Abstract
Soil is an important source of atmospheric carbon dioxide (CO2) and nitrous oxide (N2O). Studies of CO2 and N2O emissions from bare soil may explain annual changes in carbon (C) in soil organic matter (SOM) and help analyze N2O production from SOM. Therefore, CO2 and N2O emissions associated with the decomposition of SOM from bare soil are important factors for assessing the C budget and N2O emission in agricultural fields. We conducted a study over 7 years to assess the controlling factors of CO2 and N2O emissions from unplanted and unfertilized soil in Mikasa, Hokkaido, Japan. The CO2 flux increased in summer and there were significant positive correlations between the CO2 flux and soil temperature in the first 4 years. However, apparent relationships between CO2 flux and water-filled pore space, soil NH4 and NO3 concentrations were not observed. The slope of monthly CO2 emission against mean monthly temperature was positively correlated with monthly precipitation. These results suggest that the response of CO2 production to increases in soil temperature became more sensitive in wet soils. The average CO2 emission during the study period was 2.53 Mg C ha−1 year−1, and uncertainty in the annual CO2 emission was 24%. Annual precipitation explained the yearly variation (CO2 emission [Mg C ha−1 year−1] = 0.0021 × annual precipitation [mm year−1] −0.0499, R = 0.976, P < 0.001). Nitrous oxide flux increased from July to October and was positively correlated with CO2 flux. Based on the ratio of N2O-N : NO-N of fluxes, N2O appeared to be the main product of denitrification. The average N2O emission over the study period was 4.88 kg N ha−1 year−1, and uncertainty in the annual N2O emission was 58.5%. Strong relationships between the monthly emissions of CO2 and N2O suggest that N2O production by denitrification is strongly affected by SOM decomposition. Unlike CO2 emission, a relationship between N2O emission and precipitation was not observed because of the multiple pathways of nitrification and denitrification for N2O production induced by SOM decomposition.
Introduction
The contributions of atmospheric carbon dioxide (CO2) and nitrous oxide (N2O) to global warming are reported to be 60 and 6%, respectively. Since the Industrial Revolution, concentrations of these greenhouse gases in the atmosphere have increased at a rate of 1.4 p.p.m. year−1 and 0.8 p.p.b. year−1, respectively (CitationIntergovernmental Panel on Climate Change 2007). Carbon (C) emissions that have originated as a result of changes in land use from 1980 to 1989 contribute 24% of the global annual CO2 emission. Similarly, the emission of N2O originating from agricultural fields in 1989 contributed 24% of the total global N2O emission (CitationMosier et al. 1998). Recently, annual N2O emissions from fertilized cropland and grassland at a global level have been estimated to be 3.3 and 0.8 Tg N year−1, respectively (CitationStehfest and Bouwman 2006).
A number of studies have reported that there is a reduction in C from agricultural soils because microorganisms decompose soil organic matter (SOM) and emit CO2 (CitationHu et al. 2004; CitationKoga et al. 2006; CitationKoizumi et al. 1993; CitationMu et al. 2006, 2008; CitationShimizu et al. 2009). A reduction in C from soil has been estimated by the difference in soil C over 20 years in a 0–30 cm soil surface (CitationIntergovernmental Panel on Climate Change 2006; CitationPaustian et al. 1997). In recent years, the global warming potential (GWP) has been used to evaluate the effect of agricultural activities on global warming (CitationChu et al. 2007; CitationJones et al. 2006; CitationKoga et al. 2006; CitationMosier et al. 2005; CitationMu et al. 2006; CitationRobertson and Grace 2004; CitationSix et al. 2004). Therefore, it is necessary to estimate the annual reduction in soil C to evaluate the annual effect of agricultural activities on global warming. However, annual reductions in soil C are difficult to detect from investigations of annual change in the amount of soil C, because soil C is usually very large compared with C emission from the soil. In agricultural fields, decomposition of SOM can be measured as the CO2 emission from bare fields in which root respiration is excluded (CitationHanson et al. 2000; CitationHu et al. 2004; CitationMu et al. 2006, 2008; CitationShimizu et al. 2009; CitationSubke et al. 2006). As the decomposition of SOM results from microbial activities, it is influenced by chemical and physical conditions, such as soil temperature, water conditions and pH. As a result of spatial variability in these factors (CitationYanai et al. 2003), it is difficult to investigate the factors affecting the decomposition of SOM at a field level. Understanding the factors affecting the decomposition of SOM, quantifying the annual CO2 emission from bare fields and clarifying the factors affecting temporal variations in annual CO2 emissions from an unfertilized bare soil are needed to estimate C loss from soil. An understanding of these factors will provide beneficial information for the study of the effect of agricultural activities on C cycle or global warming over the long term for eco-balance or life cycle analyses (CitationKimura et al. 2007; CitationKoga et al. 2006).
Nitrous oxide is produced by nitrification and denitrification processes in the soil. Therefore, N2O emission is affected by the chemical and physical conditions of the soil (CitationBremner 1997; CitationColbourn and Dowdell 1984; CitationMosier et al. 1998; CitationStehfest and Bouwman 2006). Because ammonium (NH4 +) is used in the nitrification process and nitrate (NO3 −) is used in the denitrification process, N2O emission from agricultural fields is usually in proportion to the N application rate (CitationBouwman 1996). Based on this, methods of estimating N2O emissions from agricultural fields have been proposed (CitationIntergovernmental Panel on Climate Change 2006). In Tier 1 and 2 of the Intergovernmental Panel on Climate Change (IPCC) Guidelines for National Greenhouse Gas Inventories (CitationIntergovernmental Panel on Climate Change 2006), the emission factor (EF) is the ratio of N-induced N2O emission, which is the difference between N2O emissions from N-fertilized and unfertilized fields, divided by the amount of applied N fertilizer. However, to calculate original EF values, the N2O emission originating from soil organic N should be considered because N2O emission from the soil in agricultural fields is usually composed not only of N2O emissions originating from the applied N, but also of N2O emissions induced by decomposition of SOM. The N2O emission from bare fields is regarded as the emission originating from the soil organic N. Several studies have reported annual N2O emissions from unfertilized bare fields (CitationAkiyama et al. 2006; CitationClayton et al. 1997; Citationvan Groenigen et al. 2004; CitationKamp et al. 1998; CitationKoga et al. 2004; CitationZou et al. 2005). In addition, temporal variation in N2O emission from agricultural fields has been reported in several studies (CitationDrury et al. 2006; CitationKusa et al. 2002; CitationTakakai et al. 2006; CitationZou et al. 2005). These studies, however, have not focused on the factors that affect N2O flux or annual N2O emission from SOM. Very few studies have focused on analyzing the mechanisms of N2O production and emission induced by SOM decomposition in agricultural bare fields over the long term. Knowledge about the controlling factors of N2O flux and annual emissions will be useful for long-term studies examining the effect of agricultural activities on the C cycle or global warming.
