Abstract
Pollution by heavy metals such as cadmium (Cd) is hazardous to soil microbial function, including enzyme activities. However, the addition of clay to soils may control the negative effect of Cd by both adsorption of the metal ion onto the clay and stabilization of the soil enzymes. We investigated the effects of clay addition on the activities of soil proteases in the presence of Cd using a short-term laboratory incubation experiment. The clays were separated from the soil samples used in the study. Our experiment was done at three levels of clay and Cd addition (0, +5 and +10% of original clay content, and 0, 10 and 50 mg Cd kg−1 soil, respectively) to two surface soil samples of Andosols obtained from a forest and a cultivated field in an upland area of Japan’s Kanto district. We determined the protease activities, protein content, soil pH and exchangeable Cd in both soil samples after 0, 2, 10 and 40 days of incubation. Mineralogical analysis by dissolution with acid ammonium oxalate solution on the clay fractions from both the forest and the cultivated field soil samples showed that they were largely composed of allophane (probably including imogolite) and ferrihydrite. Allophanic clay had a significant positive effect on proteases activities even in the presence of Cd, although the addition of Cd decreased protease activities, protein contents and soil pH in both soil samples in all clay treatments during incubation. Protease activities were negatively correlated with the amounts of exchangeable Cd, but the addition of clay did not have a significant recovery effect on inhibition by Cd. These results indicate that allophanic clay enhances the activity of proteases owing to stabilization of the enzyme, and that allophanic clay has little capacity to adsorb Cd2+.
Introduction
Cadmium (Cd) is known to have deleterious effects on the density, activity and diversity of soil microflora (CitationKandeler et al. 1996), and has a clear negative influence on biologically mediated soil processes (CitationLee et al. 2002). Such an effect has been reported after assaying soil enzyme activities following the fresh addition of Cd to soil samples (CitationEffron et al. 2004; CitationShahriari et al. 2009). However, extraction, sorption and immobilization of Cd in soils appear to be effective factors in the detoxification of Cd-contaminated soils (CitationPérez-de-Mora et al. 2006).
Cadmium in soils and sediments is often associated with the various soil solids fractions. Among these, clay minerals are considered to play an important role in the accumulation, sorption/desorption and exchange processes of heavy metals (CitationRybicka et al. 1995), mainly as a result of their very large specific surface area and ionic nature (CitationProst and Yaron 2001). Clay minerals may have strong effects on the bioavailability of heavy metals because the toxicity of heavy metals in soils depends mainly on their bioavailability and, therefore, on their chemical forms (CitationLeita et al. 1999). Among the different chemical forms of Cd, soluble and exchangeable fractions are the most important (CitationUsman et al. 2005). CitationUsman et al. (2005) reported that the addition of clay minerals (Na-bentonite and Ca- bentonite) led to a significant decrease in water-soluble and exchangeable Cd and increased the respiration and microbial biomass carbon of soil from Urbic Anthrosol.
Activity measurements of different soil enzymes in soils contaminated with heavy metals, including Cd, have shown that enzyme activities are affected by soil physicochemical properties, including clay content, soil pH (CitationKelleher et al. 2003) and soil organic matter (CitationTipping et al. 2003), not only as a result of the effect of these factors on Cd availability for soil microorganisms, but also through the adsorption of soil enzymes and probably changes in the microbial communities.
Soil enzymes have a high affinity for colloidal clay components, mainly because of their adsorptive and possibly catalytic capabilities (CitationKelleher et al. 2002, 2003). A review of preceding studies has shown that clay may have two different effects on soil enzyme activity. It may stabilize and protect enzymes against their degradation and denaturation. For example, CitationAllison (2006) found that clay minerals, particularly allophane, enhanced potential enzyme activities in young volcanic soils of the Hawaiian Islands. However, the addition of clay may also inhibit soil enzyme activity owing to association with and blocking of the active site. For example, CitationKobayashi and Aomine (1967) and CitationTietjen and Wetzel (2003) have reported that reductions in enzyme activities were observed after the addition of allophanic and montmorillonitic clays, respectively. Adsorption of enzymes onto the surface of clay might occur through more than one mechanism, and enzyme activities may differ as a result of variations in the adsorption mechanisms (CitationAomine and Kobayashi 1966). Moreover, most of the preceding studies have focused on the interaction between purified enzymes and clay specimens (CitationAllison 2006; CitationTietjen and Wetzel 2003), and also on the adsorption of heavy metals onto well-characterized clay particles (CitationDavis and Upadhyaya 1996; CitationKooner 1993). Less attention has been given to the response of native extracellular soil enzymes to the addition of clay minerals to soils, particularly in the presence of Cd. The potential toxicity of Cd to soil microorganisms is assumed to be reduced and the soil enzyme activities enhanced as a result of the association of Cd and soil enzymes with added clay minerals. This may give rise to a reduction in Cd uptake by soil microorganisms.
It is well known that proteases are important proteolytic enzymes found in a wide diversity of plant, animal and microorganism taxa, and their activity and persistence are influenced by physical and chemical aspects of the soil environment (CitationFuka et al. 2008). We have previously demonstrated that protease activities in soil samples from Andosols are more sensitive to Cd addition than β-glucosidase activity (CitationShahriari et al. 2009). This may suggest that the activities of proteases are a useful indicator of soil contamination and/or improvement in Andosols. However, more information is needed on the capacity of clay minerals, as an important physicochemical factor, to adjust the negative effect of Cd on soil protease activities. Our objective in the present study was to elucidate the effects of clay addition on the activities of proteases in Andosols in the presence of Cd in a short-term incubation experiment. Our hypothesis was that the addition of clay would reduce the solubility of Cd and increase soil protease activities.
