating the cost-effectiveness of fungicides. No correlation was found between the Zn efficiency of the wheat genotypes and Fusarium root-rot disease severity in this solution culture experiment.
Introduction
Zinc (Zn) plays numerous roles in the physiology and biochemistry of higher plants, including stabilization of the sulfhydryl groups in the membrane proteins involved in ion transport processes (CitationCakmak 2000; CitationMarschner 1995; CitationWelch and Norvell 1993), maintenance of the structural stability of plant organelles, biosynthesis of various cellular proteins, and metabolism of plant hormones such as indole acetic acid (IAA) and gibberellic acid (GA3) (CitationAlloway 2004; CitationEl-Tohamy and El-Greadly 2007; CitationSuge et al. 1986). Zinc is an integral component of approximately 300 enzymes, including dehydrogenases, aldolases, isomerases, transphosphorylases, RNA and DNA polymerases, and various synthetases (CitationVallee and Falchuk 1993).
Zinc also plays an important role in maintaining root cell membrane integrity and ion transport processes in wheat root cell membranes (CitationBroadley et al. 2007; CitationCakmak and Marschner 1990; CitationMarschner 1995; CitationWelch et al. 1982). Under Zn deficiency, leakage can significantly increase from roots (CitationBroadley et al. 2007; CitationKhoshgoftarmanesh et al. 2006a,b; CitationMarschner 1995). CitationWelch and Norvell (1993) reported that the roots of Zn-deficient barley seedlings lost greater quantities of Mn, Cu and Cl than did the roots of Zn-adequate barley seedlings.
Fusarium solani L. is a soil-borne fungal pathogen of wheat that is often found associated with other root diseases. It is also a major problem for farmers in arid and semiarid regions in central Iran and causes >10% reduction in wheat production (CitationHeidarian and Ershad 2001). The result of a survey in the Chaharmahal-va-Bakhtiari province of Iran showed that between 33% and 82% of wheat plants sampled at maturity were infected with Fusarium (CitationHeidarian and Ershad 2001). In Iran, Fusarium has also been isolated from infected roots of wheat and barley grown in the Mazandaran (CitationDarvishnia et al. 2007), Fars, Khuzestan (CitationVafaei et al. 2001), Hamedan, Golestan, Lorestan, Isfahan and Kermanshah provinces (CitationZare and Ershad 1997). Reports have shown that the Fusarium root-rot disease is caused by a complex of pathogens that differ in importance among fields and also from season to season within fields (CitationDarvishnia et al. 2007). Fusarium solani is a dominant species in Iran that is introduced as a complex species (CitationDarvishnia et al. 2007).
Zinc-sufficient plants have been found to be more tolerant to root infection by Fusarium fungus than Zn-deficient plants (CitationMarschner 1995; CitationStreeter et al. 2001). This probably results from the fungitoxicity of Zn and the role of Zn in stabilizing the membranes of root cells (CitationMarschner 1995).
Zinc deficiency results in low levels of superoxide dismutase (CitationCakmak and Marschner 1988a; CitationCakmak et al. 1997b), causing high levels of superoxide radicals with the peroxidation of membrane lipids. Membranes are then impaired and their permeability is increased under Zn-deficiency conditions (CitationCakmak 2000; CitationCakmak and Marschner 1988b,c; CitationWelch and Norvell 1993; CitationWelch et al. 1982). Furthermore, the accumulation of free amino acids and amides is enhanced under Zn deficiency. Together with the increased leakiness of the root cell membranes, this may explain the observed increase in free amino acids in the root exudates of Zn-deficient plants (CitationCakmak and Marschner 1988b; CitationKastrup et al. 1996). Increased root exudation of amino acids by Zn-deficient plants has been found to accelerate root-rot development in wheat (CitationThongbai et al. 1993). A relationship between Zn nutrition and root rot has also been observed in medic plants infected by Rhizoctonia solani L. (CitationStreeter et al. 2001). Zinc application did not directly inhibit infection by R. solani nor did it reduce its pathogenicity. The protection conferred by Zn was attributed to a strong increase in root growth.
Root and crown rotting pathogens, in particular Fusarium, are causing major losses in wheat production in central Iran, where most of the soils are calcareous and Zn deficient. Zinc deficiency may be a major reason for this problem. More than 40% of arable lands in Iran, particularly alkaline calcareous soils, are suffering from Zn deficiency (CitationKhoshgoftarmanesh et al. 2006b). Although Fusarium root-rot disease and Zn deficiency in Iran are widespread, there is a paucity of studies establishing links between the Zn efficiency of wheat genotypes and their response to root-rot diseases. The aim of the present study was to investigate the response of six wheat genotypes differing in Zn efficiency to infection by F. solani under Zn-sufficient and Zn-deficient conditions.