The objective of the present study was to clarify the controlling factors of CO2 and N2O emissions from agricultural bare soil based on 7 years of monitoring in central Hokkaido, Japan.
Materials and methods
Site description
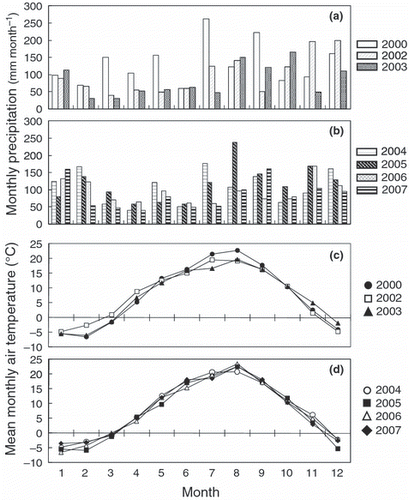
The present study was conducted in Mikasa, central Hokkaido, Japan (43°14.4′N, 141°50′E). The soil type is Gray Lowland soil (Gleysol; FAO/UNESCO). The mean annual temperature at the study site is 7.5°C and the mean annual precipitation is 1,164 mm, of which 32% is in the form of snow. The soil does not freeze during winter. The temperature usually increases in August and precipitation increases from July to October (). At a depth of 0–10 cm, the soil pH (H2O) was 5.8 and the cation exchange capacity was 25.5 cmolc kg−1. The soil was comprised of sand (12.1%), silt (51.2%) and clay (36.7%). The concentrations of soil C and N were 32.1 and 2.8 g kg−1, respectively (CitationToma and Hatano 2007) and the C:N ratio was 11.5 at a depth of 0–10 cm in 1996. Soil C density was recorded as 108 Mg C ha−1 at a depth of 0–30 cm.
Experimental design
We set up an unfertilized bare plot (5 m × 8 m) inside a 2-ha onion field owned by a farmer in Mikasa in 1999 and conducted monitoring in 2000, from 2002 to 2005, and in 2007. In 2006, the unfertilized bare plot was shifted to another location in the same field by the farmer owing to unavoidable circumstances. Conventional management practices for onion cultivation were carried out on the studied plot every year, although chemical and organic fertilizers or residues were not applied. Most weeds were removed from the monitoring plot regularly. Onion is usually cultivated from May to September in Mikasa. The soil is usually tilled before fertilization and plowed to a depth of 15 cm at the end of April. Onion roots are cut for harvest from mid to late August. The onion harvest is followed by plowing, which is carried out to incorporate the residues into the soil from the end of September to early October.
Measurement of CO2, N2O and NO fluxes
Fluxes of CO2, N2O and NO were measured using a closed-chamber method (three or four replicates) using two types of cylindrical stainless steel chambers from 10.00 to 14.00 hours. In 2000, chambers measuring 30 cm in diameter and 35 cm in height were used (CitationKusa et al. 2002, 2006); chambers measuring 20 cm in diameter and 25 cm in height were used from 2002 to 2007. The cover of the chamber was made of acryl and was equipped with a sample collector, a pressure regulating bag and a Tedlar bag (0.5 L). In 2000 and from 2002 to 2004, we inserted the chambers directly into the soil to a depth of 2 cm and began taking measurements after 15 min. However, in 2005, 2006 and 2007, we used a chamber-base made of stainless steel with a diameter of 20 cm. The upper part of the chamber-base had a slight depression, which was filled with water to seal it during the measurement (CitationToma and Hatano 2007; CitationToma et al. 2007). The base was kept on the ground, except during plowing and was reset 1 day before the next sampling.
Gas samples were taken at time 0 min from inside the chamber and 6 min (for CO2) or 15 min (for N2O and NO) after closing the chamber (CitationNakano et al. 2004; CitationToma and Hatano 2007). Using a 25 mL syringe, 10 gas samples were taken (total volume of 250 mL) and each gas sample was injected into a Tedlar bag (0.5 L). The bags were then taken to the laboratory and 20 mL of each gas sample was immediately transferred into glass vials (10 mL). From the samples in the bags, CO2 and NO were analyzed using an Infrared CO2 Analyzer (Model ZFP5YA3I; Fuji Electric, Tokyo, Japan) and a Chemoluminescence Nitrogen Oxide Analyzer (Model 265P; Kimoto Electric, Osaka, Japan), respectively. From the samples in the vials, N2O was analyzed with an ECD Gas Chromatograph (Model GC-14B; Shimadzu, Kyoto, Japan).
The gas fluxes were calculated using the following equation:
A positive value of F indicates gas emission from the soil to the atmosphere and a negative value indicates gas uptake by the soil from the atmosphere. Considering the precision of the machinery, N2O flux values ranging from −6 to 6 μg N m−2 h−1, CO2 flux values from −3.7 to 3.7 mg C m−1 h−1 and NO flux values from −0.2 to 0.2 μg N m−2 h−1 were regarded as 0 μg N or C m−2 h−1. Annual CO2 and N2O emissions were calculated assuming linear changes between two sampling occasions.
The value of Q10, which is a relative increase in CO2 flux for a 10°C change in soil temperature, was calculated based on the Arrhenius equation and the following equation (CitationHu et al. 2004):
Measurement of other variables and the frequency of sampling
Soil temperature at a depth of 5 cm was measured five times at the same time as the CO2, N2O and NO fluxes were measured. Five replicate disturbed soil samples (0–5 cm depth) were taken and combined to form one composite sample. Soil solutions were extracted using distilled water throughout the study period and NH4 +-N, NO3 −-N and water-soluble organic carbon (WSOC) concentrations were analyzed by colorimetry with indophenol-blue, ion chromatography (QIC Analyzer, Dionex, Osaka, Japan) and a total organic carbon analyzer (Model TOC-5000A; Shimadzu), respectively. In 2007 the soil NH4 +-N concentration extracted by distilled water was not measured, and in 2006 and 2007 the soil NH4 +-N concentration extracted with 2 mol L–1 KCl was analyzed. The WSOC was not measured in 2000. Three replicate undisturbed soil samples were collected using a steel cylinder (100 mL) and the water-filled pore space (WFPS) was measured. Daily or annual meteorological data were obtained from the local Iwamizawa Weather Station (43°12.6′N, 141°47.3′E).
After plowing, all samples were collected three times per week from May to June, three times per month from July to August and once or twice per month during the snow-cover season. During the period from snowmelt to onion cultivation and from mid October to snow cover, sampling was conducted two or three times per month.
Statistical analyses
Relationships between CO2 and N2O fluxes and soil physical and chemical properties, or annual CO2 and N2O emissions and mean annual air temperature or annual precipitation were analyzed using linear or quadratic curve regressions. Variations in annual CO2 or N2O emissions during the study period were evaluated using Tukey–Kramer tests. A least significant difference test was used to determine significant differences (P < 0.05).