Materials and methods
Study sites and soil samples
Soil samples were collected from a forest and a cultivated field at the Agricultural and Forestry Research Center, University of Tsukuba, located in an upland area of the Kanto district, Japan. The forest was a natural mixed wood stand of warm-temperate trees (mainly Quercus serrata and Pinus densiflora) with no management, and the field has been continuously cultivated with corn for more than 10 years with annual tillage and intermittent fallow, and normal application of NPK fertilizer. These sites have a mean annual temperature of 14°C and receive approximately 1,400 mm precipitation annually. The two sites are 200 m apart. Both soils are classified as Umbric Andosols (CitationFAO, World Reference Base for Soil Resource 2006) and Typic Hapludands (CitationUSDA 2006). Parent materials are mostly volcanic ashes. Soil samples (approximately 3 kg total) were taken from the top 10 cm of soil from each site (from the A1 horizon in the forest and from the Ap horizon in the cultivated field). We collected soil samples from three points each in the forest and the cultivated field, approximately 25 m × 25 m, and each sample was thoroughly mixed. For the forest soil, the litter layer was removed before sampling. Roots were removed from the samples, which were then sieved through a 2-mm mesh and kept moist in plastic containers for 1 week at room temperature (20–25°C) before use.
Analyses of soil physicochemical properties
Soil texture and cation exchange capacity (CEC) were determined according to the sedimentation method (CitationGee and Bauder 1986) and the semi-micro Schollenberger method (CitationRhoades 1982), respectively. Soil pH was determined with a glass electrode (pH meter HM-30R; DKK-TOA, Tokyo, Japan) in H2O (CitationBlakemore et al. 1987). Total carbon and total nitrogen were measured with a Sumigraph NC-900 NC analyzer (Sumika Analytical Center, Tokyo, Japan) (CitationNelson and Sommers 1982). Exchangeable bases in CH3COONH4 (1 mol L−1) extracts of soils were determined by atomic absorption spectroscopy (AA 6200 Atomic Absorption Spectrophotometer; Shimadzu, Tokyo, Japan) (CitationThomas 1982).
Separation of clay fractions from the soil samples and determination of total carbon
Clay fractions were separated from the soil samples using the sedimentation method after H2O2 treatment to decompose soil organic matter. The fractions were then dispersed by sonication and the pH was adjusted to approximately pH 4 by the addition of HCl. Clay fractions were repeatedly collected until no dispersion was observed and then the suspensions of the separated clay fractions were flocculated with NaCl. The flocculated clay fractions obtained by centrifugation were further treated with mixed solutions of Ca, Mg, Na and K ions, prepared from the chloride salts. The concentrations of each ion treated for the forest clay were 10.0, 6.07, 1.54 and 2.88 mmol L−1, respectively; and those for the field clay were 100, 28.23, 8.12 and 17.2 mmol L−1, respectively. This treatment was done to convert exchangeable cations on the separated clays from Na+ back to the approximate ionic composition of the original exchangeable cations of the soil samples. The clay fractions were then washed successively with water–methanol (1:1) and methanol–acetone (1:1) and kept as suspensions in water at room temperature (20–25°C). The total carbon of the separated clay fractions was measured with an NC analyzer (Sumika Analytical Center).
Mineralogical analysis of the separated clay fractions from the soils
The separated clay fractions were subjected to X-ray diffraction (XRD) and FT-IR (Fourier Transform-Infrared Spectroscopy) analyses. To remove iron and aluminum oxides and hydroxides approximately 500 mg of the separated clay fraction was placed into a centrifuging tube and 30 mL of citrate reagent (0.26 mol L−1) and 5 mL of sodium bicarbonate (1 mol L−1) were added. The mixture was stirred and then placed in a water bath at approximately 90°C. After the addition of 1 g solid sodium dithionite, the tube was left in the water bath for 15 min, and then centrifuged for 15 min at 1,700 g (DCB treatment). Crystalline clay minerals in the separated clay fraction after the DCB treatment were analyzed on oriented samples using an X-ray diffractometer (RAD-X; Rigaku, Tokyo, Japan) with Cu-Kα radiation (λ = 1.54 Å). The XRD analyses of the samples were conducted after separate treatments by air drying (Mg-saturated; K-saturated), ethylene glycol (Mg-saturated) and heating to 350°C and 550°C (both K-saturated). Infrared spectroscopic analysis of Mg-saturated clays was also carried out using a FT-IR spectrometer (FT-720; Horiba, Tokyo, Japan) over the range 400–4,000 cm−1.
Extraction of aluminum and iron from the separated clay fractions
Chemical analyses of Fe (Fed) and Al (Ald) extracted by DCB treatment (CitationMehra and Jackson 1960) and also Si (Sio), Fe (Feo) and Al (Alo) extracted by acid ammonium oxalate solution (0.3 mol L−1, pH 3) were done by inductively coupled plasma-atomic emission spectrometry (ICP-AES) (ICAP-757; Nippon Jarrell-Ash, Tokyo, Japan). Weight loss (%) arising from treatment with ammonium oxalate was also calculated.