Materials and methods
Seedling growth in solution culture
Seedlings of five bread wheat genotypes (Triticum aestivum L. cvs Rushan, Kavir, Cross, Falat and Pishtaz) and one durum wheat genotype (Triticum durum L. cv. Yav79) were grown in nutrient solutions. These wheat genotypes are Iranian genotypes that were collected from the germplasm bank of the Iranian Institute of Seed and Seedling Preparation. The composition of the nutrient solution is shown in . Two Zn treatments (0 and 1 μmol L–1 Zn in the form of ZnSO4.7H2O, denoted as “Zn-free” and “Zn-sufficient” treatments, respectively) and two F. solani inoculum levels (0 and 106 spore mL–1) were used in the present study. Seedlings were inserted through holes in 6-L polyethylene pots containing nutrient solution. The pot surfaces were covered with black polyethylene film to prevent light from entering the pots. The nutrient solutions were aerated. There were four pots per Zn and Fusarium treatment combination with three seedlings per pot. The nutrient solutions were replaced 14 and 28 days after seedling insertion. After 35 days, the seedlings were harvested and divided into roots and shoots. The samples were washed with deionized water and dried at 70°C for 48 h. The dried shoot and root samples were ground, ashed at 550°C for 8 h and the ash was dissolved in 2 mol L–1 HCl (CitationChapman and Partt 1961). The concentrations of Zn, Fe, Mn and Cu in the digest solutions were determined by atomic absorption spectrometry (AAS) (PerkinElmer 3400; PerkinElmer, Wellesley, MA, USA). The potassium concentration in the extracts was measured using a flame photometer (Sherwood Scientific Ltd., Model 410; Cambridge, UK).
High-purity deionized water and analytical grade reagents were used in all experiments. Before use, all glass and plastic ware was carefully cleaned using the procedures described by CitationNorvell and Welch (1993).
Table 1 Composition of the nutrient solution used in the Zn-free experiment
The Zn seed concentration of the Rushan, Kavir, Cross, Falat, Pishtaz and Yav79 cultivars was 17.5, 16.8, 18.2, 17.9, 17.0 and 16.4 mg kg−1, respectively.
Inoculum preparation
Fusarium-infected wheat plants were collected from a wheat field in Qom province, central Iran. A potato dextrose agar (PDA) medium was used for the isolation of F. solani according to the method described by CitationNelson et al. (1983). A conidial suspension was prepared in sterile water. The number of conidia was counted using a hemocytometer (×10) microscope field when a drop from a 3-mm diameter loop was examined on a slide. A spore density of 106 spores mL−1 was obtained by diluting centrifuged spores with sterile distilled water prior to the inoculation experiment. Two weeks after the wheat seedlings had been planted, they were inoculated with a conidial suspension of the pathogen by pin stabbing (CitationDhingra and Sinclair 1995).
Pathogen isolation and disease assessment
After harvest, F. solani infection was assessed based on the progressive development of necrotic symptoms on the roots. The roots of each plant were segmented and the segments were divided into three groups: (1) nil, no obvious infection, (2) moderate, <25% of the root system discolored, (3) severe, >25% of the root system discolored. The severity of the F. solani infection was reported as a disease score, that is, the percentage of discolored roots.
To isolate F. solani, freshly infected roots were washed with tap water and sterilized using 0.2% of sodium hypochloride (Vitex) for 5 min. After surface sterilization, the material was washed with sterilized distilled water. A small piece of root was sliced off each end, placed on an agar plate and stored at 25°C for 48 h. The white mycelium grew sufficiently from the cut ends to be collected and subcultured in PDA medium.
Reactive sulfhydryl group assay
Selected root samples of intact wheat seedlings were analyzed for sulfhydryl (SH) groups using the method described by CitationWelch and Norvell (1993) using sulfhydryl-reactive reagent 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB). The DTNB reacts with the SH groups to form nitromercaptobenzoic acid anions, giving an intense yellow color. Infected seedlings and non-infected roots were immersed in 100 mL of sulfhydryl reaction buffer (0.2 mol L–1 Tris–HCl plus 0.02 mol L–1 Na-ethylenediaminetetraacetic acid adjusted to pH 8.2 with NaOH). One milliliter of 10 mmol L–1 DTNB, dissolved in absolute methanol, was added to the reaction buffer at time zero. After 15 min incubation, the roots were removed from the reaction buffer. Aliquots of 2.0 mL incubated solution were then immediately assayed for SH groups by means of spectrophotometry for increasing absorption at 412 nm. Sulfhydryl concentrations were calculated from standard curves prepared from fresh cystein.