Uncertainties were calculated using the following equation:
The two-sided 95% confidence intervals of the CO2 and N2O emissions were calculated using the following equation:
Results
Seasonal variation in CO2 and N2O fluxes
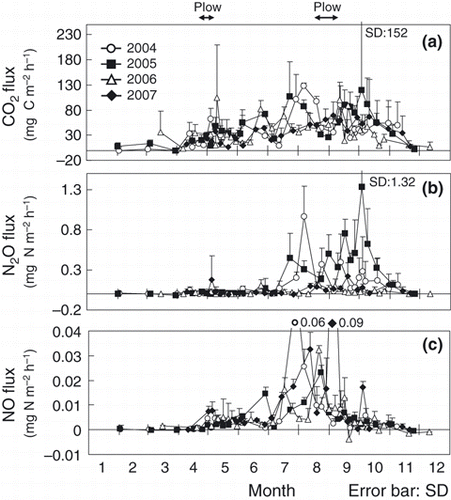
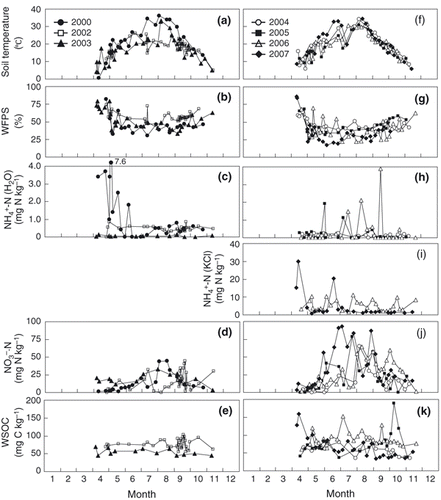
The CO2 flux peaked from August to October (, ), whereas the N2O flux increased from the end of July to October in all years (,). Soil temperature increased in August and this trend was similar to that of air temperature (). The WFPS was high in early April and November, and low in August (). From August to October, the WFPS was as low as approximately 50%. Soil NH4 +-N concentrations extracted by distilled water ranged from 0 to 7.6 mg N kg−1 each year and did not exhibit any consistent seasonal pattern (). Although the NH4 +-N concentration extracted with 2 mol L–1 KCl in 2006 and 2007 did not show a distinct seasonal pattern (), the range of concentrations (1.67–11.3 mg N kg−1 in 2006, 0.84–29.9 mg N kg−1) was higher than the values obtained through extraction with water. Soil NO3 −-N concentrations increased from August to September each year (). Fluctuations in soil WSOC concentrations were small throughout the year ().
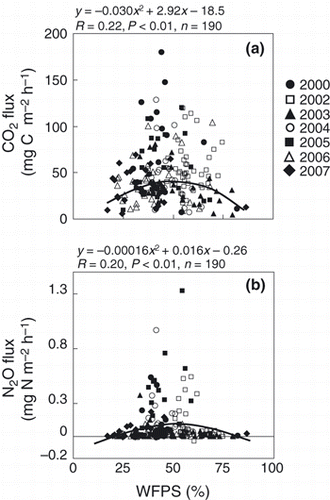
Correlation coefficients between the CO2 or N2O fluxes and soil physical and chemical properties are given in . The CO2 flux was significantly and positively correlated with soil temperature from 2000 to 2004 (), and reached its maximum at 48.8% of WFPS (). Although the N2O flux was significantly and positively correlated with soil temperature in 2000 and 2004, N2O flux was significantly and positively correlated with CO2 flux from 2000 to 2006. Carbon dioxide and N2O fluxes were significantly correlated with soil NO3 −-N concentration, and N2O flux was significantly correlated with soil temperature in 2000 and 2004. However, no significant correlation was observed between CO2 or N2O fluxes and soil chemical or physical properties over the study period. There were no significant correlations between N2O flux and WFPS over the study period except for 2003, but N2O showed high fluxes when WFPS ranged from 30 to 60% and reached its maximum at 48.4% ().
Figure 2 Seasonal variations in (a) CO2, (b) N2O and (c) NO fluxes in 2000, 2002 and 2003. Arrows indicate the timing of plowing. sd, standard deviation.
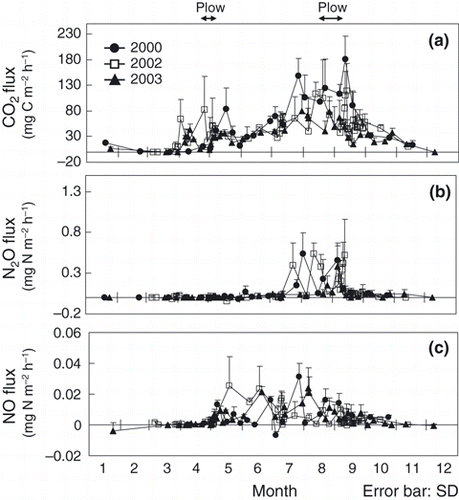
Figure 3 Seasonal variations in (a) CO2, (b) N2O and (c) NO fluxes from 2004 to 2007. Arrows indicate the timing of plowing. sd, standard deviation.
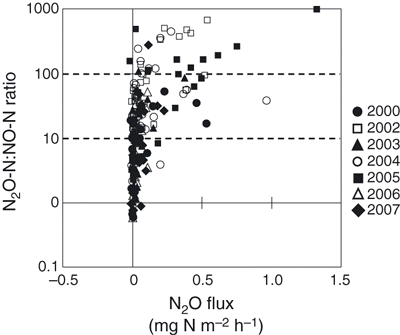
The relationship between N2O flux and the N2O-N:NO-N ratio is given in . The N2O flux increased with an increase in the N2O-N:NO-N ratio, with 18 out of 49 samples of N2O flux over 0.079 mg N m−2 h−1, which resulted in a ratio of N2O-N:NO-N over 100. The average N2O flux was 0.079 mg N m−2 h−1. Therefore, approximately 37% of the N2O fluxes were higher than the average value.