Experimental design and measurement of protease activities and protein contents
In the present study, two levels of clay fraction were added to the soils, designated as low and high concentrations (LC and HC, respectively). Experiments were also carried out with soils containing the original concentration of clay (OC: no addition of separated clay fraction). Initial sample sizes were the fresh soil equivalents of 0.25 g as dry weight of forest soil or 0.75 g as dry weight of cultivated field soil. Different amounts of soil were used to ensure comparable levels of enzyme activities in the forest and cultivated field soil samples and hence the formation of similar optical densities. Separated clay fractions were added in their water suspensions to the LC and HC systems, giving, respectively, 5 and 10% higher clay contents than OC. As the clay fractions were added to the soil samples as water suspensions, the moisture contents of the soil samples with clay fractions were higher than the original soils. To compensate for this, the soil samples were pre-incubated with the separated clay fractions for 1 week at 25°C (natural evaporation from unsealed containers) in the absence of Cd to reduce the moisture content to approximately 50% (on a fresh soil basis), which was comparable to the normal level in natural Japanese Andosols. Then doses of zero, onefold and fivefold of the critical Cd level, 10 mg Cd kg−1 soil (CitationChang and Broadbent 1981; CitationEffron et al. 2004), were added to the soil samples using solutions of Cd(NO3)2 at different concentrations, designated, respectively, as Cd0, Cd10 and Cd50. Natural concentrations of Cd at the two sites were too low to detect by ICP-AES analysis on the extract with 0.05 mol L−1 Ca(NO3)2. Thus, the soil samples prepared with a moisture content of 50% after pre-incubation with clay were further incubated at 25°C after the addition of Cd, with periodical moisture adjustment by weight. The incubation periods were 0, 2, 10 and 40 days, designated as d0, d2, d10 and d40, respectively.
Protease activity was measured after 0, 2, 10 and 40 days of incubation based on the method of CitationLadd and Butler (1972), with slight modifications (CitationOhse et al. 2003). The soil samples were mixed and reacted with 0.1 mol L−1 Tris–HCl buffer (pH 8.0) and N-benzyloxycarbonyl L-phenylalanine L-leucine (ZFL) as the substrate at 40°C for 1 h in the dark. Protease activities were measured after the addition of ninhydrin by colorimetric determination of leucine released from the substrate. The absorbance at 570 nm was measured using a spectrophotometer (U-3210; Hitachi, Tokyo, Japan). We measured protease activities in OC, LC and HC control systems, but without the addition of the substrate (ZFL).
Protein extraction was carried out based on the method of CitationOgunseitan (1993), where 100 μL of protease inhibitor cocktail and 1 mL of buffer solution (pH 7.58) containing 50 mmol L−1 Tris–HCl (pH 7.6), 10% sucrose, 4 mmol L−1 ethylenediaminetetraacetic acid (EDTA) 2 mmol L−1 dithiothreitol and 0.1% polyoxyethylene (20) cetyl ether (Brij 58) were added to the soil samples. The samples were incubated for 1 h at 0°C over ice. Lysis was completed by four 10-min freeze–thaw cycles (37°C to dry-ice bath). Cellular debris and other particulate matter were removed from the protein preparations by centrifugation at 4°C for 45 min at 16,000 g. The concentrations of proteins in the extracts were determined using the Bio-Rad DC protein assay (Bio-Rad Laboratories, Tokyo, Japan) according to the manufacturer’s protocol. The absorbance at 750 nm was measured using a spectrophotometer (U-3210; Hitachi).
Measurement of soil pH and exchangeable cadmium
The pH values of the soil samples (n = 3) suspended in water (soil : distilled water ratio of 1:2.5) were determined after 1 h of standing using a glass electrode (pH meter HM-30R; DKK-TOA) (CitationBlakemore et al. 1987). Incubated soil samples were also subjected to analysis of exchangeable Cd based on the method of CitationSadamoto et al. (1994), where extraction of Cd with Ca(NO3)2 (0.05 mol L−1), with a soil : solution rate of 1:10, was done after shaking (end to end, 200 r.p.m.) for 24 h at 30°C. The supernatant obtained by centrifuging at 1,700 g for 10 min was filtered through a 0.2 μm filter (Millipore, Bedford, MA, USA) and exchangeable Cd was measured by ICP-AES (Optima 5300 DV, Tokyo, Japan).
Statistical analysis
The experiment was carried out using a factorial split-plot design based on complete randomization with three replicates. All statistical analyses were carried out using the program SAS (Ver. 9.2, SAS Institute Inc, NC, USA). The biological and chemical data were analyzed using a 3-way anova with time, clay level and Cd level as the variables, and a Duncan’s test was used to classify the means (**P < 0.01; *P < 0.05). The percentage of inhibition by Cd of enzyme activities was calculated using the following equation: ![]() , where x is the enzyme activity in the absence of Cd and y is the enzyme activity under different doses of Cd (CitationEffron et al. 2004).
, where x is the enzyme activity in the absence of Cd and y is the enzyme activity under different doses of Cd (CitationEffron et al. 2004).