Root leakage conductivity: an indicator of root membrane permeability
Root cell membrane permeability was assessed using a modification of the method described by CitationYan et al. (1996). Approximately 10 g of washed infected and non-infected root segments (1 cm in length) were put into a beaker containing 10 mL deionized water and incubated at 30°C for 3 h. The electrical conductivity (EC) of the solution was measured at 25°C, the samples were boiled for 2 min and cooled to room temperature (25°C) before their EC was measured again. The electrolyte leakage (EL) was calculated as follows:
Data analysis
A factorial experiment (2 × 2 × 6) with and without F. solani and with and without Zn using six genotypes with four replicates of each type was conducted. Treatments effects were analyzed by ANOVA. Means were compared using a least significant difference (LSD) test at P < 0.05 (CitationSAS Institute 2000). Means of root infection were compared using Student’s t-tests.
Results
Visual deficiency symptoms
After 2 weeks of growth, the leaves of plants grown in solutions with no Zn developed a light green leaf color and brown necrotic blotches with a yellow border in older leaves. These symptoms differed among wheat genotypes and were absent or only slightly developed in the Falat and Pishtaz genotypes. In contrast, the Kavir and Yav79 genotypes grown without the addition of Zn displayed severe deficiency symptoms, and these symptoms became even more severe in the presence of F. solani. No such effect was found for the other wheat genotypes. These visual symptoms suggest the relative sensitivity of the Kavir and Yav79 genotypes to Zn deficiency, particularly in the presence of the root-rot disease.
Dry matter production and Zn efficiency
Shoot dry matter varied significantly (P < 0.05) among the wheat genotypes in all treatments of Zn addition and Fusarium infection (). In the absence of Zn, Yav79 plants had the lowest shoot dry weights (1.61 g pot−1); the Falat genotype produced the lowest shoot dry matter (1.90 g pot−1) in the presence of Zn. The effect of Zn on shoot dry matter production paralleled that on leaf Zn-deficiency symptoms. Zinc deficiency significantly decreased shoot dry matter in the genotypes Yav79, Kavir, Cross and Falat, but had no significant effect on the shoot growth of the Pishtaz genotype (). On average, shoot dry matter decreased by 36, 21 and 18% in the genotypes Yav79, Kavir and Cross, respectively, under Zn deficiency.
Table 2 Shoot and root dry weights of the wheat genotypes in relation to the Zn and Fusarium treatments
Wheat genotypes also differed in root dry matter (). Cross plants produced the highest root dry matter both in the presence (0.48 g pot−1) and absence (0.42 g pot−1) of Zn. Zinc application increased the root growth of all wheat genotypes except Pishtaz and Falat ().
Under Zn deficiency, F. solani significantly decreased the shoot growth of Yav79 and Kavir, but had no effect on the other genotypes (). In the presence of Zn, F. solani had no significant effect on shoot and root growth. Zinc application increased the shoot mass and partly compensated for the negative effects of F. solani on plant growth.
Zinc efficiency (i.e. the ratio of shoot dry matter produced under Zn-deficient conditions to that produced with applied Zn) varied significantly (P < 0.05) among wheat genotypes (). The highest and lowest reductions in shoot dry matter production were found in the Yav79 and Falat genotypes, respectively.
Root membrane permeability
Root membrane permeability varied among the wheat genotypes (). The Falat and Pishtaz plants had higher percentages of root membrane permeability in the Zn-free treatment than the other genotypes. Zinc deficiency significantly increased root membrane permeability in all wheat genotypes. On average, root membrane permeability increased by 14.8, 23.1, 28.2, 31.0, 33.0 and 35.5% from treatments with Zn to treatments without Zn in the Pishtaz, Cross, Kavir, Falat, Rushan and Yav79 cultivars, respectively.
Figure 1 Zinc efficiency (ratio of shoot dry matter produced in the Zn-free treatment to the Zn treatment) of the studied genotypes. Error bars indicate standard error.
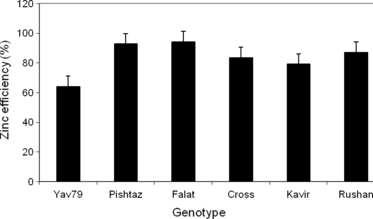
Table 3 Root membrane permeability, reactive sulfhydryl group concentration in the root and the Fusarium root-rot disease score in the different wheat genotypes as affected by the Zn and Fusarium treatments
Fusarium root rot significantly increased root membrane permeability in all genotypes and in both Zn treatments ().
Reactive sulfhydryl group concentration
The root samples significantly (P < 0.05) varied in reactive SH group concentration among the genotypes (). The highest and lowest SH group concentrations were found in the Rushan and the Falat genotypes, respectively. There was no significant relationship between Zn efficiency and root SH group concentration.
In all genotypes, the concentration of the non-protein SH group was higher in the treatments with Zn than in the treatments without Zn ().
Fusarium infection decreased the concentrations of reactive SH groups in all genotypes (). This decrease was larger in the Zn-free treatment than in the Zn-sufficient treatment.