Cumulative CO2 and N2O emissions
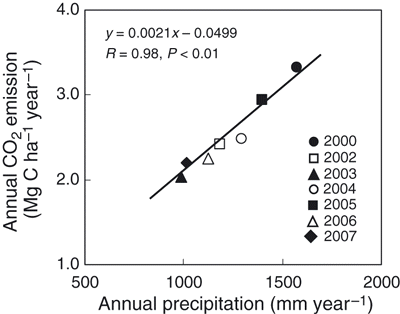
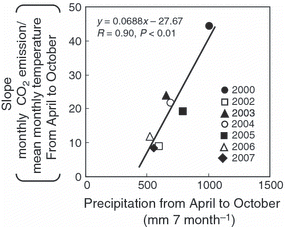
shows the annual CO2 and N2O emissions and Q10 values. Annual CO2 emissions ranged from 2.04 to 3.32 Mg C ha−1 year−1 and were significantly correlated with annual precipitation (; y = 0.0021x −0.0499, R = 0.98, P < 0.01). The average CO2 emission during the study period was 2.53 Mg C ha−1 year−1 and uncertainty in the annual CO2 emission was 24%. The Q10 values ranged from 1.11 to 2.38. There was no significant correlation between the Q10 value and annual precipitation (y = 0.0012x + 0.1918, R = 0.56, P = 0.11). However, the slope of the monthly CO2 emission against mean monthly temperature from April to October was significantly correlated with precipitation from April to October (; y = 0.0688x − 27.67, R = 0.90, P < 0.01). Carbon dioxide flux in the present study was measured only during the daytime, and annual CO2 emission was calculated assuming linear changes between two sampling occasions. That is, the decrease in CO2 flux during the night, when the temperature decreased, was not considered when calculating the annual CO2 emission from 2000 to 2004. The difference in the maximum and minimum air temperatures from July to September, which was the period of high air temperature, was less than 10°C (8.8°C). In addition, the average Q10 value from 2000 to 2004 was 1.99 (). If we assume that the CO2 flux during the maximum daily air temperature is 1.99-fold higher than the CO2 flux during the minimum daily air temperature, the measured annual CO2 emission would be 1.33-fold higher than the annual CO2 emission that was calculated by considering daily change in the CO2 flux.
Figure 4 Seasonal variations in (a,f) soil temperature, (b,g) water-filled pore space (WFPS), (c,h,i) soil NH4+-N, (d,j) NO3−-N and (e,k) water-soluble organic carbon (WSOC) concentrations.
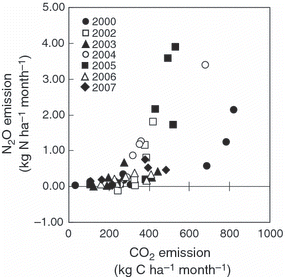
Annual N2O emission ranged from 1.62 to 12.1 kg N ha−1 year−1. The average N2O emission during the study period was 4.88 kg N ha−1 year−1 and uncertainty in the annual N2O emission was 58.5%. The uncertainties in yearly variation in CO2 and N2O emissions were 17.9 and 76.1%, respectively. Although correlations between N2O emission and annual air temperature or precipitation were not significant, N2O emission increased with an increase in CO2 emission (). Significant differences in annual CO2 and N2O emissions among the study years indicate yearly variation in CO2 and N2O emissions at the study site ().
Discussion
Key factors determining the CO2 flux in bare soil
Several studies have reported that CO2 flux from soil shows an exponential increase with an increase in soil temperature (CitationBoone et al. 1998; CitationHu et al. 2001; CitationJones et al. 2006; CitationSchindlbacher et al. 2009). In the present study, there were significant positive correlations between CO2 flux and soil temperature (in 2000 and in 2002–2004) (). In contrast, there was no significant correlation between CO2 flux and WFPS, except in 2003 (). CitationGulledge and Schimel (1998) reported that microbial respiration was directly proportional to the water-holding capacity (10–60%). Moreover, CitationLinn and Doran (1984) and CitationGulledge and Schimel (1998) reported that the maximum CO2 production by microorganisms was at 60% WFPS. In our study, CO2 flux peaked at 49.1% of WFPS. Therefore, soil moisture might not influence the production or emission of CO2 linearly, and there might be an appropriate condition of soil moisture for CO2 production in relation to soil ventilation. In contrast, significant positive correlations between annual CO2 emission and annual precipitation and between the slope of monthly CO2 emission against mean monthly temperature from April to October and precipitation from April to October suggested that the production of CO2 was greater in wet conditions even if temperatures were similar (, ). Therefore, CO2 production increased with an increase in soil temperature, but CO2 production at the same temperature was enhanced in wet conditions. High moisture content prevents soil gas from diffusing from the soil to the atmosphere. This means that even if CO2 was produced in the soil under appropriate conditions for CO2 production, CO2 might not necessarily be diffused at that time. There could be possibilities of high CO2 flux to be detected during the season of high precipitation. Differences in the timing between CO2 production and diffusion from the soil to the atmosphere could possibly be explained by the lack of correlation between CO2 flux and WFPS and by a significant positive correlation between annual CO2 emission and precipitation (; ).
Table 1 Correlation coefficients for the relationships between CO2 or N2O fluxes and soil physical and chemical properties
Table 2 Annual CO2 and N2O emissions from unfertilized bare soil in an onion field in Mikasa
Key factors determining N2O flux from bare soil
Because N2O is mainly produced by the processes of nitrification and denitrification in soil (CitationTiedje 1994), N2O flux is often affected by soil NH4 +-N or NO3 −-N concentrations (CitationLivesley et al. 2009; CitationThornton and Valente 1996; CitationToma et al. 2007). However, no apparent correlations between N2O flux and soil NH4 +-N or NO3 −-N concentrations were observed in our study. Instead, we observed significant positive correlations between N2O and CO2 fluxes in all years, except 2007 (). Similar positive correlations between N2O and CO2 fluxes were observed when crop residues were mixed with surface soil (CitationHuang et al. 2004; CitationToma and Hatano 2007). In the present study, the substrates for nitrification and denitrification originated from SOM because no fertilizer was applied in the experimental plot, suggesting that N2O production was strongly related to SOM decomposition. Therefore, one of the factors controlling the production of N2O could be the supply of C and inorganic N from SOM decomposition. A number of studies have reported that soil temperature is one factor that affects N2O production (CitationKamp et al. 1998; CitationMori et al. 2005; CitationSmith et al. 1998; CitationTokuda and Hayatsu 2004). As the CO2 flux increased with an increase in soil temperature in our study (), soil temperature might affect not only the activity of N2O production, but also the supply of substrates for N2O production. CitationBouwman (1990) summarized the results presented by CitationAnderson and Levine (1986) and CitationLipschultz et al. (1981) and reported that a N2O-N:NO-N ratio below 1.0 indicates that N2O was produced in the soil mainly by nitrification, whereas a value above 100 indicates that denitrification was the dominant process for N2O production. In our study, approximately 37% of the N2O fluxes that were higher than the average value occurred when the N2O-N:NO-N ratio was above 100. In general, an increase in soil moisture contributes to N2O production by denitrification (CitationLinn and Doran 1984). Studies have shown that a WFPS of 50–60% is suitable for the decomposition of soil organic matter and that these conditions accelerate denitrification as a result of the consumption of oxygen (CitationMaag and Vinther 1999). Moreover, N2O is mainly produced by denitrification when the WFPS is 50–60% (CitationDavidson et al. 2000). In our study, the WFPS increased from approximately 40 to 60% from the end of July to early October, and the N2O flux was highest when the WFPS was at 46.5% (,). CitationSawamoto and Hatano (2000) reported that an increase in N2O production in autumn might be caused by nitrate transportation and diffusion inside soil aggregates after rainfall and by the development of denitrification areas inside the aggregates of well-structured soil. Therefore, there is a possibility of aerobic and anaerobic conditions occurring simultaneously inside the aggregates and inter-aggregate pores in the well-structured soil examined in the present study. This would suggest that the high N2O fluxes were induced not only by denitrification, but also by nitrification. Nitrous oxide was the main product of the denitrification process in the present study.
