Abstract
Silicon (Si) is the second most abundant element in soil and effectively counteracts the effects of various abiotic stresses, such as drought, heavy metal toxicity and salinity, on plants. In the present study the ameliorating effects of Si nutrition supplied as 2 mmol L−1 sodium silicate were investigated on hydroponically grown canola (Brassica napus L.) plants under salinity stress (i.e. 150 mmol L−1 sodium chloride). Salinity decreased plant growth parameters such as tissue fresh and dry weights. These decreases were accompanied by increased lignin contents, Na+ ion accumulation, increased lipid peroxidation and decreased chlorophyll contents in plants. Silicon nutrition, however, enhanced plant growth parameters and led to the prevention of lignin and the Na+ accumulation in shoots, reduced levels of lipid peroxidation in the roots and higher levels of chlorophyll. As a result of salinity, catalase activity in the whole plant and both soluble and cell wall peroxidase activities in the shoots decreased. Silicon nutrition, however, increased the reactive oxygen species scavenging capacity of salt-stressed plants through increased catalase and cell wall peroxidase activities. Thus, silicon nutrition ameliorated the deleterious effects of salinity on the growth of canola plants through lower tissue Na+ contents, maintaining the membrane integrity of root cells as evidenced by reduced lipid peroxidation, increased reactive oxygen species scavenging capacity and reduced lignification.
Introduction
Salinity is a major factor limiting plant growth and crop productivity. Approximately one-third of the world’s irrigated lands already suffer from excess salinity (CitationSzabolcs 1994). It is assumed that salt stress imbalances cellular ions, and this imbalance results in ion toxicity and osmotic stress, compromising plant growth and survival (CitationTester and Davenport 2003). Salt stress through an increase in reactive oxygen species (ROS), such as singlet oxygen, superoxide anion (O2˙−), hydrogen peroxide (H2O2) and hydroxyl radical (OH˙), also leads to oxidative stress (CitationAlscher et al. 1997; CitationMittler 2002; CitationNeill et al. 2002). Reactive oxygen species can alter normal cellular metabolism through oxidative damage to lipids, proteins and nucleic acids (CitationAlscher et al. 1997; CitationImlay 2003; CitationMcKersie and Leshem 1994). Reactive oxygen species scavenging enzymes, such as superoxide dismutase, convert superoxide anions to H2O2. Subsequently, hydrogen peroxide is scavenged by catalase and different classes of peroxidases (CitationMcKersie and Leshem 1994; CitationNoctor and Foyer 1998). The activities of some ROS scavenging enzymes involved in lignin biosynthesis like cell wall bound peroxidases and some other enzymes related to lignification are affected by salinity (CitationGunes et al. 2007; CitationVaidyanathan et al. 2003; CitationWang et al. 1997). Salinity leads to increased activities of ROS scavenging enzymes like peroxidase in Chloris gayana (Rhodes grass) and Atriplex prostrata plants associated with greater tissue lignification (CitationOrtega et al. 2006; CitationSánchez-Aguayo et al. 2004; CitationWang et al. 1997), which in turn restricts further plant growth.
Canola (Brassica napus L.) is an important crop for edible oil production (CitationFrancois 1994; CitationQasim et al. 2003). Recent efforts have focused on the use of canola oil to produce clean-burning fuel (i.e. biodiesel) and canola is currently the third most important crop after soybean and maize for biodiesel production (CitationVasudevan and Briggs 2008). Most studies on canola have reported a reduction in leaf area, shoot and root biomass under salinity and have compared the salt tolerance of this species with other species of Brassica (CitationAshraf and McNeilly 1990; CitationFrancois 1994; CitationQasim et al. 2003). It appears that canola plants are moderately salt tolerant (CitationAshraf and McNeilly 1990; CitationFrancois 1994). As salt affected areas in the world are increasing (CitationSzabolcs 1994; CitationTester and Devenport 2003), the adoption of economic strategies to extend canola cultivation in saline areas through both the development of genetically engineered plants and other conventional methods like breeding is justified (CitationPurty et al. 2008; CitationZhang et al. 2001). Although the exogenous application of compatible solutes, such as proline or glycine–betaine, may reduce the deleterious effects of salt stress on canola plants (CitationAthar et al. 2009), the use of other non-costly ubiquitous compounds that result in more economic production is favored.