Fusarium infection
Infection with Fusarium root rot differed significantly among the wheat genotypes (). Both in the absence and in the presence of Zn, the Pishtaz genotype was the most affected genotype, whereas the Rushan genotype was the least affected one (). There was no relationship between Zn efficiency and infection score, but Zn application reduced Fusarium infection in all wheat genotypes ().
Table 4 Shoot Zn concentrations in the different wheat genotypes as affected by the Zn and Fusarium treatments
Table 5 Total amounts of Zn, Mn, Cu and K in the shoots of the different wheat genotypes as affected by the Zn and Fusarium treatments
Shoot Zn concentration
Shoot Zn concentrations varied significantly among the wheat genotypes in both Zn treatments (). In the Zn-free treatment, the Falat and Cross genotypes tended to accumulate more Zn in the shoots than all other genotypes, whereas the Cross genotype accumulated most Zn (30.7 mg kg−1 dry matter) in the presence of Zn. Application of Zn significantly increased the shoot Zn concentration in all wheat genotypes ().
There was no significant relationship between Zn efficiency and shoot Zn accumulation. Fusarium infection significantly reduced the Zn concentrations in the shoots. This decrease was largest in the Kavir genotype. In the absence of Zn, this genotype showed the lowest Zn accumulation if infected with Fusarium. In the treatment with both Zn and Fusarium infection, the Yav79 genotype had the lowest shoot Zn concentration of all of the studied wheat genotypes.
Total amount (content) of mineral ions
As with the Zn treatment, the total amounts of K, Zn and Mn taken up by the shoots differed significantly among the various genotypes (). The Cross genotype accumulated the highest amounts of K, Mn and Zn in its shoots. The application of Zn to the nutrient solution significantly increased the total amount of Zn, whereas it sharply reduced the total amount of Mn taken up (). There was significant improvement in shoot K content in the Zn treatment compared with the Zn-free treatment. The total amount of Cu increased in some genotypes and decreased in others in the Zn treatment ().
Fusarium infection significantly reduced the shoot contents of K, Mn and Zn in all wheat genotypes, particularly Yav79 and Kavir ().
Discussion
Large variations in Zn efficiency among wheat genotypes have been reported in previous studies (CitationCakmak et al. 1997a; CitationKalayci et al. 1999; CitationMarschner 1995). The relative sensitivity of the studied genotypes to Zn deficiency decreased in the order Yav79 > Kavir > Cross > Rushan > Pishtaz > Falat. In our study, this ranking in Zn efficiency was somewhat in contrast to the sensitivities to Zn deficiency reported previously under field (CitationKhoshgoftarmanesh et al. 2004) and greenhouse conditions (CitationKhoshgoftarmanesh et al. 2006a,b). Only the Yav79 genotype showed a considerably lower Zn efficiency than the other genotypes. These differences can probably explained by the fact that the experimental conditions in our study differed from the previous studies, that is, the present study was conducted with nutrient solutions and not with soil. In soil, the Cross and Rushan genotypes have been reported to be the most Zn-efficient genotypes, whereas they were relatively inefficient in the nutrient solution. The Zn efficiency of these genotypes in soil may be related to the increased proton and solubilization of Zn owing to the release of organic acids by the roots (CitationKhoshgoftarmanesh et al. 2006a). The main reason why these genotypes can tolerate low Zn availability better than other genotypes appears to be their ability to absorb more Zn from the solid phase. In nutrient solution, this mechanism cannot work because there is no Zn-binding solid. This result indicates that studies conducted in nutrient solution need to be shortened to fully evaluate the tolerance of wheat genotypes to Zn deficiency.
The positive effect of Zn on reducing the Fusarium root-rot infection () indicates that Fusarium is genetically controlled, but that the severity of this disease is reduced by Zn nutrition. In previous studies, Zn deficiency intensified the severity of crown rot disease caused by Fusarium graminearum in wheat, whereas Zn sufficiency suppressed the infection (CitationGraham et al. 1993; CitationGrewal et al. 1996; CitationSparrow and Graham 1988). Zinc nutrition has also been found to be beneficial in reducing the occurrence of phyllody virus in white clover (CitationCarr and Stoddart 1963), take-all in wheat (CitationBrennan 1992), Rhizoctonia root rot in wheat (CitationThongbai et al. 1993), Fusarium root rot in chickpea (CitationGaur and Vaidya 1983), and charcoal rot development in maize (CitationPareek and Pareek 1999). When considering the role of Zn nutrition in reducing the intensity of Fusarium, it appears that before evaluating the cost-effectiveness of fungicides, Zn deficiency has to be corrected through appropriate approaches.