Results and discussion
Physicochemical properties of the soil samples
The physicochemical properties of the soil samples are shown in . Both samples had Loam texture. Total C, total N, C/N ratio and CEC in the forest soil were distinctly higher than the values in the cultivated field soil, and the pH in H2O was lower for the forest soil than for the cultivated field soil. Higher exchangeable cations in the cultivated field soil sample probably resulted from the application of chemical fertilizers. All these soil properties are representative of Andosols in the upland of the Kanto district, Japan.
Mineralogical characterization of the separated clay fractions
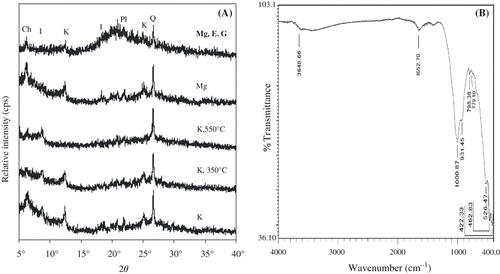
The X-ray diffraction patterns of the oriented clay fractions from the forest soil sample are shown in , where the peaks at 7.06 and 3.53 Å remained unaffected with the ethylene glycol treatment and on heating to 350°C, but were destroyed on heating to 550°C. This indicates the presence of kaolin minerals. The presence of chlorite, marked by a 14.02 Å reflection that remained unaffected through treatment with ethylene glycol and heating, was also identified in all treatments. Peaks of quartz (3.33 Å) were detected in all diffractograms. Minor abundances of illite (9.97 Å) and plagioclase (4.04 Å) were also detected. Thus, the crystalline layer silicates of the separated clay fraction are composed of kaolinite, illite and chlorite in addition to the primary minerals of quartz and feldspar. The FT-IR spectrum of clay separated from the forest soil sample is shown in , where AlOH stretching vibrations of kaolinite (3648.66 cm−1), Si-O stretching vibrations of quartz (798.38 cm−1), a broad absorption band at 1625.70 cm−1 resulting from adsorbed water and the band at 526.47 cm−1 resulting from silicate minerals were all observed. The intense vibration bands at 1000.87 and 931.45 cm−1 could be assigned to Si-O-(Si) and Si-O-(Al) vibrations in layer silicates (CitationMontarges-Pelletier et al. 2005). Identical mineralogy was also found for the clay fraction from the cultivated field soil samples. However, the separated clay fraction also contained a considerable amount of allophane (probably including imogolite) and ferrihydrite, which dissolved with acid ammonium oxalate treatment.
Table 1 Physicochemical properties of the soil samples
Figure 1 Mineralogical characterization of the clay fractions. (A) X-ray diffraction patterns of the oriented clay fraction from the forest soil sample. Mg, magnesium saturated; K, potassium saturated; E.G, treated with ethylene glycol; Ch, chlorite (14.02 Å); I, illite (9.97 Å and 4.41 Å); K, kaolinite (7.06 Å and 3.53 Å); Pl, plagioclase (4.04 Å): Q, quartz (3.33 Å). (B) FT-IR spectra of the clay fraction from the forest soil sample.
Chemical characterization of the separated clay fractions
shows the total C content, weight loss (%) after treatment with acid ammonium oxalate and the amounts of Al and Fe extracted by DCB treatment and acid ammonium oxalate solution from the separated clay fractions of forest and cultivated field soil samples. Even after the harsh decomposition treatment with H2O2, both separated clays showed the presence of very small amounts of total C derived from soil organic matter. However, this would not affect the experimental results in the present study because in each incubation tube there were only 0.25 and 0.20 mg C in LC, and 0.50 and 0.40 mg C in HC for the forest and the cultivated field soil samples, respectively. As for selective dissolution, more Fe was extracted by DCB than acid ammonium oxalate because most of the crystalline iron oxide and hydroxide is dissolved by the DCB treatment. There was a large difference between the amount of Al extracted by the DCB and oxalate treatments of the soil samples, and Sio ranged from 56 to 59 g kg−1. Moreover, the atomic ratios of Al/Si extracted by acid ammonium oxalate were 2.02 and 1.95 for the forest and the cultivated field soil samples, respectively. Considering the total weight loss (%) in the acid oxalate treatment (56 and 61%, respectively), the two separated clay fractions were largely composed of allophane (probably including imogolite) and ferrihydrite in addition to the layer silicates.
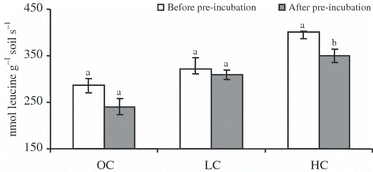
Protease activity before and after pre-incubation of the forest soil sample with added clay
In the present study, microbial activities including protease production might have changed during 1 week pre-incubation owing to alterations in the microbial communities. Thus, the effect of the addition of separated clay fractions on protease activities was measured before and after the pre-incubation period in the forest soil sample. The measurement before pre-incubation was done 5 h after the clay addition. As shown in , protease activities increased significantly after the addition of clay, probably as a result of the stabilization of enzymes and the protection of proteases against their degradation by binding to the added clay. CitationTietjen and Wetzel (2003) demonstrated that a 5-h incubation was long enough for soil enzymes to be adsorbed onto the clay. Enhancement in enzyme activity may result, in part, from the production of new enzymes. The activities of the proteases decreased after 1 week of pre-incubation. However, the decrease was not significant, except at high concentrations of clay. These results may indicate that clay addition moderately stabilizes newly produced proteases during pre-incubation, probably in association with some changes in soil microflora.