Silicon (Si) is the second most abundant element in soil. It is also a major structural component of the cell walls in some monocotyledonous species, such as rice and barley, and readily accumulates as a compression-resisting element analogous to lignin (CitationInanaga and Okasaka 1996; CitationRaven 1983). Although Si is not usually considered to be an essential element, it has been shown to be beneficial for plant growth and production (CitationEpstein 1994; CitationLiang et al. 2006). Silicon fertilizers are applied as slag, compost, rice straw, calcium and sodium silicates (CitationMa and Takahashi 2002). For example, the Si application rate in rice fields amounts to 2.5 t ha−1 of calcium silicate slag with 20% Si content (CitationAlvarez and Datnoff 2001) and 9 t ha−1 in the form of rice chaff with 4% Si content (CitationMa and Takahashi 2002; CitationAkbar Hossain et al. 2001). Silicon has been shown to ameliorate the adverse effects of salinity in several plants, including barley (CitationLiang et al. 1996; CitationLiang 1998), wheat (CitationAhmad et al. 1992), mesquite (Prosopis juliflora) (CitationBradbury and Ahmad 1990), spinach and tomato (CitationGunes et al. 2007). A reduction in the accumulation of sodium in the shoots of several plants, such as barley (CitationLiang et al. 1996) and rice (CitationYeo et al. 1999), under salinity has been reported following the application of Si. It has been suggested that Si deposition in the exodermis and endodermis reduces sodium uptake under salinity through a reduction in apoplastic transport across the root (CitationGong et al. 2006; CitationYeo et al. 1999). The amelioration of salt toxicity effects by Si nutrition in maize (CitationMoussa 2006), barley (CitationLiang et al. 2003, 2006), cucumber (CitationZhu et al. 2004), spinach and tomato (CitationAl-aghabary et al. 2004; CitationGunes et al. 2007) has been attributed to decreased oxidative damage.
To the best of our knowledge, no reports have examined the interaction of salinity and Si nutrition in canola or how the beneficial effects of Si in salt-stressed canola plants (if any) are exerted. Accordingly, in the present work, growth, changes in ion concentrations, phenolics, lignin and the activities of some ROS scavenging enzymes were studied in canola plants (Brassica napus L.) under salinity supplied with or without sodium silicate to examine the possibility of extending canola cultivation into saline soils.
Materials and methods
Plant culture and growth conditions
The experiments were conducted during 2006–2007 in a greenhouse at the Gorgan University of Agricultural Sciences and Natural Resources. Seeds of canola (Brassica napus L. cv. Hayola) were obtained from Iran Oilseeds Research Institute and after screening for uniform size and color, the seeds were sterilized with a 2.5% sodium hypochlorite solution. The seeds were incubated in a moistened paper towel and germinated in the dark at 25 ± 5°C for 48 h. Healthy seedlings of uniform size were selected for hydroponic culture. Each plant was supported in the centre of a grey foam lid and the roots were immersed in a 10 L black plastic container filled with continuously aerated Hoagland nutrient solution. The experiment was carried out in a factorial completely randomized design. Factor one was salinity, which was applied to the root medium as NaCl (0 and 150 mmol L−1), and factor two was silicon nutrition, which was supplied as sodium silicate (0 and 2 mmol L−1). Treatments were started 3 weeks after transplanting the seedlings to hydroponic culture with 25 mmol L−1 NaCl and increased in three steps over the next 3 days to 100 mmol L−1 to avoid osmotic shock. The pH of the nutrient solution was adjusted daily to remain at 6.0 ± 0.2 and the nutrient solution was refreshed weekly. During the experiment, the midday irradiance in the greenhouse was approximately 900 μmol photons m−2 s−1, the maximum and minimum air temperatures were 27°C and 18°C, respectively, and the mean relative humidity was 74%. Plants were harvested 25 days after starting the treatments and used to assess growth parameters and for chemical analyses. Samples from the stems (including petioles), leaves and roots were weighed, oven-dried for 3 days at 70°C, re-weighed and ground to determine the mineral contents. Fresh samples or deep-frozen samples were used for the biochemical assays.
Determination of sodium, potassium and silicon
Potassium and sodium concentrations were determined in the roots and shoots after digesting 100 mg powder of the oven-dried tissues in a mixture of concentrated nitric acid and perchloric acid (3:1; v/v) at 175°C. The potassium and sodium contents of the digested extracts were quantified using a flame photometer (model Jenway PFP7, Essex, UK). The Si in the plant tissues was determined spectrophotometrically after digestion of the plant material with H2O2 and NaOH in an autoclave as described by CitationElliot and Synder (1991).
Assay of enzymatic activity
Extracts for the enzymatic assays were prepared as described by CitationKar and Mishra (1976). Fresh leaf samples (0.05 g) were homogenized with 2 mL phosphate buffer (100 mmol L−1, pH 6.8) and centrifuged at 17,000 g for 15 min. The clear supernatant was subsequently used as an enzyme source of catalase, soluble peroxidase and polyphenol oxidase.
The remaining 17,000 g pellets were washed four times with the extraction buffer until no peroxidase activity could be detected. The pellet from the last centrifugation step was mixed with 1 mol L−1 NaCl solution and the mixture was centrifuged at 17,000 g for 20 min at 4°C. The supernatant containing cell wall proteins was saved. Soluble and cell wall peroxidase activities were determined using the guaiacol–peroxidase reaction. For the peroxidase assay, the 3 mL reaction mixture consisted of 25 mmol L−1 phosphate buffer (pH 6.8), 20 mmol L−1 guaiacol, 40 mmol L−1 H2O2 and 10 μL of the enzyme extract. The reaction was initiated by the addition of H2O2 and changes in the absorbance at 470 nm were recorded for 2 min. Activity of peroxidase was calculated assuming an extinction coefficient of 26.6 (mmol L−1)−1 cm−1 for tetraguaiacol (CitationChen et al. 2000; CitationKar and Mishra 1976). Enzyme activity was expressed as μmol H2O2 g−1 fresh weight (FW) min−1.
