Abstract
The aim of the present study was to verify the effect of inosine on plant growth. Rice (Oryza sativa L.), tomato (Solanum lycopersicum L.), onion (Allium cepa L.), sunflower (Helianthus annuus L.) and soybean (Glycine max (L.) Merr.) seedlings were grown in a hydroponic system with different added concentrations of inosine (0, 7.2, 72 and 373 μmol L−1) in a greenhouse. The application of inosine improved the growth of all plant parts, particularly roots, except for soybean. The optimum inosine concentration for plant growth was 72 μmol L−1. Furthermore, in rice, tomato and sunflower, treatment with inosine increased root length. To confirm the effect of inosine, an aseptic experiment was carried out using rice plants in a hydroponic system and using tomato in a rhizobox system with sterile soil. The positive effect of inosine was also confirmed under aseptic conditions in rice and tomato, indicating that inosine directly enhances plant growth without microbial decomposition.
Introduction
Inosine is a purine nucleoside that is widely found in plants, animals and other forms of living organisms. It is composed of the purine-base hypoxanthine and the sugar D-ribose. Nucleosides and nucleotides are precursors of nucleic acids (DNA and RNA), metabolites that participate in bioenergetic processes (ATP) and in the synthesis of macromolecules, including polysaccharides, phospholipids and glycolipids (CitationStasolla et al. 2003); they are also constituents of cytokinins, which control plant growth and development (CitationD’Agostino and Kieber 1999). CitationLetham (1994) indicated that the root tip is the main part of the plant used for the synthesis of cytokinins. Therefore, the application of nucleosides, such as inosine, as a cytokinin precursor could promote the growth of shoot meristems (CitationTaiz and Zeiger 2006). For example, CitationNagao et al. (2005) reported that foliar application of proline and nucleic acid fertilizer, including 2% inosine diluted in water, stimulated second flush growth of Italian ryegrass (Lolium multiflorum Lam).
The aim of the present study was to verify the effect of inosine on plant growth, particularly root growth, using a hydroponic system and soil in a rhizobox. We speculated that other compound(s), such as inorganic nitrogen (N) as an N source or plant-growth-promoting substances, derived from inosine degradation can affect plant growth. Thus, we also conducted an aseptic experiment to confirm whether inosine application directly or indirectly (e.g. contribution of microbial decomposition) affected plant growth.
Materials and methods
Non-aseptic hydroponic experiment
Five species, rice (Oryza sativa L. cv. Kirara 397), tomato (Solanum lycopersicum L. cv. Momotarou), sunflower (Helianthus annuus L. cv. Takii), onion (Allium cepa L. cv. Ikoru) and soybean (Glycine max (L) Merr. cv. Iwakuro) were grown hydroponically under greenhouse conditions at the Graduate School of Agriculture, Hokkaido University, Sapporo, Japan (43°3′N, 141°2′E, 17 m a.s.l.; maximum temperature 32°C; minimum temperature 16°C) in July–August 2006. The seeds were sown in vermiculite, except for the rice seedlings, which were germinated on a net floating on tap water. Seedlings were transplanted to nutrient solution containing 0.42 mmol L−1 NH4NO3, 0.0128 mmol L−1 NaH2PO4•2H2O, 0.154 mmol L−1 K2SO4, 0.24 mmol L−1 CaCl2•2H2O, 0.164 mmol L−1 MgSO4•7H2O, 7.2 μmol L−1 FeSO4•7H2O, 1.82 μmol L−1 MnSO4•5H2O, 9.2 μmol L−1 H3BO3, 0.62 μmol L−1 ZnSO4•7H2O, 0.032 μmol L−1 CuSO4•5H2O and 0.0104 μmol L−1 (NH4)6•Mo7O24•4H2O. The inosine concentrations applied to the nutrient solution were 0 (control), 7.2, 72 and 373 μmol L−1. The solution pH was adjusted to 5.2 by HCl or NaOH daily. Seedlings were hydroponically cultivated for 21 days in all species except for sunflower and soybean; the duration for sunflower and soybean was 15 days. Three plants were grown in each 5-L pot with 4 L of nutrient solution. There were three replicate pots for each treatment.