There was also no correlation between the Zn efficiency of the wheat genotypes and the severity of the Fusarium root-rot disease (). For example, Zn-efficient Pishtaz was more affected by the disease without Zn treatment than the other genotypes. This is in contrast to the findings of CitationGrewal (2001), which indicated a negative correlation between the Zn efficiency of alfalfa genotypes and Phytophtora root-rot disease severity. In another study, CitationStreeter et al. (2001) found that Zn-sufficient plants were more tolerant to the effects of root pruning by R. solani than Zn-deficient medic (Medicago truncatula) plants and suggested that increased tolerance to R. solani infection resulted from increased root growth. The difference between the results of the present study and the findings of CitationGrewal (2001) and CitationStreeter et al. (2001) may again result from different experimental conditions. In soil, Zn-efficient genotypes may absorb enough Zn to maintain resistance to the fungus, whereas in hydroponics no additional Zn can be mobilized from a solid phase.
Figure 2 Correlation between the root membrane permeability and the severity of the Fusarium disease.
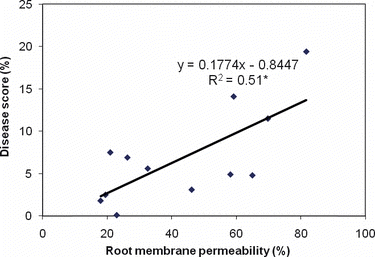
Zinc deficiency significantly decreased the shoot and root dry matter production of the Yav79, Kavir, Cross and Falat genotypes, whereas no such effect was found in the Pishtaz genotype (). In contrast, Zn nutrition significantly decreased Fusarium infection in all genotypes. These results suggest that the increased resistance of the plants to disease results from both increased plant vigor and root growth through improved Zn nutrition, and from Zn decreasing the infection by stabilizing root cell membranes, as indicated by the significant decrease in root membrane permeability in the Zn treatment (). Accordingly, a negative correlation (R 2 = 0.51) was found between the severity of Fusarium disease and root membrane permeability ().
This result agrees with CitationThongbai et al. (1993), who also reported a relationship between Zn nutrition and the severity of R. solani disease in wheat and hypothesized that the effect was not the result of the fungitoxicity of Zn, but rather a result of the role of Zn in the integrity of the host plant’s membranes. In addition, other authors (CitationBettger and O’Dell 1993; CitationBroadley et al. 2007; CitationCakmak 2000; CitationWelch 1995; CitationWelch and Norvell 1993) have suggested that the effect of Zn nutrition on disease suppression is likely to result from the protective role of Zn in the structural and functional integrity of cell plasma membranes. Enhanced photo-oxidative damage in Zn-deficient plants has been reported (CitationCakmak 2000; CitationSharma et al. 2004). Zinc deficiency is likely to enhance O−2 generation by enhancing NADPH-dependent oxidase activity (CitationCakmak 2000; CitationCakmak and Marschner 1988a) because Zn inhibits NADPH oxidase and decrease the NADP/NADPH ratio as a result of the decreased uptake and photosynthetic fixation of CO2 (CitationCakmak 2000; CitationCakmak and Marschner 1988a; CitationSharma et al. 2004). The loss of membrane integrity resulting from the attack of reactive oxygen species (ROS) is a primary effect of Zn deficiency (CitationCakmak and Marschner 1988b,c; CitationKastrup et al. 1996; CitationWelch and Norvell 1993).
Zinc deficiency impairs protein synthesis and enhances the accumulation of free amino acids and amides and, as a result, increases free amino acids in the root exudates (CitationCakmak and Marschner 1988b; CitationKastrup et al. 1996). The increased exudation of amino acids by Zn-deficient plants may attract pathogens (CitationFlentje et al. 1963) or pests (CitationSikora and Hoffmann-Hergarten 1992) to the root and accelerate the infection process.
In addition, Zn deficiency caused a decrease in non-protein SH groups in the roots of all wheat genotypes, with the magnitude of the increase depending on the genotype (). Zinc may protect SH groups in membrane proteins from undergoing oxidative reactions with free radicals and transition metals to disulfides at the exterior surface of animal cell plasma membranes (CitationBettger and O’Dell 1993). Similar to the results of the present study, CitationWelch and Norvell (1993) found that Zn-deficient barley roots contained lower concentrations of reactive sulfhydryls when compared with roots of Zn-sufficient barley seedlings. These results are in line with the proposed role of Zn2+ ions to protect reactive SH groups in higher plant root cell exterior surface membrane proteins from oxidative reactions with free radicals (superoxides or hydroxyl free radicals) and redox-active transition metals (CitationWelch and Norvell 1993).