To assay catalase activity, 100 μL of the supernatant was added to a reaction mixture in a final volume of 3 mL that consisted of 50 mmol L−1 phosphate buffer (pH 6.8) and 15 mmol L−1 H2O2 as substrate. The decrease in the absorbance at 240 nm (owing to H2O2 destruction) was recorded for 2 min. Assuming an extinction coefficient of 40 (mmol L−1)−1 cm−1 for H2O2, catalase activity was determined. Enzyme activity was expressed as μmol H2O2 decomposed g−1 FW min−1.
Polyphenol oxidase activity was determined in a 3 mL reaction mixture consisting of 25 mmol L−1 phosphate buffer (pH 6.8), 10 mmol L−1 pyrogallol and 100 μL of the enzyme extract. Changes in the absorbance of the solution at 420 nm were monitored for 2 min. Activity of polyphenol oxidase was expressed as the amount of purpurogallin formed, assuming an extinction coefficient of 2.47 (mmol L−1)−1 cm−1 (CitationResende et al. 2002).
Other analytical methods
Soluble protein was quantified spectrophotometrically at 595 nm using the CitationBradford (1976) method after extracting plant material with 0.1 mol L−1 phosphate buffer at pH 6.8 (CitationKar and Mishra 1976). For chlorophyll determination, the tissue was briefly ground in cold 80% acetone solution and the absorbance was measured at 645 and 663 nm using the method of CitationArnon (1949). The chlorophyll a and chlorophyll b contents were calculated accordingly.
Phenolic compounds were extracted with ethanol as described by CitationFukoda et al. (2003) and quantified according to CitationLavid et al. (2001). Plant phenolic extracts (50 μL) were diluted with 3 mL of H2O. To this, 100 μL of 50 mmol L−1 FeCl3 in 0.1 N HCl plus 100 μL of 0.008 mol L−1 K3Fe(CN)6 were added and incubated at ambient temperature for 20 min. The absorbance at 720 nm was determined against the reagent blank mixture with ethanol rather than plant extract. Using gallic acid, a standard curve was constructed to estimate phenolics.
The extent of tissue lipid peroxidation was determined as described by CitationHeath and Packer (1968) and expressed as the amount of malondialdehyde (MDA) equivalents produced. Plant tissue was homogenized with 0.1% (w/v) trichloroacetic acid (TCA) and centrifuged at 6000 g for 15 min. The obtained supernatant (250 μL) was mixed with 2 mL thiobarbituric acid reagent (0.25% thiobarbituric acid (TBA) in 10% TCA). After heating for 30 min at 95°C in a water bath, the mixture was cooled and centrifuged at 6000 g for 10 min. The absorbance was recorded at 532 nm and corrected for non-specific absorbance at 600 nm and 440 nm (CitationHodges et al. 1999).
Lignin was extracted with ethanolic HCl (absolute ethanol : 1 mol L−1 HCl; 1:1; v/v) after pre-extracting the plant tissues three times with 50% methanol at 60°C to remove any phenolic compounds (CitationZimmer 1999). The lignin content was determined spectrophotometrically at 488 nm using phloroglucinol according to CitationZimmer (1999).
Table 1 Effect of salinity (control versus 150 mmol L−1 NaCl) on the fresh and dry weights of canola plants grown for 25 days with or without 2 mmol L−1 supplementary silicon as Na2SiO3 in the nutrient solution
Table 2 Na+, K+ and Si concentrations in the roots and shoots of canola plants grown for 25 days at 150 mmol L−1 NaCl with or without 2 mmol L−1 supplementary silicon as Na2SiO3 in the nutrient solution
Statistical analyses
Statistical analyses of the data were carried out using SAS statistical software (CitationSAS Institute 2004). All data were subjected to ANOVA and a comparison of the means was carried out using a least significant difference test.
Results
Growth
Salinity led to a significant reduction in both the fresh and dry weights of the plants. The total dry weight of the plants decreased by approximately 68% as a result of salinity (). The dry weight of the plants improved significantly under saline conditions when Si was added to the medium; however, it was still lower than the control plants. Silicon alone (i.e. under non-saline conditions) did not increase the dry matter. Under salinity, plants grown without Si had approximately 50% lower total dry weight compared with plants fed with 2 mmol L−1 Si. The decline in shoot dry weight was greater than the decline in root dry weight under salinity and consequently the ratio of shoot to root decreased more than 60%. Silicon nutrition partly recovered this ratio.