Aseptic hydroponic experiment
All materials used in this experiment, including the nutrient solution, glass pots and plastic bags, were sterilized by autoclave (120°C for 40 min). The pericarp of the rice seeds (cv. Nipponbare) was removed and sterilized with 70% ethanol for 1 min and 10% sodium hypochlorite for 15 min twice and then washed thoroughly with sterilized water. The seeds were then sown in MS medium (CitationMurashige and Skoog 1962) and the pH was adjusted to 5.3. Seedlings were transferred to glass pots (two seedlings per pot) containing 450 mL of nutrient solution and covered with a plastic bag. The nutrient solution was changed every 3 days and the pH was adjusted to 5.3. The plants were precultured for 3 weeks in a growth chamber at a constant temperature of 25°C and a photoperiod of 16/8 h light/dark. After preculture, the plants were transferred to treatment solutions with different inosine concentrations (72 or 144 μmol L−1) and grown for 2 weeks until sampling; maintaining the above-mentioned conditions. The experiment had four replicates, with two rice seedlings in each pot.
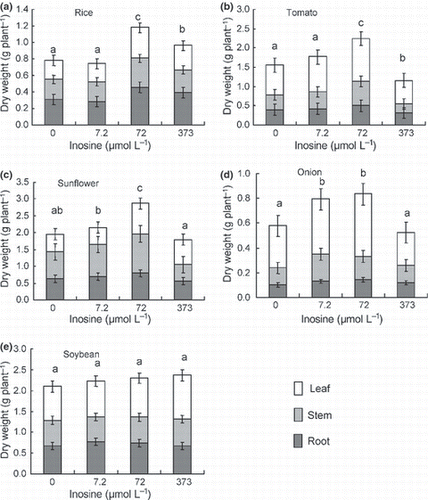
Figure 1 Dry weight (g plant−1) of the leaves, stems and roots in (a) rice, (b) tomato, (c) sunflower, (d) onion and (e) soybean grown in a hydroponic system with 0 (Control), 7.2, 72 or 373 μmol L−1 of inosine. Error bars indicate the standard error. Different letters denote significant differences in total dry weight using a Tukey’s test (P < 0.05).
Rhizobox experiment
Tomato seedlings (cv. Momotarou) were grown in a rhizobox containing 0.880 kg of sterilized or non-sterilized vermiculite with sand (vermiculite : sand = 1:3 v/v). The soil was sterilized by gamma (γ) radiation at 25 kGy before the experiment (Koka Laboratory, Japan Radioisotope Association, Shiga, Japan). For fertilization, 3.57 mmol (NH4)2SO4, 1.4 mmol Ca (H2PO4)2 and 1.16 mmol K2SO4 were added at the beginning of the experiment to each rhizobox as a NPK + Ca source. The remaining nutrients were supplemented by irrigation with 50 mL nutrient solution per rhizobox containing 0.081 mmol L−1 MgSO4•7H2O, 7 μmol L−1 FeSO4•7H2O, 2 μmol L−1 MnSO4•5H2O, 8 μmol L−1 H3BO3, 1.2 μmol L−1 ZnSO4•7H2O, 0.063 μmol L−1 CuSO4•5H2O and 0.004 μmol L−1 (NH4)6•Mo7O24•4H2O. Tomato seeds were sterilized and grown as per the aseptic hydroponic experiment. Seedlings were transplanted to the rhizobox on a clean bench when the seedlings were 2 weeks old. The plants were grown for 5 weeks in a growth room at a constant temperature of 26°C and a photoperiod of 16/8 h light/dark. Inosine was applied once per week. The treatments were divided between aseptic and non-aseptic soils. Inosine treatments were used as a subtreatment. The total quantity of inosine per rhizobox used during the experiment was 37 μmol. To maintain aseptic conditions, water and nutrient solutions were applied after filtration through a membrane filter (pore size = 0.22 μm; Millipore, Cork, Ireland) connected to the rhizobox with 20 pieces of filter on one side of the surface of rhizobox evenly. The experimental design was four rhizoboxes per treatment and two plants per rhizobox. Each rhizobox was made from acrylic material and measured 38 cm × 17 cm × 2.5 cm.