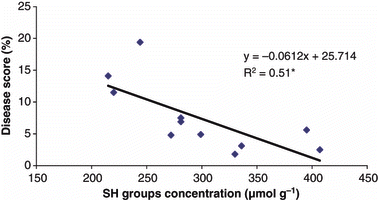
Reactive root SH groups decreased in all genotypes in the Fusarium treatment (). This decrease was larger under Zn-deficient conditions. The genotypes with higher root SH concentrations were more tolerant to Fusarium disease (). The root SH concentrations was also positively correlated (R 2 = 0.58) to the Fusarium disease score () These results support the hypothesis that reactive SH groups are involved in Fusarium tolerance in wheat. These results also agree with the findings of CitationCakmak (2000), who found that Zn ions can partially prevent the loss of SH groups under stress conditions. This protective effect may result from preventing sulfhydryl oxidation by physically capping the SH groups.
Figure 3 Correlation between the root sulphydryl group concentrations and the severity of the Fusarium disease.
CitationWelch and Norvell (1993) suggested that Zn can act as an anti-oxidant protecting SH groups in certain membrane proteins against oxidation and the formation of disulfides at the outer membrane surfaces. In fact, Zn may protect membrane protein SH groups from oxidative damage. The higher concentration of SH groups and the lower root permeability in the presence of Zn confirms the role of Zn in improving the cell membrane integrity of roots infected with Fusarium.
The variation recorded in Zn accumulation among the studied genotypes is comparable to that found in previous studies (CitationCakmak et al. 1997a; CitationKalayci et al. 1999; CitationKhoshgoftarmanesh et al. 2004, 2006a). Zinc uptake may be more closely controlled by Zn-efficient genotypes than in Zn-inefficient genotypes (CitationKhoshgoftarmanesh et al. 2006a). Differences in shoot Zn concentration between Zn-efficient and Zn-inefficient genotypes may result from different Zn uptake from the soil or different translocation from root to shoot (CitationHacisalihoglu and Kochian 2003; CitationKhoshgoftarmanesh et al. 2006a). Greater soil Zn uptake by Zn-efficient genotypes might, in part, result from a high affinity Zn2+ transporter in these genotypes (CitationHacisalihoglu et al. 2001).
A negative correlation (r 2 = 0.56) between plant tissue Zn concentration and Fusarium infection was observed (,). A negative correlation between fungal infection and plant tissue Zn concentration has also been observed in panicle blast in rice (CitationFilippi and Prabhu 1998).
Zinc application sharply reduced the total amount of Mn and significantly increased the total amount of Zn (). The reduction in Mn uptake in Zn-sufficient plants compared with Zn-deficient plants results in part from a marked reduction in the Mn concentration in wheat shoots in Zn-sufficient plants as a result of an increase in shoot dry matter (dilution effect).
The reduction in the Mn uptake in the Zn treatments agrees with reports showing that Zn deficiency stimulates the uptake of several mineral nutrients, including Mn (CitationLoneragan and Webb 1993). The role of Zn in ion uptake regulation is not totally clear, but it may involve a Zn function in root cell plasma membrane ion transport processes (CitationNorvell and Welch 1993). Reduced net growth and membrane damage may also explain why the shoot K, Zn and Mn contents were significantly lower in the Fusarium treatments.
Conclusion
The present study showed that Zn nutrition can reduce Fusarium root-rot infection in wheat. The positive effect of Zn on crop tolerance to F. solani resulted, in part, from improved integrity of the cell membranes and lower oxidative damage of membrane lipids indicated by non-protein SH groups in the roots. Decreased root membrane permeability in the presence of Zn highlights the role that Zn nutrition plays in improving cell membrane integrity and, thus, higher tolerance to the disease. There was no relationship between Zn efficiency and Fusarium root-rot disease severity, but the various genotypes differed in their ability to tolerate Zn deficiency and Fusarium infection. These associations may have widespread implications in managing the establishment and growth potential of wheat in regions where Zn deficiency occurs. The practical implication of the present study is that growing or breeding Zn-efficient wheat cultivars along with the use of Zn fertilizers can help sustain wheat production in Zn-deficient conditions.
References
- Alloway , BJ . 2004 . Zinc in Soils and Crop Nutrition , Brussels, Belgium : International Zinc Association . Box 4, B-1150
- Bettger , WJ and O’Dell , BL . 1993 . Physiological roles of zinc in the plasma membrane of mammalian cells . J. Nutr. Biochem. , 4 : 194 – 207 .
- Brennan , RF . 1992 . Effect of zinc fertilizer on take-all and grain yield of wheat grown on zinc deficient soils of the Esperance region, Western Australia . Fer. Res. , 31 : 215 – 216 .
- Broadley , MR , Broadley , PJ , White , J , Hammond , P , Zelko , I and Lux , A . 2007 . Zinc in plants . New Phytol. , 173 : 677 – 702 .
- Cakmak , I . 2000 . Possible roles of zinc in protecting plant cells from damage by reactive oxygen species . New Phytol. , 146 : 185 – 205 .
- Cakmak , I , Ekiz , H Yilmaz , A . 1997a . Differential response of rye, bread and durum wheats to zinc deficiency in calcareous soils . Plant Soil , 188 : 1 – 10 .