Table 2 Chemical characterization of the separated clay fractions
Protease activity after the addition of separated clay fractions in the presence of cadmium
As shown in , the addition of separated clay to both forest and cultivated field soil samples significantly increased protease activities (P < 0.01) compared with the control (OC: no clay addition) after each incubation time (d0, d2, d10 and d40). This increase in activity was observed at all levels of Cd concentration (Cd0, Cd10 and Cd50), and protease activities were primarily in the order of HC > LC > OC. In the forest soil sample (), the highest increase in activity compared with OC (115.6%) was observed for HC after 10 days of incubation (d10). In the cultivated field soil sample (), protease activities were roughly one-tenth of those for the forest soils and increased by 54.6% in LC for Cd10 after 2 days of incubation (d2) and by 108.8% in HC for Cd0 after 40 days of incubation (d40). The percentages given above are the relative increases in protease activities after the addition of clay. Although soil microbial communities and populations, as the influential factors governing enzyme activities, may change during incubation (CitationPérez-de-Mora et al. 2006), the addition of clay fractions would result in the stabilization of proteases. However, activity enhancement did not occur linearly with the increase in clay addition levels, and fluctuated over the differing incubation periods.
Figure 3 Protease activities and protein contents of forest and cultivated field soil samples after the addition of clay and cadmium. (A) Protease activities in the forest soil samples, (B) protease activities in the cultivated field soil samples, (C) protein contents in the forest soil samples and (D) protein contents in the cultivated field soil samples. OC, original concentration; LC, low concentration of clay; HC, high concentration of clay; d0, 0 days of incubation; d2, 2 days of incubation; d10, 10 days of incubation; d40, 40 days of incubation. Samples without Cd are shown by solid lines, and samples incubated with 10 mmol L−1 Cd and 50 mmol L−1 Cd are shown by dashed lines and dotted lines, respectively. Error bars indicate standard errors (n = 3).
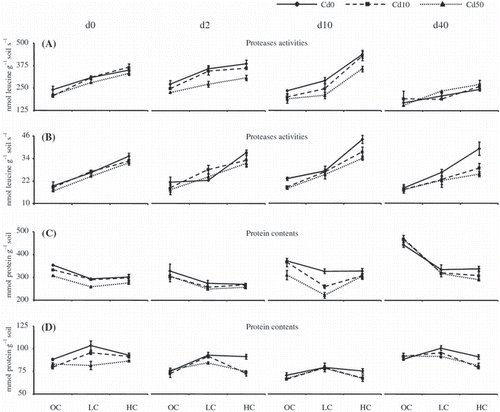
Protease activities were significantly inhibited by the addition of Cd for all incubation periods (P < 0.01; ) in most OC, LC and HC treatments for both soil samples (). In OC inhibition percentages ranging from 0 to 28.4% were found for the forest soil samples, and from 0 to 20.3% for the cultivated field soil samples. However, the addition of allophanic clay fractions did not lead to a regular decrease in the inhibition percentage of protease activities in the presence of Cd. In the forest soil samples (), the clearest decrease in the inhibition percentage as a result of clay addition occurred after 0 and 40 days of incubation in both LC and HC, whereas in the cultivated field soil samples it occurred after 0 and 2 days of incubation in both LC and HC. Moreover, the effect of time on protease activities was significant (P < 0.01; ), but this effect fluctuated over the incubation periods. In general, protease activities decreased in the presence of Cd during the incubation for all clay treatments, except for the 40 days of incubation of the forest soil sample. Protease activities in the presence of Cd appeared to increase after 2 and 10 days, and then decrease after 40 days in both soil samples. This may relate to changes in the microbial community over the incubation (CitationPérez-de-Mora et al. 2006).
Table 3 anova showing the F-values of different factors affected by cadmium, clay fractions and time
At all incubation times the addition of clay separated from Andosols clearly enhanced protease activities in both soil samples in a way approximately proportional to the added amounts, even in the presence of Cd. This result provides evidence that clay minerals stabilize or enhance protease activities in these soils. As allophane can complex with and stabilize organic material, these minerals may have a strong effect on the stability and activity of soil enzymes (CitationAllison 2006; CitationTyler et al. 1989; CitationWada 1989). Other studies have also reported that enzyme activities are increased by the addition of clay minerals (CitationAllison 2006; CitationRao et al. 2000; CitationTietjen and Wetzel 2003), although the adsorption process may reduce catalytic activity depending on differences in the mineralogy of the added clay (CitationKelleher et al. 2003; CitationQuiquampoix et al. 2002; CitationSarkar et al. 1989; CitationTietjen and Wetzel 2003).
Protein contents after the addition of separated clay fractions in the presence of cadmium
The addition of different amounts of separated clay fractions caused different responses in the protein concentrations between the forest and cultivated field soil samples. In the forest soil samples (), protein concentrations, in general, decreased with the addition of clay, particularly for LC, whereas in the cultivated field soil samples () the protein concentrations increased in LC for all incubation periods, and tended to decrease again in HC. The decrease in the protein contents () in both samples might be caused by strong binding of the soil proteins to clay minerals, leading to reduced extraction efficiency from soils, despite an increase in protease activities ().