Nitrous oxide fluxes were significantly correlated with the CO2 flux, and production of CO2 was affected by soil temperature and precipitation. However, a significant correlation between N2O flux or annual N2O emission and WFPS or annual precipitation was not recorded. This might be because N2O production in soil is a complex process. In the present study, the source of inorganic N might be mainly SOM. Therefore, NH4 and NO3, which are substrates for nitrification and denitrification, could have been produced by mineralization and nitrification processes in the soil. In a study of N2O emission from organic fertilizer, similar correlations between N2O and CO2 emission were observed (CitationHayakawa et al. 2009). When N originates from organic matter, decomposition of the organic matter is probably essential for the production of N2O. In any case, significant correlations between CO2 flux and N2O flux or monthly emissions of CO2 and N2O suggest that climate change, which will affect SOM decomposition, might greatly affect N2O emission induced by SOM decomposition.
Annual CO2 emission and its spatial and temporal variations
The annual CO2 emission associated with decomposition of SOM ranged from 2.04 to 3.32 Mg C ha−1 year−1 (). The CO2 emission values from 2000 to 2004, however, might have been overestimated by approximately 1.33-fold because daily variation in CO2 flux was not considered. Therefore, if we consider that the CO2 emission was overestimated then annual CO2 emission ranged from 1.11 to 2.95 Mg C ha−1 year−1. CitationMu et al. (2006) reported that CO2 emitted from SOM in bare treatments under several types of land use (e.g. wheat, maize, onion) ranged from 3.01 to 5.68 Mg C ha−1 year−1 in Hokkaido, Japan. CitationJacinthe et al. (2002) reported that 4.37 Mg C ha−1 year−1 of CO2 was emitted from a bare plot in Ohio, USA. Decomposition of SOM in these two studies might have been overestimated similar to our study because they used the trapezoidal rule to calculate annual CO2 emission. CitationKoizumi et al. (1993) reported that heterotrophic respiration ranged from 7.16 to 10.5 Mg C ha−1 year−1 in three double-cropping agro-ecosystems (rice–barley, peanut–wheat and dentcorn–italian ryegrass). They calculated SOM decomposition as the difference between total soil respiration and plant respiration by calculating soil and root respirations using continuous data of soil temperature and correlations between soil temperature and soil or root respirations. CitationKoga et al. (2006) reported average SOM decomposition over a 20-year time scale from 1981 to be 1.04 to 1.34 Mg C ha−1year−1 from the difference in total soil C within a depth of 20 cm in Hokkaido, Japan. The decomposition rate of SOM reported by Koizumi et al. (1994) and CitationKoga et al. (2006) might not have been overestimated, and their values were larger and smaller than the values in our study, respectively. Differences in SOM decomposition may result from variations in location, cultivation system and soil type at the study site. The mean annual temperature (13.1°C) of the study site reported by Koizumi et al. (1994) was higher than that at our study site. In addition, there were frequent tillage operations because of a double-cropping system. High temperature and frequency of plowing may have increased SOM decomposition in the study reported by Koizumi et al. (1994). In contrast, the study site of CitationKoga et al. (2006) was located at a similar latitude (42°53′N) to our study. Therefore, differences in SOM decomposition between our study and the study conducted by CitationKoga et al. (2006) might result from differences in crop management tillage and/or soil type. CitationMu et al. (2008) reported that CO2 emission from bare soil reached its maximum when the silt and clay content of the soil was 60–70%. Moreover, CitationBellamy et al. (2005) reported that the amount of C over 25 years decreased with an increase in soil C content. In these studies, however, Andisols were not included. Further study examining differences in SOM decomposition in various soil types is required.
Carbon dioxide emission was measured at the same location at our study site from 2000 to 2005. Over this period the average annual CO2 emission was 2.84 Mg C ha−1 year−1, and 17 Mg C ha−1 was estimated to be released from the soil in 2000–2005. Because the mass of C in the surface (0–30cm) soil was 108 Mg C ha−1, a C loss of 17 Mg C ha−1 corresponds to 15.7% of the surface soil C. We did not measure changes in soil C storage over the study period so the actual reduction in soil C could not be determined in the present study. If changes in soil C were measured in our study then the soil C mass could possibly have been detected. CitationBellamy et al. (2005), however, reported that loss of soil C was not detectable over 10 years when the soil C content was lower than 50 g kg−1. Detecting changes in soil C at our study site might be difficult. There are several ways for C input in agricultural fields, including rainfall, weeds and algae. However, very little weed or algae existed during the study period. Carbon input from rainfall was not included in the IPCC guidelines for calculating soil C change (CitationIntergovernmental Panel on Climate Change 2006). Thus, there might be no major C inflow that supplemented soil C loss by SOM decomposition. Further study is required to match the reduction in soil C and CO2 emission from unfertilized bare soil.
Large variation in CO2 fluxes and annual CO2 emissions among replicates was observed owing to spatial variation in the CO2 flux (,; ). Average uncertainty in annual CO2 emission (24%) was higher than of the uncertainty in annual CO2 emission (17.9%). In general, the decomposition of SOM is affected by soil properties (CitationKoizumi et al. 1993; CitationMu et al. 2008), method of cultivation (CitationKoga et al. 2006) or land-use practices (CitationMu et al. 2006). CitationMu et al. (2008) reported a significant correlation between CO2 emission and clay and silt content in a bare field in the same district as our study site. In the study field, spatial variations in total C content, the soil C:N ratio and microbial biomass C have been reported (CitationYanai et al. 2003). These factors possibly cause large uncertainty in the annual CO2 emission. Using a larger chamber or taking more CO2 flux measurements might be required to improve the accuracy of annual CO2 emission values.
Significant differences in annual CO2 emissions from the bare field during the study period show that there is yearly variation in annual CO2 emission that is induced by SOM decomposition (). Large uncertainly (17.9%) in the average annual CO2 emission during the study period shows that continuous monitoring of CO2 emission is required to determine a representative value of annual CO2 emission. This yearly variation in annual CO2 emission suggests that climate will have a great impact on yearly CO2 emission from SOM. CitationRaich and Schlesinger (1992) reported that soil respiration, including root respiration, increased with an increase in annual precipitation or mean annual air temperature. Our study also showed that annual precipitation affects CO2 emission from mineralization of SOM.