- Cakmak , I and Marschner , H . 1988a . Increase in membrane permeability and exudation of roots of zinc deficient plants . J. Plant Physiol. , 132 : 356 – 361 .
- Cakmak , I and Marschner , H . 1988b . Zinc-dependent changes in ESR signal, NADPH oxidase and plasma membrane permeability in cotton roots . Physiol. Plant. , 73 : 182 – 186 .
- Cakmak , I and Marschner , H . 1988c . Enhanced superoxide radical production in roots of zinc-deficient plants . J. Exp. Bot. , 39 : 1449 – 1460 .
- Cakmak , I and Marschner , H . 1990 . Decease in nitrate uptake and increase in proton release in zinc deficient cotton, sunflower, and buck wheat plant . Plant Soil , 129 : 261 – 268 .
- Cakmak , I , Ozturk , L , Eker , S , Torun , B , Kalfa , H and Yilmaz , I . 1997b . Concentration of zinc and activity of copper/zinc-superoxide dismutase in leaves of rye and wheat cultivars differing in sensitivity to zinc deficiency . J. Plant Physiol. , 151 : 91 – 95 .
- Carr , AJH and Stoddart , JL . 1963 . The ameliorating effect of zinc on symptoms of phyllody virus in white clover . Ann Appl Biol , 51 : 259 – 268 .
- Chapman , HD and Partt , PF . 1961 . Methods of Analysis for Soil, Plant and Water , Riverside, , California : Division of Agriculture Science, California Univ .
- Darvishnia , M , Alizadeh , A and Mohammadi Goltapeh , E . 2007 . Three new Fusarium taxa isolated from gramineous plants in Iran . Rostaniha , 7 ( 2 ) : 193 – 205 .
- Dhingra , OD and Sinclair , JB . 1995 . Basic Plant Pathology Methods , 2nd edn , Florida : CRC Press .
- El-Tohamy , WA and El-Greadly , NHM . 2007 . Physiological responses, growth, yield and quality of snap beans in response to foliar application of yeast, vitamin E and zinc under sandy soil conditions . Aust. J. Basic Appl. Sci. , 1 ( 3 ) : 294 – 299 .
- Filippi , MC and Prabhu , AS . 1998 . Relationship between panicle blast severity and mineral nutrient content of plant tissue in upland rice . J Plant Nutr , 21 : 1577 – 1587 .
- Flentje , NT , Dodman , RL and Kerr , A . 1963 . The mechanism of host penetration by Thanatephorus cucumeris . Aust. J. Biol. Sci. , 16 : 784 – 799 .
- Gaur , RB and Vaidya , PK . 1983 . Reduction of root rot of chickpea by soil application of phosphorus and zinc . International Chickpea Newsletter , 9 : 17 – 18 .
- Graham , RD , Neate , SM and Webb , MJ . 1993 . Interaction between zinc nutritional status of cereals and Rhizoctonia root rot severity . Plant Soil , 153 : 215 – 222 .
- Grewal , HS . 2001 . Zinc influences nodulation, disease severity, leaf drop and herbage yield of alfalfa cultivars . Plant Soil , 234 (13) : 47 – 59 .
- Grewal , HS , Graham , RD and Rengel , Z . 1996 . Genotypic variation in zinc efficiency and resistance to crown rot disease (Fusarium graminearumSchw. Group 1) in wheat . Plant Soil , 186 : 219 – 226 .
- Hacisalihoglu , G , Hart , JJ and Kochian , LV . 2001 . High- and low-affinity zinc transport systems and their possible role in zinc efficiency in bread wheat . Plant Physiol. , 125 : 456 – 463 .
- Hacisalihoglu , G and Kochian , LV . 2003 . How do some plants tolerate low levels of soil zinc? Mechanisms of zinc efficiency in crop plants . New Phytol. , 159 : 341 – 350 .
- Heidarian , A and Ershad , J . 2001 . Identification of wheat root and crown fungi in Charmahal-Bakhtiari province . Iran. J. Plant Pest Disease. , 37 : 97 – 112 .
- Kalayci , M , Torun , B , Eker , S , Aydin , M , Ozturk , L and Cakmak , I . 1999 . Grain yield, zinc efficiency and zinc concentration of wheat cultivation grown in a zinc-deficient calcareous soil in field and greenhouse . Field Crops Res. , 63 : 87 – 98 .
- Kastrup , BV , Steiger , S , Luttge , U and Fischer-Schlibes , E . 1996 . Regulatory effects of zinc on corn root plasma membrane H+-ATPase . New Phytol. , 134 : 467 – 473 .
- Khoshgoftarmanesh , AH , Shariatmadari , H , Kalbasi , M , Karimian , N and Khajepour , MR . 2004 . Salinity and Zn applicatrion effect on phytoavailability of Cd and Zn . Soil Sci. Soc. Am. J. , 68 : 1885 – 1889 .