With the addition of Cd, protein concentrations significantly decreased (P < 0.05) in Cd10 and Cd50 for both forest and cultivated field soil samples. This may reflect the response of soil microbial communities to Cd. According to CitationSingleton et al. (2003), changes in soil protein levels may result from shifts in microbial communities and the measurement of soil protein may be indicative of the soil microbial responses to Cd contamination. Our observations showed a relatively small drop in protein contents in both soil samples under high exposure to Cd (up to 50 mg kg−1) probably as a result of the presence of clay.
Soil pH and exchangeable cadmium in the presence of clay fractions and cadmium
The forest soil sample had a lower pH compared with the cultivated field soil sample (). In both soil samples, soil pH significantly decreased after the addition of the separated clay fractions (P < 0.05). Exchangeable Cd () increased in proportion to the total added Cd in both the forest and cultivated field soil samples. However, in contrast to our hypothesis, in both soil samples there was little difference in exchangeable Cd following the addition of clay or with increased incubation time.
Exchangeable Cd in the forest soil samples was higher than that in the cultivated field soil samples (). This can be attributed to the difference in pH between these soils because soil pH has a serious effect on the availability and mobility of metal cations (CitationMartínez and Motto 2000). In general, because of the reduced solubility of Cd as the pH increases there is a negative correlation between the concentration of exchangeable Cd and soil pH (CitationAdriano 2001; CitationAguilar et al. 2004; CitationPérez-de-Mora et al. 2006). This is roughly what was observed in the forest soil samples in the present study (). However, exchangeable Cd in the forest soil samples showed no clear change in LC and HC, and appeared to fluctuate between the different incubation times. In the cultivated field soil samples the addition of clay resulted in a significant decrease in exchangeable Cd, particularly for Cd50, but again there was some fluctuation. The higher amount of exchangeable Cd in the forest soil sample () may be attributed, in part, to either its lower pH or to the presence of much greater amounts of dissolved organic matter () because soil organic matter is highly important in controlling Cd sorption and desorption (CitationGray et al. 1998).
Pearson’s correlations between protease activity and other factors
The values of the correlation coefficients between protease activity and protein content, pH and exchangeable Cd are shown in . A significant negative correlation was found between protease activity and protein concentration only in the case of no addition of separated clay fraction (OC) in both soil samples and in LC of the forest soil sample, although protease activities and protein concentrations decreased with the addition of Cd in all clay treatments. A significant positive correlation was observed between protease activity and pH for all clay concentrations for both soil samples, except for LC with the cultivated field soil sample. Protease activity correlated negatively with exchangeable Cd, and the coefficient tended to decrease as the concentration of added clay increased. This pattern was more consistent in the forest soil sample.
Figure 4 Soil pH and exchangeable cadmium of forest and cultivated field soil samples after the addition of clay and Cd. (A) pH of the forest soil samples, (B) pH of the cultivated field soil samples, (C) exchangeable Cd of the forest soil samples and (D) exchangeable Cd of the cultivated field soil samples. OC, original concentration; LC, low concentration of clay; HC, high concentration of clay; d0, 0 days of incubation; d2, 2 days of incubation; d10, 10 days of incubation; d40, 40 days of incubation. Samples without Cd are shown by solid lines, and samples with 10 mmol L−1 Cd and 50 mmol L−1 Cd are shown by dashed lines and dotted lines, respectively. Error bars indicate standard errors (n = 3).
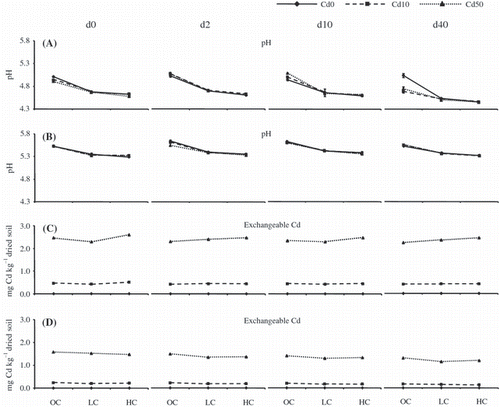
Table 4 Pearson’s correlation coefficients between protease activity and protein content, pH and exchangeable Cd
Variation in the correlation coefficients between protease activities and other factors () was assumed to be related to the ability of clay minerals to adsorb Cd2+and soil enzymes or protein. As, in the present study, almost 60% of the separated clay was composed of allophane (probably including imogolite) and ferrihydrite, the presence of protonated sites, such as Al-OH2 + and Fe-OH2 +, on the outer surface of these minerals (CitationParfitt 1980) would be an important factor influencing the correlations examined.
Conclusions
In the present study, the data obtained for both forest and cultivated field soil samples of Andosols indicate that the addition of allophanic clay potentiates a strong stabilization of proteases in the presence of Cd, and also that the inhibitory effect of Cd on proteases tended to be reduced by the addition of clay. However, the increase in the activity by the addition of the clay may result, in part, from microbial community alteration. Exchangeable Cd did not show any apparent trend over the different incubation periods, suggesting that allophanic clay has a low ability to adsorb Cd2+. Thus, the response of enzyme activities to the addition of separated clay fractions in the presence of Cd could depend on both the chemical properties of the clay and the adsorption mechanisms of the enzymes, including the particular disposition of their active sites. Further studies are needed for soils with different physicochemical properties and clay mineralogy from Andosols.