Previous studies have reported that the effect of chemical fertilizer application on microbial respiration is negligible (CitationGinting et al. 2003; CitationHu et al. 2004; CitationJacinthe et al. 2002). Hence, CO2 emissions obtained in the present study could be an approximation of SOM decomposition in a fertilized field. Several studies have reported that soil C decreases in agricultural fields with low levels of organic matter application (CitationGinting et al. 2003; CitationHu et al. 2004; CitationJacinthe et al. 2002; CitationMu et al. 2008; CitationShimizu et al. 2009). The loss of soil C is reported to have a greater influence on global warming than the emissions of N2O or CH4 (CitationJones et al. 2006; CitationKoga et al. 2006; CitationMosier et al. 2005; CitationMu et al. 2006). Although it is difficult to calculate an annual loss of C by measuring soil C reduction, it could be done by measuring CO2 emission from bare soil in an agricultural field. Assessing CO2 emissions from bare fields, using the correlation between CO2 emission and available environmental factors such as meteorological data or soil type, will be useful for cultivation management or planning of farm activities at a regional scale to mitigate C loss from soil.
Annual N2O emission and its spatial and temporal variations
Annual N2O emission in the present study ranged from 1.62 to 12.1 kg N ha−1 year−1 (average 4.88 kg N ha−1year−1). These values are higher than most reported values for bare fields (1.00 kg N ha−1 year−1 [CitationIntergovernmental Panel on Climate Change 2006], −0.02–0.13 kg N ha−1year−1 [CitationKoga et al. 2004], 0.14–1.52 kg N ha−1 year−1 [Citationvan Groenigen et al. 2004] or 0.36–0.14 kg N ha−1year−1 [CitationAkiyama et al. 2006], 4.80 kg N ha−1 year−1 [CitationKamp et al. 1998] or 4.25 kg N ha−1 year−1 [CitationZou et al. 2005]). This difference in annual N2O emission might result from soil physical and chemical properties. The N content in the soil may affect N2O production because there is no N source for a substrate of N2O in bare soil except for the N in SOM. CitationKlemedtsson et al. (2005) reported a significant negative relationship between N2O emission and soil C:N ratio in forested peat soil in Europe, indicating that N2O emission increased with an increase in relative N to C in peat. Nitrogen contents of the soils in the study sites of CitationKamp et al. (1998) (1.7 g kg−1) and CitationZou et al. (2005) (1.1 g kg−1), in which N2O emissions exceeded 4.0 kg N ha−1, were lower than the value recorded at our study site (2.8 g kg−1). Production of N2O in poorly drained soil increases as a result of anaerobic conditions in the soil (Citationvan Groenigen et al. 2004). The N2O emission reported by CitationKoga et al. (2004) was lower than the value recorded here even when the N content at their study site (2.4–3.1 g kg−1) was similar to that at our site. This could possibly be because of the drainage quality of the soil. The soil type at the study site of CitationKoga et al. (2004) was a well-drained volcanic ash soil. Therefore, N concentration as a source of substrate for N2O and drainage conditions, which might control the aerobic and anaerobic conditions of the soil, might be an indicator of the potential N2O source from unfertilized bare fields.
Large variations in flux and annual emission of both N2O and CO2 were observed in our study (,; ). There is considerable uncertainty in the amount of N2O emission from unfertilized bare fields (CitationAkiyama et al. 2006; CitationZou et al. 2005). Both CO2 and N2O emissions are influenced by soil properties (Citationvan Groenigen et al. 2004) and cultivation methods (CitationKoga et al. 2004). At our study site, high spatial variability (CV 217%) in the N2O flux has been reported (CitationYanai et al. 2003). CitationYanai et al. (2003) also reported that soil organic matter and soil pH were the main soil-related determining factors for N2O flux.
Temporal variation in N2O was confirmed to be similar to that recorded in a number of previous studies (CitationDrury et al. 2006; CitationKusa et al. 2002; CitationTakakai et al. 2006; CitationZou et al. 2005). In our study, neither N fertilizer nor crop residue was applied to the experimental plot from 1999 to 2005. Therefore, it is expected that the substrate required for the production of N2O had been reduced. However, annual N2O emission did not consistently decrease from 2000 to 2005, resulting in large uncertainty in annual N2O emission (). This suggests that the effect of climatic factors on N2O emission might be greater than the reduction in substrate required for N2O production. Annual N2O emission tended to increase with an increase in annual precipitation. The relationship between annual N2O emission and annual precipitation was not significant, whereas annual CO2 emission was significantly correlated with annual precipitation (); this may have occurred because there is a difference in the environmental factors that are required for mineralization, nitrification and denitrification. In mineralization and nitrification processes, oxygen is required for oxidation of organic C or NH4. However, anaerobic conditions are required for denitrification because NO3 is used for electron capture (CitationBouwman et al. 2001). As N2O was produced through these three biological processes in the soil, a correlation between annual N2O emission and annual precipitation may not be significant.
Acknowledgments
This study was financially supported by the Global Environmental Research Program of the Ministry of the Environment of Japan (No. S-2). The authors thank Takeshi Morimoto for allowing us to conduct the study on his farm.
References
- Akiyama , H , Yan , XY and Yagi , K . 2006 . Estimations of emission factors for fertilizer-induced direct N2O emissions from agricultural soils in Japan: summary of available data . Soil Sci. Plant Nutr. , 52 : 774 – 787 .
- Anderson , IC and Levine , J . 1986 . Relative rates of nitric oxide and nitrous oxide productio by nitrifiers, denitrifiers, and nitrate respirers . Appl. Environ. Microbiol. , 51 : 938 – 945 .
- Bellamy , PH , Loveland , PJ , lan Bradley , R , Lark , RM and Kirk , GJD . 2005 . Carbon losses from all soils across England and Wales 1978-2003 . Nature , 437 : 245 – 248 .
- Boone , RD , Nadelhoffer , KJ , Canary , JD and Kaye , JP . 1998 . Roots exert a strong influence on the temperature sensitivity of soil respiration . Nature , 396 : 570 – 572 .
- Bouwman , AF . 1990 . “ Nitric oxide and nitrogen dioxide ” . In Soils and the Greenhouse Gas Effect , Edited by: Bouwman , AF . 120 – 124 . Great Britain : John Wiley & Sons .
- Bouwman , AF . 1996 . Direct emission of nitrous oxide from agricultural soils . Nutr. Cycl. Agroecosyst. , 46 : 53 – 70 .
- Bouwman , A , Boumans , LJM and Batjes , NH . 2001 . Global estimates of gaseous emissions of NH3 , Rome, , Italy : NO and N2O from agricultural land, Food and agriculture organization of the unites nations .