- Khoshgoftarmanesh , AH , Shariatmadari , H and Karimian , N . 2006b . Responses of wheat genotypes to zinc fertilization under saline soil conditions . J. Plant Nutr. , 29 ( 9 ) : 1 – 14 .
- Khoshgoftarmanesh , AH , Shariatmadari , H , Karimian , N and van der Zee , SEATM . 2006a . Cadmium and zinc in saline soil solutions and their concentrations in wheat . Soil Sci. Soc. Am. J. , 70 : 582 – 588 .
- Loneragan , JF and Webb , MJ . 1993 . “ Interactions between zinc and other nutrients affecting the growth of plants ” . In Zinc in Soils and Plants , Edited by: Robson , AD . 119 – 134 . Dordrecht, , The Netherlands : Kluwer Academic Publishers .
- Marschner , H . 1995 . Mineral Nutrition of Higher Plants , 2nd edn , London, , United Kingdom : Academic Press .
- Nelson , PE , Toussoun , TA and Marasa , WFO . 1983 . Fusarium Species: An Illustrated Manual for Identification , University Park, , Pennsylvania : Pennsylvania State Univ. Press .
- Norvell , WA and Welch , RM . 1993 . Growth and nutrient uptake by barley (Hordeum vulgareL. cv. Herta): studies using an N-(2-hydroxyethyl) ethylene dinitrilo triacetic acid-buffered nutrient solution technique. I. Zinc ion requirements . Plant Physiol. , 101 : 619 – 625 .
- Pareek , S and Pareek , S . 1999 . Effect of macro-and micro-nutrients on charcoal rot disease development of maize induced by Macrophominea phaseolina . Ann. Agric Res. , 20 : 129 – 131 .
- SAS Institute . 2000 . SAS/STAT user’s guide , Cary, NC : SAS Inst . Version 9
- Sharma , PN , Kumar , P , Kumar , R and Tewari , L . 2004 . Early signs of oxidative stress in wheat plants subjected to zinc deficiency . J. Plant Nutr. , 27 : 451 – 463 .
- Sikora , RA and Hoffmann-Hergarten , S . 1992 . Importance of plant health-promoting rhizobacteria for the control of soil-borne fungal diseases and plant parasitic nematodes . Arab. J. Plant Prot. , 10 ( 4 ) : 53 – 58 .
- Sparrow , DH and Graham , RD . 1988 . Susceptibility of zinc-deficient wheat plants to colonization by Fusarium graminearumSchw. Group 1 . Plant Soil , 112 : 261 – 266 .
- Streeter , TC , Rengel , Z , Neate , SM and Graham , RD . 2001 . Zinc fertilization increases tolerance to Rhizoctonia solani(AG 8) in Medicago truncatula . Plant Soil , 228 : 233 – 242 .
- Suge , H , Takahashi , H , Arita , S and Takaki , H . 1986 . Gibberellin relationships in zinc- deficient plants . Plant Cell Physiol. , 27 : 1010 – 1012 .
- Thongbai , P , Hannam , RJ , Graham , RD and Webb , MJ . 1993 . Interaction between zinc nutritional status of cereals and Rhizoctoniaroot rot severity . Plant Soil , 153 : 207 – 214 .
- Vafaei , H , Farokhinejad , R and Darvishnia , M . 2001 . Fusarium species associated with root and crown of wheat and barley in Khuzestan Province . Sci. J. Agri. , 24 : 101 – 125 .
- Vallee , BL and Falchuk , KH . 1993 . The biochemical basis of zinc physiology . Physiol. Rev. , 73 : 79 – 118 .
- Welch , RM . 1995 . Micronutrient nutrition of plants . Crit. Rev. Plant Sci. , 14 : 49 – 82 .
- Welch , RM and Norvell , WA . 1993 . Growth and nutrient uptake by barley (Hordeum vulgareL. cv Herta): studies using an N-(2-Hydroxyethyl) ethylenedinitrilotriacetic acid-buffered nutrient solution technique . Plant Physiol. , 101 : 627 – 631 .
- Welch , RM , Webb , MJ and Loneragan , JF . Zinc in Membrane Function and its Role in Phosphorous Toxicity . 9th Conf. Commonweath Agricultural Bureau, Colloquium . Warwick, England. pp. 710 – 715 .
- Yan , B , Dai , Q , Liu , X , Huang , S and Wang , Z . 1996 . Flooding-induced membrane damage, lipid oxidation and activated oxygen generation in corn leaves . Plant Soil , 179 : 261 – 268 .
- Zare , R and Ershad , D . 1997 . Fusarium species isolated from cereals in Gorgan area . Iran. J. Plant Pathol. , 33 : 1 – 14 .