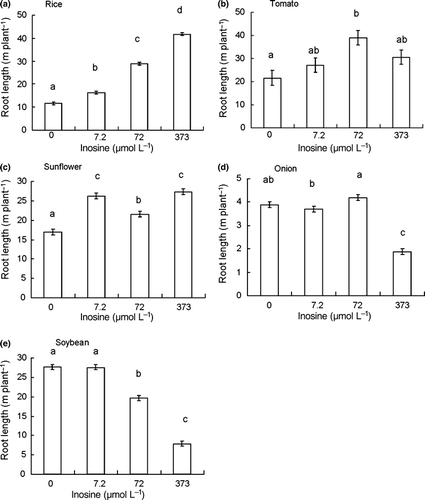
Figure 2 Total root length (m plant−1) for (a) rice, (b) tomato, (c) sunflower, (d) onion and (e) soybean grown in a hydroponic system with 0 (Control), 7.2, 72 or 373 μmol L−1 inosine. Error bars indicate the standard error. Different letters denote significant differences using a Tukey’s test (P < 0.05).
Contamination check
In the aseptic cultivation (hydroponic and rhizobox), sterility was checked using the soybean–casein–digest (Nihon Seiyaku, Tokyo, Japan) plate technique before and after the experiment. The plots (hydroponic pots or rhizoboxes) were regarded as aseptic if microbial growth did not occur after 7 days of incubation at 30°C.
Plant analyses
After measuring the fresh weights, samples of leaves, stems and roots were dried in an air-forced oven at 105°C for 72 h. Fresh roots were stained with Lugol’s solution (Merck, Darmstadt, Germany) for the root hair observation. Root hair development was evaluated under a microscope (200× magnifications) equipped with a camera (Coolpix 4500; Nikon, Tokyo, Japan). Root length was determined using the method of CitationNewman (1966). This methodology consists of indirect measures using a magnifying glass with a straight-lined box, but does not consider the root hair. The number of intersections between the roots and the straight lines determines the root length, in combination with a mathematical equation. The lateral root number was counted at the same as the root length was measured. The N content was determined using the Kjeldahl method (Büchi, Flawil/Schweiz, Switzerland) (CitationHind 1993). In the rhizobox, root growth observations and measurements were done through the opposite side of the rhizobox. The rhizobox was scanned and the root surface area was determined using high-performance numeric computation and visualization software (version 6.5) (The Math Works; Matlab, Natick, Massachusetts, USA) (CitationFan and Neumann 2004).
Table 1 Complementary data for root hair density (number of root hairs mm−1) in rice, tomato, sunflower and soybean grown in a hydroponic system with 0 (Control), 7.2, 72 or 373 μmol L−1 inosine
Statistical analyses
All data were analyzed with ANOVA and Tukey’s multiple comparison tests (P < 0.05) using SPSS version 11.5 (SPSS, Chicago, IL, USA). A Student’s t-test was used for the rhizobox experiment (P < 0.05).
Results and discussion
Effect of inosine under non-aseptic conditions
There was a clear and positive effect of inosine on plant dry weight for rice, tomato, sunflower and onion at concentrations of 7.2–72 μmol L−1 (). However, when the inosine concentration was increased to 373 μmol L−1 there was a positive effect on dry weight for rice only (). Inosine had no effect on the dry weight and root length of soybean (, ), indicating that the effect of inosine was different for soybean. It is possible that inosine produces different responses in fabaceous plants.