- Bremner , JM . 1997 . Sources of nitrous oxide in soils . Nutr. Cycl. Agroecosyst. , 49 : 7 – 16 .
- Chu , H , Hosen , Y and Yagi , K . 2007 . NO, N2O, CH4and CO2fluxes in winter barley field of Japanese Andisol as affected by N fertilizer management . Soil Biol. Biochem. , 39 : 330 – 339 .
- Clayton , H , McTaggart , IP , Parker , J , Swan , L and Smith , KA . 1997 . Nitrous oxide emissions from fertilised grassland: a 2-year study of the effects of N fertiliser form and environmental conditions . Biol. Fertil. Soils , 25 : 252 – 260 .
- Colbourn , P and Dowdell , RJ . 1984 . Denitrification in field soils . Plant Soil , 76 : 213 – 226 .
- Davidson , EA , Keller , M , Erickson , HE , Verchot , LV and Veldkamp , E . 2000 . Testing a conceptual model of soil emissions of nitrous and nitric oxides . Bioscience , 50 : 667 – 680 .
- Drury , CF , Reynolds , WD , Tan , CS , Welacky , TW , Calder , W and McLaughlin , NB . 2006 . Emissions of nitrous oxide and carbon dioxide: influence of tillage type and nitrogen placement depth . Soil Sci. Soc. Am. J. , 70 : 570 – 581 .
- Ginting , D , Kessavalou , A , Eghball , B and Doran , JW . 2003 . Greenhouse gas emissions and soil indicators four years after manure and compost applications . J. Environ. Qual. , 32 : 23 – 32 .
- van Groenigen , JW , Kasper , GJ , Velthof , GL , van den Pol-van Dasselaar , A and Kuikman , PJ . 2004 . Nitrous oxide emissions from silage maize fields under different mineral nitrogen fertilizer and slurry applications . Plant Soil , 263 : 101 – 111 .
- Gulledge , J and Schimel , JP . 1998 . Moisture control over atmospheric CH4consumption and CO2production in diverse Alaskan soils . Soil Biol. Biochem. , 30 : 1127 – 1132 .
- Hanson , PJ , Edwards , NT , Garten , CT and Andrews , JA . 2000 . Separating root and soil microbial contributions to soil respiration: a review of methods and observations . Biogeochemistry , 48 : 115 – 146 .
- Hayakawa , A , Akiyama , H , Sudo , S and Yagi , K . 2009 . N2O and NO emissions from an Andisol field as influenced by pelleted poultry manure . Soil Biol. Biochem. , 41 : 521 – 529 .
- Hu , R , Hatano , R , Kusa , K and Sawamoto , T . 2004 . Soil respiration and net ecosystem production in an onion field in central Hokkaido, Japan . Soil Sci. Plant Nutr. , 50 : 27 – 33 .
- Hu , RG , Kusa , K and Hatano , R . 2001 . Soil respiration and methane flux in adjacent forest, grassland, and cornfield soils in Hokkaido, Japan . Soil Sci. Plant Nutr. , 47 : 621 – 627 .
- Huang , Y , Zou , J , Zheng , X , Wang , Y and Xu , X . 2004 . Nitrous oxide emissions as influenced by amendment of plant residues with different C:N ratio . Soil Biol. Biochem. , 36 : 973 – 981 .
- Intergovernmental Panel on Climate Change . 2006 . “ IPCC Guidelines for National Greenhouse Gas Inventories, Vol. 4. Agriculture, Forestry and Other Land Use, N2O emission from managed soils, and CO2 emission from lime and urea application ” . Vol. 4 , The Institute for Global Environmental Strategies (IGES) fro the IPCC . http://www.ipcc-nggip.iges.or.jp/public/2006gl/vol4.html
- Intergovernmental Panel on Climate Change . 2007 . “ IPCC Forth Assessment Report: climate Change 2007 ” . the Physical Science Basis, Technical Summaryhttp://www.ipcc.ch/publications_and_data/publications_ipcc_fourth_assessment_report_wg1_report_the_physical_science_basis.htm
- Jacinthe , PA , Lal , R and Kimble , JM . 2002 . Carbon budget and seasonal carbon dioxide emission from a central Ohio Luvisol as influenced by wheat residue amendment . Soil. Till. Res. , 67 : 147 – 157 .
- Jones , SK , Rees , RM , Kosmas , D , Ball , BC and Skiba , UM . 2006 . Carbon sequestration in a temperate grassland; management and climatic controls . Soil Use Manag. , 22 : 132 – 142 .
- Kamp , T , Steindl , H , Hantschel , RE , Beese , F and Munch , JC . 1998 . Nitrous oxide emissions from a fallow and wheat field as affected by increased soil temperatures . Biol. Fertil. Soils , 27 : 307 – 314 .
- Kimura , SD , Mu , ZJ , Toma , Y and Hatano , R . 2007 . Eco-balance analysis of six agricultural land uses in the Ikushunbetsu watershed . Soil Sci. Plant Nutr. , 53 : 373 – 386 .
- Klemedtsson , L , Arnold , KV , Weslien , P and Gundersen , P . 2005 . Soil CN ratio as a scalar parameter to predict nitrous oxide emissions . Glob. Change Biol. , 11 : 1142 – 1147 .
- Koga , N , Sawamoto , T and Tsuruta , H . 2006 . Life cycle inventory-based analysis of greenhouse gas emissions from arable land farming systems in Hokkaido, northern Japan . Soil Sci. Plant Nutr. , 52 : 564 – 574 .
- Koga , N , Tsuruta , H , Sawamoto , T , Nishimura , S and Yagi , K . 2004 . N2O emission and CH4uptake in arable fields managed under conventional and reduced tillage cropping systems in northern Japan . Glob. Biogeochem. Cycl. , 18 : GB4025 doi http://dx.doi.org/10.1029/2004GB002260 10.1029/2004GB002260
- Koizumi , H , Usami , Y and Satoh , M . 1993 . Carbon dynamics and budgets in 3 upland double-cropping agro-ecosystem in Japan . Agric. Ecosyst. Environ. , 43 : 235 – 244 .
- Kusa , K , Hu , RG , Sawamoto , T and Hatano , R . 2006 . Three years of nitrous oxide and nitric oxide emissions from silandic andosols cultivated with maize in Hokkaido, Japan . Soil Sci. Plant Nutr. , 52 : 103 – 113 .
- Kusa , K , Sawamoto , T and Hatano , R . 2002 . Nitrous oxide emissions for 6 years from a gray lowland soil cultivated with onions in Hokkaido, Japan . Nutr. Cycl. Agroecosyst. , 63 : 239 – 247 .
- Linn , DM and Doran , JW . 1984 . Effect of water-filled pore-space on carbon-dioxide and nitrous-oxide production in tilled and nontilled soils . Soil Sci. Soc. Am. J. , 48 : 1267 – 1272 .