Na+, K+ and Si contents of the tissues
The concentration of Na+ increased significantly under salinity in both shoots and roots (). Silicon nutrition decreased the accumulation of Na+ in shoots under salinity, that is, in the 2 mmol L−1 Si treatment the Na+ content was approximately 56% less compared with plants grown without supplemental Si.
The concentration of K+ decreased in both roots and shoots, particularly in the shoots in plants under salinity (). The tissue K+ content was not affected by Si nutrition. A significant increase in Si concentration in both roots and shoots occurred when Si was supplied to the plants (). Salinity did not affect the Si concentration in the shoots; however, the content of Si in Si-supplied roots grown in a non-saline condition was 46% greater than the content in roots grown under salinity.
Figure 1 Effect of salinity (control versus 150 mmol L−1 NaCl) on the malondialdehyde concentrations as a measure of lipid peroxidation in the (a) shoots and (b) roots of canola plants grown for 25 days with or without supplementary silicon. Error bars represent the standard error. FW, fresh weight. Different small letters on histograms represent statistically significant differences at P < 0.05.
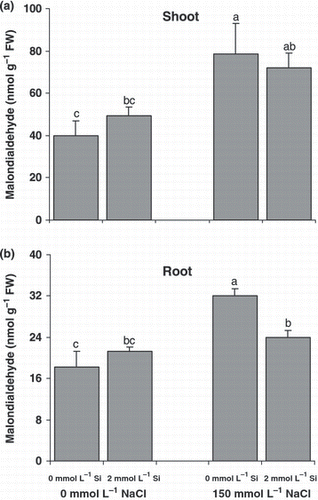
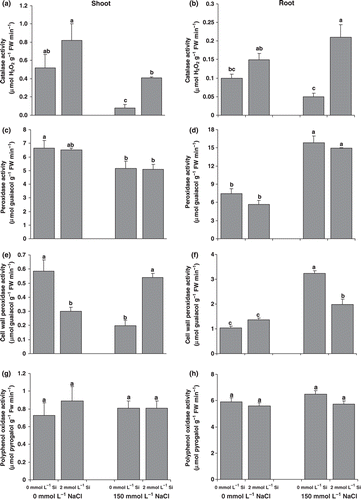
Figure 2 Effect of salinity (control versus 150 mmol L−1 NaCl) on the activity of (a,b) catalase, (c,d) perosidase, (e,f) cell wall perosidase and (g,h) polyphenol oxidase in the roots (a,c,e,g) and shoots (b,d,f,h) of canola plants grown for 25 days with or without supplementary silicon. Error bars represent the standard error. Different small letters on histograms represent statistically significant differences at P < 0.05.
Biochemical factors
Lipid peroxidation increased markedly under salinity (). In the 150 mmol L−1 NaCl treatment, it increased approximately twofold in both roots and shoots compared with the control plants. A significant reduction in lipid peroxidation occurred only in the roots of salt-treated plants following Si application.
Catalase and various peroxidases scavenge tissue hydrogen peroxide and in this way control the severity of oxidative stress. Catalase activity decreased in both the roots and shoots under salt treatment; however, Si nutrition led to a significant increase in the activity of this enzyme in salt-treated plants (), so that in the 150 mmol L−1 NaCl treatment Si-fed plants had 3.4-fold and 5.1-fold greater catalase activity in the roots and shoots, respectively, compared with plants grown without Si. In the salt treatment, soluble peroxidase activity decreased significantly in the shoots, whereas in the roots it increased to a great extent (). Silicon nutrition could not bring about any significant changes in soluble peroxidase activity. In the absence of Si nutrition, cell wall peroxidase activity decreased in the shoots, but increased in the roots in salt-treated plants. Silicon nutrition, however, increased the activity of this enzyme in the shoots and decreased it in the roots. Polyphenol oxidase activity did not change significantly with either salinity or silicon nutrition ().
Phenolics are one of the most important groups of secondary metabolites with anti-oxidative properties. The amount of soluble phenolics increased significantly in the NaCl treatment in both roots and shoots (). Silicon nutrition did not have any significant effect on phenolics in the roots and shoots. Salinity significantly increased the tissue lignin content in the absence of Si (). The lignin content in the shoots and roots of salt-treated plants was 43% and 34% greater, respectively, when compared with the controls. However, Si application decreased the lignin contents of both shoots and roots under salinity.
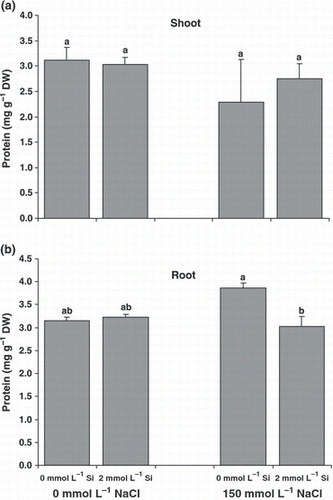
Leaf and root soluble proteins did not change significantly with NaCl nutrition. Silicon nutrition induced a marked decrease in leaf soluble proteins only in roots under NaCl treatment (). The leaf chlorophyll content decreased significantly in the salt treatment. The contents of chlorophyll a and chlorophyll b were 53% and 41% less, respectively, in salt-treated plants compared with control plants (). Under salinity, Si nutrition could recover the leaf chlorophyll content of the plants so that the contents of chlorophyll a and chlorophyll b in the Si-fed plants were 85% and 50% more, respectively, compared with plants grown without Si.