The results showed that inosine can increase root length in rice, tomato and sunflower (). In rice, the root length increased with increases in the concentration of inosine; the longest root length of approximately 40 m was observed at 373 μmol L−1 (). Root length also increased at 7.2 μmol L−1 inosine in rice and sunflower (). In tomato, root length also increased with an increase in the concentration of inosine; the longest root length was observed at 72 μmol L−1 inosine (). In sunflower, root length increased with an increase in the concentration of inosine and the longest root lengths were observed at 7.2 and 373 μmol L−1 (). However, in onion, root length decreased with increases in inosine concentration ().
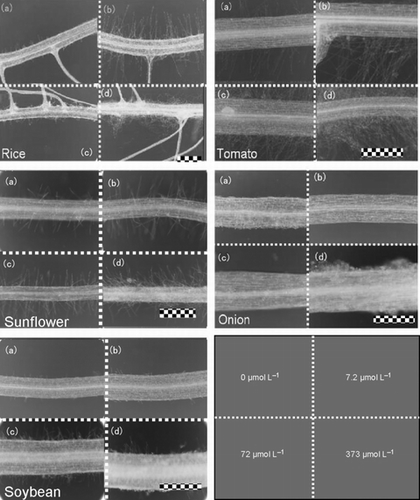
Root hair density increased with an increase in inosine concentration in all plant species, except for onion, which does not develop root hairs (; ). Even at the low inosine concentration (7.2 μmol L−1), there was a slight increase in root hair density (; ). Root hairs are very important for nutrient uptake. In general, root hairs contribute up to 70–80% of the total root surface area (CitationItoh and Barber 1983). In all sizes of roots, the most important roots for nutrient uptake are the thinnest roots; CitationSullivan et al. (2000) found no effect on nitrate uptake rate with larger numbers of thick roots (diameter > 0.5 mm). Root hairs are most representative to uptake N and P (CitationWang et al. 2006). Thus, there could be a strong connection between root morphology and nutrient uptake as affected by inosine application.
Figure 3 Root hair microscopy images (200× magnification) from rice, tomato, sunflower, onion and soybean grown in nutrient solutions with (a) 0, (b) 7.2, (c) 72 and (d) 373 μmol L−1 inosine. The scale bar is 0.5 mm.
Inosine might be metabolized to other compounds, such as plant-growth-promoting hormones (CitationAkiyoshi et al. 1987). Examples of plant hormones that affect root growth are auxins (CitationSantelia et al. 2005), cytokinins (CitationLaplaze et al. 2007), abscisic acid (CitationSmet et al. 2003) and ethylene (CitationNegi et al. 2008). Inosine contains N and is able to be degraded into other compounds by microorganisms; it is possible that the degradation products, such as inorganic N, could improve plant growth. Therefore, an aseptic experiment was used to confirm that inosine affects root growth under aseptic conditions.