- Lipschultz , F , Zafiriou , OC , Wofsy , SC , McElroy , MB , Valois , FW and Watson , SW . 1981 . Production of NO and N2O by soil nitrifying bacteria . Nature , 294 : 641 – 643 .
- Livesley , SJ , Kiese , R , Miehle , P , Weston , CJ , Butterbach-Bahl , K and Arndt , SK . 2009 . Soil-atmosphere exchange of greenhouse gases in a Eucalyptus marginatawoodland, a clover-grass pasture, and Pinus radiataand Eucalyptus globulusplantations . Glob. Chane Biol. , 15 : 425 – 440 .
- Maag , M and Vinther , FP . 1999 . Effect of temperature and water on gaseous emissions from soils treated with animal slurry . Soil Sci. Soc. Am. J. , 63 : 858 – 865 .
- Mori , A , Hojito , M , Kondo , H , Matsunami , H and Scholefield , D . 2005 . Effects of plant species on CH4and N2O fluxes from a volcanic grassland soil in Nasu, Japan . Soil Sci. Plant Nutr. , 51 : 19 – 27 .
- Mosier , AR , Halvorson , AD , Peterson , GA , Robertson , GP and Sherrod , L . 2005 . Measurement of net global warming potential in three agroecosystems . Nutr. Cycl. Agroecosyst. , 72 : 67 – 76 .
- Mosier , A , Kroeze , C , Nevison , C , Oenema , O , Seitzinger , S and van Cleemput , O . 1998 . Closing the global N2O budget: nitrous oxide emissions through the agricultural nitrogen cycle - OECD/IPCC/IEA phase II development of IPCC guidelines for national greenhouse gas inventory methodology . Nutr. Cycl. Agroecosyst. , 52 : 225 – 248 .
- Mu , ZJ , Kimura , SD and Hatano , R . 2006 . Estimation of global warming potential from upland cropping systems in central Hokkaido, Japan . Soil Sci. Plant Nutr. , 52 : 371 – 377 .
- Mu , ZJ , Kimura , SD , Toma , Y and Hatano , R . 2008 . Evaluation of the soil carbon budget under different upland cropping systems in central Hokkaido, Japan . Soil Sci. Plant Nutr. , 54 : 650 – 661 .
- Nakano , T , Sawamoto , T , Morishita , T , Inoue , G and Hatano , R . 2004 . A comparison of regression methods for estimating soil-atmosphere diffusion gas fluxes by a closed-chamber technique . Soil Biol. Biochem. , 36 : 107 – 113 .
- Paustian , K , Andren , O Janzen , HH . 1997 . Agricultural soils as a sink to mitigate CO2emissions . Soil Use Manag. , 13 : 230 – 244 .
- Raich , JW and Schlesinger , WH . 1992 . The global carbon-dioxide flux in soil respiration and its relationship to vegetation and climate . Tellus B Chem. Phys. Meteorol. , 44 : 81 – 99 .
- Robertson , G and Grace , P . 2004 . Greenhouse Gas Fluxes in Tropical and Temperate Agriculture: the need for a Full-Cost accounting of Global Warming Potentials . Environ. Dev. Sustain. , 6 : 51 – 63 .
- Sawamoto , T and Hatano , R . 2000 . N2O flux from a well-structured gray lowland soil cultivated for onion in Hokkaido, Japan . Japanese J. Soil Sci. Plant Nutr. , 71 : 659 – 665 . (In Japanese.)
- Schindlbacher , A , Zechmeister-Boltenstern , S and Jandl , R . 2009 . Carbon losses due to soil warming: do autotrophic and heterotrophic soil respiration respond equally? . Glob. Change Biol. , 15 : 901 – 913 .
- Shimizu , M , Marutani , S , Desyatkin , AR , Jin , T , Hata , H and Hatano , R . 2009 . The effect of manure application on carbon dynamics and budgets in a managed grassland of Southern Hokkaido, Japan . Agric. Ecosyst. Environ. , 130 : 31 – 40 .
- Six , J , Ogle , SM , Breidt , FJ , Conant , RT , Mosier , AR and Paustian , K . 2004 . The potential to mitigate global warming with no-tillage management is only realized when practised in the long term . Glob. Change Biol. , 10 : 155 – 160 .
- Smith , KA , McTaggart , IP , Dobbie , KE and Conen , F . 1998 . Emissions of N2O from Scottish agricultural soils, as a function of fertilizer N . Nutr. Cycl. Agroecosyst. , 52 : 123 – 130 .
- Stehfest , E and Bouwman , L . 2006 . N2O and NO emission from agricultural fields and soils under natural vegetation: summarizing available measurement data and modeling of global annual emissions . Nutr. Cycl. Agroecosyst. , 74 : 207 – 228 .
- Subke , JA , Inglima , I and Cotrufo , MF . 2006 . Trends and methodological impacts in soil CO2efflux partitioning: a metaanalytical review . Glob. Change Biol. , 12 : 921 – 943 .
- Takakai , F , Morishita , T Hashidoko , Y . 2006 . Effects of agricultural land-use change and forest fire on N2O emission from tropical peatlands, Central Kalimantan, Indonesia . Soil Sci. Plant Nutr. , 52 : 662 – 674 .
- Thornton , FC and Valente , RJ . 1996 . Soil emissions of nitric oxide and nitrous oxide from no-till corn . Soil Sci. Soc. Am. J. , 60 : 1127 – 1133 .
- Tiedje , JM . 1994 . Methods of Soil Analysis , Madison : SSSA . Part 2
- Tokuda , S and Hayatsu , M . 2004 . Nitrous oxide flux from a tea field amended with a large amount of nitrogen fertilizer and soil environmental factors controlling the flux . Soil Sci. Plant Nutr. , 50 : 365 – 374 .
- Toma , Y and Hatano , R . 2007 . Effect of crop residue C : N ratio on N2O emissions from Gray Lowland soil in Mikasa, Hokkaido, Japan . Soil Sci. Plant Nutr. , 53 : 198 – 205 .
- Toma , Y , Kimura , SD , Hirose , Y , Kusa , K and Hatano , R . 2007 . Variation in the emission factor of N2O derived from chemical nitrogen fertilizer and organic matter: a case study of onion fields in Mikasa, Hokkaido, Japan . Soil Sci. Plant Nutr. , 53 : 692 – 703 .
- Yanai , J , Sawamoto , T Oe , T . 2003 . Spatial variability of nitrous oxide emissions and their soil-related determining factors in an agricultural field . J. Environ. Qual. , 32 : 1965 – 1977 .
- Zou , JW , Huang , Y , Lu , YY , Zheng , XH and Wang , YS . 2005 . Direct emission factor for N2O from rice-winter wheat rotation systems in southeast China . Atmos. Environ. , 39 : 4755 – 4765 .