Figure 3 (a,b) Phenolics and (c,d) lignin contents in the roots (b,d) and shoots (a,c) of canola plants grown for 25 days under salinity with or without supplementary silicon. Error bars represent the standard error. DW, dry weight. Different small letters on histograms represent statistically significant differences at P < 0.05.
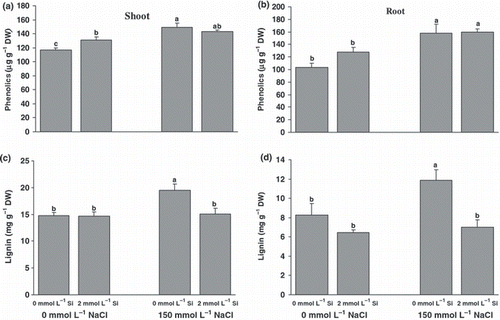
Figure 4 Soluble protein contents of the (a) shoots and (b) roots of canola plants grown for 25 days under salinity with or without supplementary silicon. Error bars represent the standard error. DW, dry weight. Different small letters on histograms represent statistically significant differences at P < 0.05.
Discussion
Salinity led to toxicity in the canola plants as evidenced by a drastic reduction in growth, greater amounts of Na+ and decreased K+ contents (,2). Reductions in growth under salinity have been reported in canola plants (CitationAshraf and McNeilly 1990; CitationFrancois 1994; CitationQasim et al. 2003). Silicon nutrition, however, decreased the Na+ concentration in canola shoots under salinity (), as has been reported in several other plants under saline conditions (CitationLiang et al. 2006; CitationMa 2004). This effect may result from Si deposition in the cell walls and endodermis, which reduces Na+ uptake through a reduction in apoplastic transport across the root and its translocation to the shoots (CitationGong et al. 2006).
Owing to decreased activity of ROS scavenging enzymes, such as catalase in the whole plant and peroxidases in the shoots, under salinity (), cell free radical metabolism might be shifted toward the production of more ROS. Catalase and peroxidases are the two major ROS scavenging enzymes and a decline in their activities under salinity has been reported by several investigators (CitationHertwig et al. 1992), which ultimately leads to oxidative stress (CitationAlscher et al. 1997; CitationMittler 2002). Increased lipid peroxidation in the salt-treated canola plants () and their reduced growth might have resulted from alterations to the activities of these ROS-scavenging enzymes. Increased lipid peroxidation under salinity has been reported in other plants (CitationVaidyanathan et al. 2003), and represents membrane oxidative damage and dysfunction (CitationImlay 2003; CitationMittler 2002). Silicon nutrition significantly increased catalase activity in the whole plant and cell wall peroxidase in the shoots. Higher catalase activity under salinity following Si application has already been reported in tomato and barley (CitationAl-aghabary et al. 2004; CitationLiang et al. 2003). It appears that under salinity Si-supplied canola plants use ROS scavenging metabolic pathways more efficiently and this might be related to the lower toxicity resulting from reduced Na+ accumulation. Greater activities of other ROS scavenging enzymes, such as superoxide dismutase (SOD), peroxidases, glutathione reductase (GR) and dehydro-ascorbate reductase (DHAR), have been reported by CitationZhu et al. (2004) in salt-stressed cucumber plants following Si application. The significant reduction in lipid peroxidation that was observed particularly in the roots of salt-grown canola plants following Si application may relate to changes in the activities of these anti-oxidative enzymes. The ROS scavenging capacity might also be improved by Si nutrition through other mechanisms, such as non-enzymatic anti-oxidants (CitationGunes et al. 2007), which prevent oxidative damage to membranes, and it may account for the reduced lipid peroxidation observed in the canola plants.
Figure 5 Effect of salinity (control versus 150 mmol L−1 NaCl) on the (a) chlorophyll a and (b) chlorophyll b contents of canola plants grown for 25 days with or without supplementary silicon. Error bars represent the standard error. DW, dry weight. Different small letters on histograms represent statistically significant differences at P < 0.05.