Effect of inosine under aseptic conditions
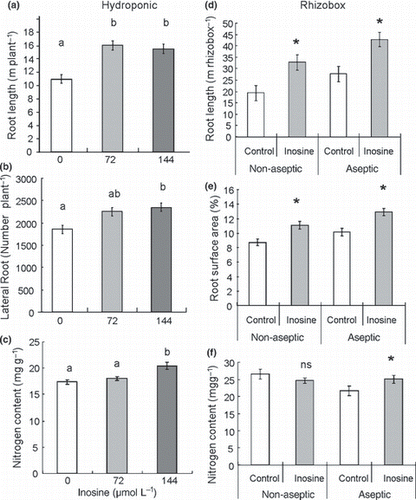
Positive effects of inosine were identified (72 and 144 μmol L−1) on rice root length and on the quantity of lateral roots under aseptic conditions (). Our results showed that root growth enhancement with inosine application can be induced without microorganisms. Thus, enhanced root growth did not occur as a result of the production of growth hormone or other compounds by microorganisms in the rhizosphere (CitationAkiyoshi et al. 1987; CitationTaiz and Zeiger 2006). Under aseptic conditions, both in rice and tomato, the N content increased with inosine application (). Although we do not have direct evidence that inosine is absorbed in its intact form by the roots, the existence of nucleoside transporter as PUP (purine transporters) families, such ENT (equilibrative nucleoside transporters) families may contribute to the transport of inosine. OsENT2, one of the ENT families, was predominantly expressed in roots and it is has been suggested that it contributes to long-distance transport of nucleosides in growing plants (CitationHirose et al. 2005). After the incorporation of inosine into the plant, it has been suggested that inosine is transformed into a N source. Inosine is known to be an intermediate compound for purine nucleotide synthesis in two different biosynthesis processes in plants: one is the purine nucleotide salvage pathway and the other is the purine nucleotide catabolism pathway (CitationStasolla et al. 2003). In the first synthesis process, inosine is converted to inosine monophosphate by non-specific nucleoside phosphotransferase (CitationGuranowski 1979) or inosine kinase (CitationCombes et al. 1989). And in the second synthesis process, inosine is metabolized to hypoxanthine by inosine nucleosidase, and then to xanthine and allantoin and then degraded to ammonia (NH3) until complete oxidation (CitationGuranowski 1982; CitationStasolla et al. 2003).
Figure 4 (a–c) Rice and (d–f) tomato grown in nutrient solution and in a rhizobox with soil, respectively. (a) Root length in cm plant−1, (b) number of lateral roots per plant and (c) the leaf nitrogen content in mg g−1 of rice grown hydroponically with different concentrations of inosine (0, 72 or 144 μmol L−1). (d) Root length of tomato in m rhizobox−1, (e) root surface area covered in terms of the percentage of the total rhizobox area and (f) the leaf nitrogen content in mg g−1. Error bars indicate the standard error. Different letters denote significant differences using Tukey’s tests (P < 0.05). *P < 0.05; ns, not significant (comparisons between the control measures and the inosine treatments according to Student’s t-tests).
Effect of inosine under soil culture conditions
Positive effects of inosine under soil culture conditions were also observed in the rhizobox experiment with tomato plants under aseptic and non-aseptic conditions (). Root length and root surface area increased under both non-aseptic and aseptic conditions (). Compared with the control, the application of inosine in the rhizosphere increased root length 1.54-fold and 1.74-fold under non-aseptic and aseptic conditions, respectively (); in addition the total root surface area increased 1.22-fold and 1.30-fold, respectively ().
Effect of inosine on nitrogen content
The N content of the plants changed with the addition of inosine to the nutrient solution (; ). When the inosine concentration was 373 μmol L−1, all of the studied plants had smaller leaf size, darker green color of leaves by SPAD (Minolta, Tokyo, Japan) (data not shown) and the internodes were shorter. We assumed that these characteristics resulted from excess N accumulation in the plants (). However, the inosine concentration was not high enough to conclude that inosine caused the N excess symptoms. The N content in 7.2, 72 and 373 μmol L−1 of inosine was equivalent to 37, 370 and 1500 μmol L−1, respectively. An increase in N accumulation was also confirmed in the aseptic hydroponic experiment (); thus N accumulation might enhance root development (CitationLiao et al. 2004; CitationLittle et al. 2005; CitationRemans et al. 2006; CitationWirén et al. 2000).