Acknowledgments
We thank Dr Tamao Hatta, Japan International Research Center of Agricultural Sciences, for clay mineral analysis and Mr Mohammad Zaman Nouri, National Institute of Crop Sciences, for his helpful discussion of the statistical analysis. We also thank Dr Maki Asano and Mr Ali Sdiri, Graduate School of Life and Environmental Sciences, University of Tsukuba, for their helpful discussion and assistance during the laboratory analysis. We thank Dr Martin Shepherd for linguistic assistance in the English editing of this manuscript.
References
- Adriano , DC . 2001 . Trace Elements in Terrestrial Environments: Biogeochemistry, Bioavailability and Risks of Metals , 2nd edn , New York : Springer .
- Aguilar , J , Dorronsoro , C Fernández , E . 2004 . Soil pollution by a pyrite mine spill in Spain: evolution in time . Environ. Pollut. , 132 : 395 – 401 .
- Allison , SD . 2006 . Soil minerals and humic acids alter enzyme stability: implications for ecosystem processes . Biogeochemistry , 81 : 361 – 373 .
- Aomine , S and Kobayashi , Y . 1966 . Effects of allophanic clays on the enzymatic activity of beta-amylase . Soil Sci. Plant Nutr. , 12 : 7 – 12 .
- Blakemore , LC , Searle , PL and Daly , BK . 1987 . Methods for Chemical Analysis of Soils , NZ Soil Bureau Scientific Report 80 11 Huff, , New Zealand : Department of Scientific and Industrial Research Lower .
- Chang , FH and Broadbent , FE . 1981 . Influence of trace metals on carbon dioxide evolution from a yolo soil . Soil Sci. , 132 : 416 – 421 .
- Davis , AP and Upadhyaya , M . 1996 . Desorption of cadmium from goethite (alpha-FeOOH) . Water Res. , 30 : 1894 – 1904 .
- Effron , D , De la Horra , AM , Defrieri , RL , Fontanive , V and Palma , RM . 2004 . Effect of cadmium, copper and lead on different enzyme activities in a native forest soil . Commun. Soil Sci. Plant Anal. , 35 : 1309 – 1321 .
- FAO, World Reference base for soil resource . 2006 . World Soil Resource Reports , 70 Rome : FAO .
- Gee , GW and Bauder , JW . 1986 . “ Particle-size analysis ” . In Methods of Soil Analysis, Part 1 Physical and Mineralogical Methods , 2nd edn , Edited by: Klute , A . 383 – 409 . Madison, WI : American Society of Agronomy, Inc. SSSA, Inc. .
- Gray , CW , McLaren , RG , Roberts , AHC and Condorn , LM . 1998 . Sorption and desorption of cadmium from some New Aeland soils: effect of pH and contact time . Aust. J. Soil Res. , 36 : 199 – 216 .
- Kelleher , BP , Oppenheimer , SF Han , FX . 2003 . Dynamical systems and phase plane analysis of protease-clay interactions . Langmuir , 19 : 9411 – 9417 .
- Kelleher , BP , Sutton , D and O’Dwyer , TF . 2002 . The effect of kaolinite intercalation on the structural arrangements of N-methylformamide and 1-methyl-2-pyrrolidone . J. Colloid Interf. Sci. , 255 : 219 – 224 .
- Kobayashi , Y and Aomine , S . 1967 . Mechanism of inhibitory effect of allophane and montmorillonite on some enzymes . Soil Sci. Plant Nutr. , 13 : 189 – 194 .
- Kooner , ZS . 1993 . Comparative study of adsorption behavior of copper, lead, and zinc onto goethite in aqueous systems . Environ. Geol. , 21 : 242 – 250 .
- Ladd , JN and Butler , JHA . 1972 . Short-term assays of soil proteolytic enzyme activities using proteins and dipeptide derivatives as substrates . Soil Biol. Biochem. , 4 : 19 – 30 .
- Lee , I-S , Kim , OK , Chang , Y-Y , Bae , B , Kim , HH and Baek , KH . 2002 . Heavy metal concentrations and enzyme activities in soil from a contaminated Korean shooting range . J. Biosci. Bioeng. , 94 ( 5 ) : 406 – 411 .
- Leita , L , Nobili , MD and Mondini , C . 1999 . Influence of inorganic and organic fertilization on soil microbial biomass, metabolic quotient and heavy metal bioavailability . Biol. Fertil. Soils , 28 : 371 – 376 .
- Martínez , CE and Motto , HL . 2000 . Solubility of lead, zinc and copper added to mineral soils[J] . Environ. Pollut. , 107 : 153 – 158 .
- Mehra , OP and Jackson , ML . 1960 . Iron Oxide removal from soils and clays by a dithionite-citrate system buffered with sodium bicarbonate . Clays. Clay Miner. , 7 : 317 – 327 .
- Montarges-Pelletier , E , Bogenez , S Pelletier , M . 2005 . Synthetic allophane-like particles: textural properties . Colloid surf. A. , 255 : 1 – 10 .
- Nelson , DW and Sommers , LE . 1982 . “ Total carbon, organic carbon, and organic matter ” . In Methods of soil analysis, Part 2 Chemical and microbiological properties , 2nd edn , Edited by: Page , LA . 539 – 579 . Madison, WI : American Society of Agronomy, Inc. SSSA, Inc. .