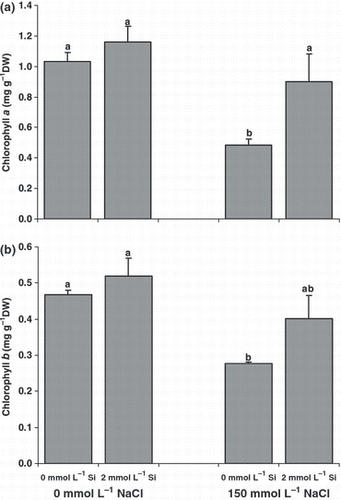
Salinity led to a reduction in leaf-soluble proteins and chlorophyll a and chlorophyll b in canola plants (). These reductions can be attributed to the oxidative stress generated by salinity as several investigators have reported increased susceptibility of proteins to proteolysis and chlorophyll depletion following salinity-induced oxidative stress (CitationBhattacharjee 2005; CitationBlokhina et al. 2003). Silicon nutrition can recover the chlorophyll content of canola plants under salinity, which suggests that it plays a role in the suppression of oxidative stress. Added Si has also been shown to improve the chlorophyll content of tomato and barley under salt stress (CitationAl-aghabary et al. 2004; CitationLiang 1998).
Salinity increased phenolics and the lignin content in canola plants (). An increase in phenolics might result from alterations to plant secondary metabolism following oxidative stress (CitationSchutzendubel and Polle 2002). Greater lignin deposition in response to salinity has already been reported in rice and tomato (CitationSánchez-Aguayo et al. 2004; CitationSeungGon et al. 2004). The reduction in growth of the canola plants under salinity might have resulted from the increased lignin and phenolics and may show harmful effects of salinity and/or plant response to salinity, which brings about greater selectivity and a reduced uptake of harmful ions via the apoplastic pathway as supposed by CitationSánchez-Aguayo et al. (2004). Cell wall loosening and plant growth would be impaired as a result of lignification. The lignin contents declined in the canola plants, particularly in the shoots, following the application of Si. Partial substitution of lignin by Si or the formation of Si–polyphenol complexes (CitationMaksimovićet al. 2007) in the cell walls may facilitate cell wall loosening and cell extension and promote the growth of plants under stress conditions. Silicon deposition has been shown to increase cell-wall extensibility in sorghum roots (CitationHattori et al. 2003). Thus, the results of the present work may reveal another unknown beneficial effect of Si nutrition in salt-stressed plants, that is, the prevention of tissue lignifications, which allows further plant growth under saline conditions.
Despite the non-essentiality of Si for dicotyledonous plants, the data reported in the present study show new aspects of the beneficial effects of Si on plants grown under saline conditions. The application of Si prevents Na+ accumulation in canola plants. This reduced Na+ accumulation improves the plant ROS scavenging capacity (our data; CitationAl-aghabary et al. 2004; CitationGunes et al. 2007; CitationLiang et al. 2003, 2006; CitationMoussa 2006), accompanied by reduced lipid peroxidation. The reduced lignification of Si-supplied canola plants grown under saline conditions found in the present study is another consequence of the restricted Na+ accumulation that allows further plant growth.
Acknowledgment
We thank GUASNR Deputy of Research and Office of Higher Education for financial support to A. Hashemi in the form of grants for MSc research projects.
References
- Ahmad , R , Zaheer , SH and Ismail , S . 1992 . Role of silicon in salt tolerance of wheat (Tritium aestivumL.) . Plant Sci. , 85 : 43 – 50 .
- Akbar Hossain , K , Horiuchi , T and Miyagawa , S . 2001 . Effects of silicate materials on growth and grain yield of rice plants grown in clay loam and sandy loam soils . J. Plant Nutr. , 24 : 1 – 13 .
- Al-aghabary , K , Zhu , Z and Shi , Q . 2004 . Influence of silicon supply on chlorophyll content, chlorophyll fluorescence, and antioxidative enzyme activities in tomato plants under salt stress . J. Plant Nutr. , 27 : 2101 – 2115 .
- Alscher , RG , Donahue , JL and Cramer , CL . 1997 . Reactive oxygen species and antioxidants: relationship in green cells . Physiol. Plant. , 100 : 224 – 233 .
- Alvarez , J and Datnoff , LE . 2001 . The economic potential of silicon for integrated management and sustainable rice production . Crop Prod. , 20 : 43 – 48 .
- Arnon , DI . 1949 . Copper enzymes in isolated chloroplasts. polyphenoloxidase in Beta Vulgaris . Plant Physiol. , 24 : 1 – 15 .
- Ashraf , M and McNeilly , T . 1990 . Responses of four Brassicaspecies to sodium chloride . Environ. Exp. Bot. , 30 : 475 – 487 .
- Athar , HR , Ashraf , M , Vahid , A and Jamil , A . 2009 . Inducing salt tolerance in canola (Brassica napusL.) by exogenous application of glycinebetaine and proline: response at the initial growth stages . Pak J. Bot. , 41 : 1311 – 1319 .
- Bhattacharjee , S . 2005 . Reactive oxygen species and oxidative stress, senescence and signal transduction in plants . Curr. Sci. , 89 : 1113 – 1121 .
- Blokhina , O , Virolainen , E and Fagerstedt , KV . 2003 . Antioxidants, oxidative damage and oxygen deprivation stress: a Review . Ann. Bot. , 91 : 179 – 194 .