Table 2 Nitrogen content of the leaves, stems and roots (mg g−1) in rice, tomato, onion, sunflower and soybean grown in a hydroponic system with 0 (Control), 7.2, 72 or 373 μmol L−1 of inosine
The treatment with 7.2 μmol L−1 of inosine in the nutrient solution increased the N content of the soybean plants, but did not change their dry weight (; ). Furthermore, although the root length in soybean decreased markedly when 72 to 373 μmol L−1 of inosine was applied, the N concentration in the leaves increased without a corresponding biomass change (,; ). In soybean, although an effect of inosine on root length was not observed, the N concentration in the leaf increased even at the lowest inosine level (7.2 μmol L−1) (). Although further investigations are necessary, we consider that the effect of inosine in soybean is partly because soybean is a fabaceous plant that has a specific mechanism to transport N as ureides (CitationTaiz and Zeiger 2006). The main biosynthesis process to transfer N between the nodules and the plant is to involve inosine as an intermediate in purine catabolism (CitationAtkins 1981; CitationMoffat and Ashihara 2002; CitationStasolla et al. 2003). As a result, it is possible that fabaceous species may have different mechanisms for transporting inosine to the shoots. Further experiments examining the form of N in the plant and on the effect of nodulation are required.
Conclusion
The present study demonstrated that the application of inosine positively and directly affects plant growth, particularly root growth. We propose that inosine participates in important metabolic processes, such as catabolism of purine nucleotides (CitationAshihara et al. 2008; CitationStasolla et al. 2003), and must be carefully considered in future studies.
Acknowledgments
This study was financially supported in part by Ajinomoto and by a scholarship from the Ministry of Education, Culture, Sports, Science and Technology of Japan. We would like to thank the Koka Laboratory, Japan Radioisotope Association, for sterilizing the soil.
References
- Akiyoshi , DE , Regier , DA and Gordon , MP . 1987 . Cytokinin production by Agrobacteriumand Pseudomonas spp . J. Bacteriol. , 169 : 4242 – 4248 .
- Ashihara , H , Luit , B , Belmonte , M and Stasolla , C . 2008 . Metabolism of nicotiamide, adenine and inosine in developing microspore-derived canola (Brassica napus) embryos . Plant Physiol. Biochem. , 46 : 752 – 759 .
- Atkins , CA . 1981 . Metabolism of purine nucleotides to form ureides in nitrogen-fixing nodules of cowpea (Vignia unguiculataL. Walp) . FEBS Lett. , 125 : 89 – 93 .
- D’Agostino , IB and Kieber , JJ . 1999 . Molecular mechanisms of cytokinin action . Curr. Opin. Plant Biol. , 2 : 359 – 364 .
- Fan , L and Neumann , PM . 2004 . The spatially variable inhibition by water deficit of maize root growth correlates with altered profiles of proton flux and cell wall pH . Plant Physiol. , 135 : 2291 – 2300 .
- Guranowski , A . 1979 . Nucleoside phosphotransferase from yellow lupin seedling cotyledons . Biochim. Biophys. Acta , 569 : 13 – 22 .
- Guranowski , A . 1982 . Purine catabolism in plants. Purification and some properties of inosine nucleosidase from yellow lupin (Lupinus luteus L.) seeds . Plant Physiol. , 70 : 344 – 349 .
- Hind , G . 1993 . “ Thylakoid components and processes ” . In Photosynthesis and Production in a Change Environment: A Field and Laboratory Manual , Edited by: Hall , DO , Scurlock , JMO , Bolhar-Nordenkampf , HR , Leegood , RC and Long , SP . 283 – 298 . London : Chapman & Hall .
- Hirose , N , Makita , N , Yamaya , T and Sakakibara , H . 2005 . Functional characterization and expression analysis of a gene, OsENT2, encoding an equilibrative nucleoside transporter in rice suggest a function in cytokinin transport . Plant Physiol. , 138 : 196 – 206 .
- Combes , A , Lafleuriel , J and Le Floc’h , F . 1989 . The inosine-guanosine kinase activity of mitochondria in tubers of Jerusalem artichoke . Plant Physiol. Biochem. , 27 : 729 – 736 .
- Moffatt , BA and Ashihara , H . 2002 . “ Purine and pyrimidine nucleotide synthesis and metabolism ” . In Arabidopsis Book 2nd Edition , Rockville, MD : American Society of Plant Biologists . Online publication. DOI/ 10.1199/tab.0018 http://aspb.org/publications/arabidopsis
- Itoh , S and Barber , SA . 1983 . Phosphorus uptake by six plant species as related to root hair . Agron. J. , 75 : 457 – 461 .