- Ogunseitan , OA . 1993 . Direct extraction of proteins from environmental samples . J. Microbiol. Methods , 17 : 273 – 281 .
- Ohse , K , Tamura , K and Higashi , T . 2003 . Influences of forest decline on various properties of soils on Mt. Hirugatake, Tanzawa Mountains, Kanto District, Japan – III. Changes in microbial biomass and enzyme activities of surface soils . Soil Sci. Plant Nutr. , 49 : 179 – 184 .
- Parfitt , RL . 1980 . “ Chemical properties of variable charge soils ” . In Soil With Variable Charge , Edited by: Theng , BKG . 167 – 294 . Lower Hutt, , New Zealand : New Zealand Soc. Soil Sci .
- Pérez-de-Mora , A , Burgos , P , Madejón , E , Cabrera , F , Jaeckel , P and Schloter , M . 2006 . Microbial community structure and function in a soil contaminated by heavy metals: effects of plant growth and different amendments . Soil Biol. Biochem. , 38 : 327 – 341 .
- Prost , R and Yaron , B . 2001 . Use of modified clays for controlling soil environmental quality . Biol. Fertil. Soils , 166 : 429 – 434 .
- Quiquampoix , H , Servagent-Noinville , S and Baron , M . 2002 . “ Enzyme adsorption on soil mineral surfaces and consequences for the catalytic activity ” . In Enzymes in the Environment , Edited by: Burns , RG and Dick , RP . 285 – 306 . New York : Marcel Dekker .
- Rao , MA , Violante , A and Gianfreda , L . 2000 . Interaction of acid phosphatase with clays, organic molecules and organo-mineral complexes: kinetics and stability . Soil Biol. Biochem. , 32 : 1007 – 1014 .
- Rhoades , JD . 1982 . “ Cation exchange capacity ” . In Methods of Soil Analysis, Part 2 Chemical and Microbiological Properties , 2nd edn , Edited by: Page , LA . 149 – 157 . Madison, WI : American Society of Agronomy, Inc. SSSA, Inc. .
- Rybicka , EH , Calmano , W and Breeger , A . 1995 . Heavy metals sorption/desorption on competing clay minerals; an experimental study . Appl. Clay Sci. , 9 : 369 – 381 .
- Sadamoto , H , Iimura , K , Honna , T and Yamamoto , S . 1994 . Examination of fractionation of heavy metals in soils . Jpn. J. Soil Sci. Plant Nutr. , 65 : 645 – 653 .
- Sarkar , JM , Leonowicz , A and Bollag , JM . 1989 . Immobilization of enzymes on clays and soils . Soil Biol. Biochem. , 21 : 223 – 230 .
- Shahriari , F , Yoshida , S , Ohse , K and Higashi , T . 2009 . Response of β-glucosidase and proteases to the Cd addition in Andosols from a forest and cultivated field . Soil Sci. , 174 ( 11 ) : 621 – 628 .
- Singleton , I , Merrington , G , Colvan , S and Delahunty , JS . 2003 . The potential of soil protein-based methods to indicate metal contamination . Appl. Soil Ecol. , 23 : 25 – 32 .
- Thomas , GW . 1982 . “ Exchangeable cations ” . In Methods of Soil Analysis, Part 2 Chemical and Microbiological Properties , 2nd edn , Edited by: Page , LA . 159 – 165 . Madison, WI : American Society of Agronomy, Inc. SSSA, Inc. .
- Tietjen , T and Wetzel , RG . 2003 . Extracellular enzyme–clay mineral complexes: enzyme adsorption, alteration of enzyme activity, and protection from photodegradation . Aquat. Ecol. , 37 : 331 – 339 .
- Tipping , E , Rieuwerts , J Pan , G . 2003 . The solid-solution partitioning of heavy metals (Cu, Zn, Cd, Pb) in upland soils of England and Wales . Environ. Pollut. , 125 : 213 – 225 .
- Tyler , G , Balsberg-Pahlson , AM , Bengtsson , G , Bååth , E and Tranvik , L . 1989 . Heavy-metal ecology of terrestrial plants, micro-organisms and invertebrates . Water Air Soil Pollut. , 47 : 189 – 215 .
- USDA, United States department of agriculture . 2006 . Keys to Soil Taxonomy , 10th edn , 86 Washington, DC : USDA-Natural Resources Conservation Service .
- Usman , A , Kazyakov , Y and Stahr , K . 2005 . Effects of clay minerals on immobilization of heavy metals and microbial activity in a sewage sludge-contaminated soil . J. Soils sediments , 5 ( 4 ) : 245 – 252 .
- Wada , K . 1989 . “ Allophane and immogolite ” . In Minerals in Soil Environments , Edited by: Dixon , JB and Weld , SB . 1051 – 1087 . Madison, WI : Soil Science Society of America .
- Kandeler , E , Kampichler , C and Horak , O . 1996 . Influence of heavy metals on the functional diversity of soil microbial communities . Biol. Fertil. Soils , 23 : 299 – 306 .
- Fuka , MM , Engel , M , Gattinger , A , Bausenwein , U , Sommer , M , Munch , JC and Schloter , M . 2008 . Factors influencing variability of proteolytic genes and activities in arable soils . Soil Biol. Biochem. , 40 : 1646 – 1653 .