- Bradbury , M and Ahmad , R . 1990 . The effect of silicon on the growth of Prosopis julifloragrowing in saline soil . Plant Soil , 125 : 71 – 74 .
- Bradford , M . 1976 . A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding . Anal. Biochem. , 72 : 248 – 254 .
- Chen , LM , Lin , CC and Kao , CH . 2000 . Copper toxicity in rice seedlings: changes in antioxidative enzyme activities, H2O2level, and cell wall peroxidase activity in roots . Bot. Bull. Acad. Sin. , 41 : 99 – 103 .
- Elliot , CL and Synder , GH . 1991 . Autoclave-induced digestion for the colorimetric determination of silicon in rice straw . Agric. Food chem. , 39 : 111 – 119 .
- Epstein , E . 1994 . The anomaly of silicon in plant biology . Proc. Natl Acad. Sci. USA , 91 : 11 – 17 .
- Francois , LE . 1994 . Growth, seed yield and oil content of canola growth under saline conditions . Agron. J. , 86 : 233 – 237 .
- Fukoda , T , Ito , H and Yoshida , T . 2003 . Antioxidative polyphenols from walnuts (Juglans regiaL.) . Phytochem. , 63 : 795 – 801 .
- Gong , HJ , Randall , DP and Flowers , TJ . 2006 . Silicon deposition in the root reduces sodium uptake in rice (Oryza sativaL.) seedlings by reducing bypass flow . Plant Cell Environ. , 29 : 1979 – 1970 .
- Gunes , A , Inal , A , Bagci , EG and Pilbeam , DJ . 2007 . Silicon-mediated changes of some physiological and enzymatic parameters symptomatic for oxidative stress in spinach and tomato grown in sodic-B toxic soil . Plant Soil , 290 : 103 – 114 .
- Hattori , T , Inanaga , S , Tanimoto , E , Lux , A , Luxová , M and Sugimoto , Y . 2003 . Silicon-induced changes in viscoelastic properties of sorghum root cell walls . Plant Cell Physiol. , 44 : 743 – 749 .
- Heath , RL and Packer , L . 1968 . Photoperoxidation in isolated chloroplasts. I. kinetics and stoichimetry of fatty acid peroxidation . Arch. Biochem. Biophys. , 125 : 189 – 198 .
- Hertwig , B , Steb , P and Feierabend , J . 1992 . Light dependence of catalase synthesis and degradation in leaves and the influence of interfering stress conditions . Plant Physiol. , 100 : 1547 – 1553 .
- Hodges , DM , Delong , JM , Forney , CF and Prange , RK . 1999 . Improving the thiobarbituric acid-reactive-substance assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds . Planta , 207 : 604 – 641 .
- Imlay , JA . 2003 . Pathways of oxidative damage . Annu. Rev. Microbiol. , 57 : 395 – 418 .
- Inanaga , S and Okasaka , A . 1996 . Calcium and silicon binding compounds in cell walls of rice shoots . Soil Sci. Plant Nutr. , 41 : 103 – 110 .
- Kar , M and Mishra , D . 1976 . Catalase, Peroxidase and polyphenolxidase activities during rice leaf senescence . Plant Physiol. , 57 : 315 – 319 .
- Lavid , N , Schwrtz , A , Yarden , O and Tel-Or , E . 2001 . The involvement of polyphenols and peroxidase activities in heavy-metal accumulation by epidermal glands of waterlily (Nymphaeaceae) . Planta , 212 : 323 – 331 .
- Liang , YC . 1998 . Effects of Si on leaf ultrastructure, chlorophyll content and photosynthetic activity in barley under salt stress . Pedosphere , 8 : 289 – 296 .
- Liang , YC , Chen , Q , Liu , Q , Zhang , WH and Ding , RX . 2003 . Exogenous silicon (Si) increases antioxidant enzyme activity and reduces lipid peroxidation in roots of salt-stressed barley (Hordeum vulgareL.) . J. Plant Physiol. , 160 : 1157 – 1164 .
- Liang , YC , Shen , QR , Shen , ZG and Ma , TS . 1996 . Effects of silicon on salinity tolerance of two barley cultivars . J. Plant Nutr. , 19 : 173 – 183 .
- Liang , YC , Sun , W , Zhu , YG and Christie , P . 2006 . Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: a review . Environ. Pollut. , 147 : 1 – 7 .
- Ma , JF . 2004 . Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses . Soil Sci. Plant Nutr. , 50 : 11 – 18 .
- Ma , JF and Takahashi , E . 2002 . Soil, Fertilizer, and Plant Silicon Research in Japan , 274 Elsevier B.V. Amsterdam .
- Maksimović , JD , Bogdanović , J , Maksimović , V and Nikolic , M . 2007 . Silicon modulates the metabolism and utilization of phenolic compounds in cucumber (Cucumis sativusL.) grown at excess manganese . J. Plant Nutr. Soil Sci. , 170 : 739 – 744 .