- Laplaze , L , Benkova , E Casimiro , I . 2007 . Cytokinins act directly on lateral root founder cells to inhibit root initiation . Plant Cell , 19 : 3889 – 3900 .
- Letham , DS . 1994 . “ Cytokinins as phytohormones-sites of biosynthesis, translocation and function of translocated cytokinin ” . In Cytokinins: Chemistry, Activity and Function , Edited by: Mok , DWS and Mok , MC . 57 – 80 . Boca Raton : CRC Press .
- Liao , MT , Fillery , IRP and Palta , JA . 2004 . Early vigorous growth is a major factor influencing nitrogen uptake in wheat . Funct Plant Biol. , 31 : 121 – 129 .
- Little , DY , Rao , H , Oliva , S , Daniel-Vedele , F , Krapp , A and Malamy , JE . 2005 . The putative high-affinity nitrate transporter NRT2.1 repress lateral root initiation in response to nutritional cues . Proc. Natl Acad. Sci. USA , 102 : 13693 – 13698 .
- Murashige , T and Skoog , F . 1962 . A revised medium for rapid growth and bioassays with tobacco tissue cultures . Physiol. Plant. , 15 : 473 – 497 .
- Nagao , K , Takeuchi , M , Miyazawa , Y , Sato , H , Yokota , HO and Kita , K . 2005 . Foliar application of fertilizer based on proline and inosine stimulates the second flush growth of Italian ryegrass (Lolium multiflorumLam.) . Grassl. Sci. , 51 : 269 – 270 .
- Negi , S , Ivanchenko , MG and Muday , GK . 2008 . Ethylene regulates lateral root formation and auxin transport in Arabidopsis thaliana . Plant J. , 55 : 175 – 187 .
- Newman , EI . 1966 . A method of estimating the total length of root in a sample . J. Appl. Ecol. , 3 : 139 – 145 .
- Remans , T , Nacry , P Pervent , M . 2006 . The ArabidopisisNRT1.1 transporter participates in the signaling pathway triggering root colonization of nitrate-rich patches . Proc. Natl Acad. Sci. USA , 103 : 19206 – 19211 .
- Santelia , D , Vincenzetti , V Azzarello , E . 2005 . MDR-like ABC transporter AtPGP4 is involved in auxin-mediated lateral root and root hair development . FEBS Lett. , 579 : 5399 – 5406 .
- Smet , ID , Signora , L , Beeckman , T , Inzé , D , Foyer , CH and Zhang , H . 2003 . An abscisic acid-sensitive checkpoint in lateral root development of Arabidopsis . Plant J. , 33 : 543 – 555 .
- Stasolla , C , Katahira , R , Thorpe , TA and Ashihara , H . 2003 . Purine and pyrimidine nucleotide metabolism in higher plant . J. Plant Physiol. , 160 : 1271 – 1295 .
- Sullivan , WM , Jiang , Z and Hull , RJ . 2000 . Root morphology and its relationship with nitrate uptake in Kentucky bluegrass . Crop Sci. , 40 : 765 – 772 .
- Taiz , L and Zeiger , E . 2006 . “ Assimilation of mineral nutrients ” . In Plant Physiology , Edited by: Taiz , L and Zeiger , E . 289 – 313 . Massachusetts : Sinauer Associates Inc, Publishers .
- Wang , H , Inukai , Y and Yamauchi , A . 2006 . Root development and nutrient uptake . Crit. Rev. Plant Sci. , 25 : 279 – 301 .
- Wirén , NV , Lauter , FR Ninnemann , O . 2000 . Differential regulation of three functional ammonium transporter genes by nitrogen in root hairs and by light in leaves of tomato . Plant J. , 21 : 167 – 175 .