- McKersie , BD and Leshem , YY . 1994 . Stress and Stress Coping in Cultivated Plants , Dordrecht : Kluwer Academic Publishes .
- Mittler , R . 2002 . Oxidative stress, antioxidants and stress tolerance . Trends Plant Sci. , 7 : 405 – 410 .
- Moussa , HR . 2006 . Infuence of exogenous application of silicon on physiological response of salt-stressed maize (Zea maysL.) . Inter. J. Agic Boil. , 8 : 293 – 297 .
- Neill , S , Desikan , R and Hancock , J . 2002 . Hydrogen peroxide signalling . Curr. Opin. Plant Biol. , 5 : 388 – 395 .
- Noctor , G and Foyer , CH . 1998 . Ascorbate and glutathione: keeping active oxygen under control . Annu. Rev. Plant Physiol. Plant Mol. Biol. , 49 : 249 – 279 .
- Ortega , L , Fry , SC and Taleisnik , E . 2006 . Why are Chloris gayanaleaves shorter in salt-affected plants? Analyses in the elongation zone . J. Exp. Bot. , 57 : 3945 – 3952 .
- Purty , RS , Kumar , G , Singla-Pareek , SL and Pareek , A . 2008 . Towards salinity tolerance in Brassica: an overview . Physiol. Mol. Biol. Plant. , 14 : 39 – 49 .
- Qasim , M , Ashraf , M , Ashraf , MY , Rehman , SU and Ma , ES . 2003 . Salt-induced changes in two canola cultivars differing in salt tolerance . Biol. Plant , 46 : 629 – 632 .
- Raven , JA . 1983 . The transport and function of silicon in plants . Biol. Reviews , 58 : 179 – 207 .
- Resende , MLV , Nojosa , GBA Cavalcanti , LS . 2002 . Induction of resistance in cocoa against Crinipellis perniciosaand Verticillium dahliaeby acibenzolar-S-methyl (ASM) . Plant Pathol. , 51 : 621 – 628 .
- Sánchez-Aguayo , I , Rodrìguez-Galán , JM , Garcìa , R , Torreblanca , J and Pardo , JM . 2004 . Salt stress enhances xylem development and expression of S -adenosyl-L-methionine synthase in lignifying tissues of tomato plants . Planta , 220 : 278 – 285 .
- SAS Institute Inc . 2004 . SAS/IML User’s Guide, Version 9.1 , Cary, NC : SAS Institute, Inc .
- Schutzendubel , A and Polle , A . 2002 . Plant response to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization . J. Exp. Bot. , 53 : 1351 – 1365 .
- SeungGon , W , JaeSung , K JinHong , K . 2004 . Effect of salinity on lignin and hydroxycinnamic acid contents in rice . Korean J. Crop Sci. , 49 : 368 – 372 .
- Szabolcs , I . 1994 . “ Soils and salinisation ” . In Handbook of Plant and Crop Stress , Edited by: Pessarakali , M . 3 – 11 . New York : Marcel Dekker .
- Tester , M and Davenport , R . 2003 . Na+tolerance Na+transport in higher plants . Ann. Bot. , 91 : 503 – 527 .
- Vaidyanathan , H , Sivakumar , P , Chakrabarty , R and Thomas , G . 2003 . Scavenging of reactive oxygen species in NaCl-stressed rice (Oryza sativaL.) differential response in salt-tolerant and sensitive varieties . Plant Sci. , 165 : 1411 – 1418 .
- Vasudevan , PT and Briggs , M . 2008 . Biodiesel production: current state of the art and challenges . J. Ind. Microbiol. Biotechnol. , 35 : 421 – 430 .
- Wang , L , Showalter , AM and Ungar , IA . 1997 . Effect of salinity on growth, ion content, and cell wall chemistry in Atriplex prostrate(chenopodiaceae) . Am. J. Bot. , 4 : 1247 – 1255 .
- Yeo , AR , Flowers , SA , Rao , G , Welfare , K , Senanayake , N and Flowers , TJ . 1999 . Silicon reduces sodium uptake in rice (Oryza sativaL.) in saline conditions and this is accounted for by a reduction in the transpirational bypass flow . Plant Cell Environ. , 22 : 559 – 565 .
- Zhang , H-X , Hodson , JN , Williams , JP and Blumwald , E . 2001 . Engineering salt-tolerant Brassicaplants: characterization of yield and seed oil quality in transgenic plants with increased vacuolar sodium accumulation . Proc. Natl Acad. Sci. USA , 98 : 12832 – 12836 .
- Zhu , ZJ , Wei , GQ , Li , J , Qian , QQ and Yu , JQ . 2004 . Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativusL.) . Plant Sci. , 167 : 527 – 533 .
- Zimmer , M . 1999 . Combined methods for the determination of lignin and cellulose in leaf litter . Sci. Soils , 4 : 14 – 21 .