Abstract
Soil Fe fractions and availability vary with landscape positions, because landscape position affects soil chemical properties and water conditions. In the present study, we investigated Fe fractions and availability at selected landscape positions in the loessial gully region of northwestern China. Four landscape positions, plateau, slope, terrace, and gully bottom were investigated. For each landscape position, soil samples were collected at 20-cm increments to a depth of 80 cm. Iron in the soil samples was fractionated by a modified sequential extraction method. Available Fe was assessed by diethylene thiamine pentacetic acid (DTPA) extraction procedure. The results showed that soil profile distributions of DTPA-Fe varied greatly with landscape position in the study area. The largest content of DTPA-Fe content was observed in the plateau soils, while the smallest content was observed in the gully bottom soils. Iron in soils existed mainly in the mineral bound fraction, which accounted for about 73 to 96% of the total Fe. The content of Fe in soil fractions varied greatly with landscape position. Exchangeable Fe and organic matter bound Fe were direct sources of available Fe, but exchangeable Fe contributed little to the total available Fe due to its low content in the soils. Oxides bound Fe was an indirect source of available Fe. The results of the present study indicate that landscape position strongly influences soil profile distribution and capacity of available Fe by influencing soil Fe fractions and organic matter distributions.
Introduction
Iron (Fe) is one of the major constituents of the lithosphere and pedosphere. The content of Fe in natural soils ranges between 7 and 42 g kg−1 (CitationKabata-Pendias 2000; CitationXing and Zhu 2003). However, plant available Fe is low in most soils. Iron in soils is mainly associated with metal oxides and hydroxides or contained in primary and secondary minerals. The percentage of soil Fe unavailable to plants can be as high as 92% (CitationKabata-Pendias 2000; CitationXing and Zhu 2003). The availability of soil Fe for plant growth varies greatly with soil conditions and depends particularly on soil redox and pH conditions. Certain soil conditions, such as high soil pH, free calcium carbonate, and low organic matter, often cause Fe deficiency in plants. Currently, approximately one-third of the soils in the world suffer Fe deficiency, which limits plant growth (CitationAbadia 1995; CitationConnolly and Guerinot 2002).
On the Loess Plateau of China, the total content of Fe in soils is 28 g kg−1 on average, and DTPA extracted Fe varies between 0.01 and 5.6 mg kg−1 with an average content of 4.2 mg kg−1 (CitationYu et al. 1991). The content of DTPA-Fe in 40% of the soils is lower than 4.5 mg kg−1, which is the critical level of Fe deficiency for winter wheat and millet (CitationYu et al. 1991). On the Loess Plateau, Fe deficiency for plants (e.g. apple, pear, grapevine, maize and soybean) is frequently observed (CitationZhang et al. 2002).
Soil iron deficiency is mainly related to soil physicochemical properties and Fe fractionation that determine the availability of Fe to plants (CitationAbadia 1995; CitationBarton and Abadia 2006; CitationZhou et al. 2004). As a valence-variable element, Fe exists in well drained soils in different forms of Fe oxides. Soil redox conditions can cause changes in Fe valence, which impacts Fe availability and fractional distribution (CitationLi et al. 2007; CitationPlekhanova 2007). Landscape position impacts soil water movement and transport of solutes and soil particles in the soil profile. As a result, landscape position impacts soil physicochemical properties and water conditions, which in turn affect Fe fractions in soils and Fe availability to plants (CitationLi et al. 2007; CitationPlekhanova 2007; CitationZhang and Zheng 2002).
The objective of the present study was to investigate soil Fe fractionation and Fe availability at selected landscape positions (e.g. plateau, terrace, slope and gully bottom) in a loessial gully watershed of northwestern China. A sequential extraction procedure modified from that described in CitationTessier et al. (1979) was used to fractionate soil Fe into exchangeable, carbonate bound, organic matter bound, oxide bound, and mineral bound fractions. We expect to discover the profile distributions of Fe in soils at the selected landscape positions, which will be of theoretical and practical importance for improving nutritional management of soil Fe in the degrading Loess Plateau environment.
Material and methods
Study area
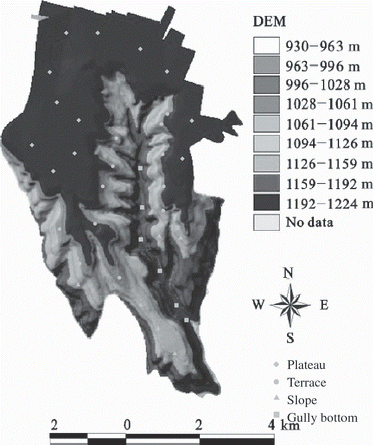
The experiment was conducted in Wangdougou watershed in Changwu County, Shaanxi Province, China (35°12′–35°16′N, 107°40′–107°42′E). The watershed is a part of the Agro-ecological Experiment Station of the Chinese Ecology Research Network (CERN), and is located in a typical gully region of the Loess Plateau with an altitude of 800–1200 m and an area of 8.5 km2. The study site has a warm-temperate zone subhumid continental climate. During the period 1984 to 2005, the average annual temperature was 9.1°C with a frost-free period of 171 days. The accumulation of temperatures higher than 0 and 10°C was 3866 and 3029°C, respectively. The average annual precipitation was 584 mm. Rainfall mainly occurs between June and September with large variation of intensity within a year and between years. The soil is a silty loam Yellow Mian soil, a Cambisol according to the International Soil Taxonomy (CitationFAO/ISRIC/ISSS 1998), which is developed in eroded loess and is influenced by humus accumulation, eluviation and illuviation.
Soil sampling and chemical analysis
Soil samples were collected from the ground surface to a depth of 80 cm in 20-cm increments using a 5-cm diameter tube auger at 12 locations in the plateau, 12 locations in the terrace, seven locations in the slope, and seven locations in the gully bottom in July of 2005. The distribution of sampling sites is described in . The plateau and terrace were mainly farmland of spring corn or winter wheat, while the slope and gully bottom were grass and forest lands, respectively. In each sampling location, three soil profiles were sampled to make a composite soil sample. After removal of large pieces of un-decomposed organic matter (e.g. plant roots), the soil samples were air-dried in a laboratory and ground to pass through 1.00 and 0.25 mm nylon screens for analysis.
Soil pH, organic matter and cation exchange capacity (CEC) were measured using the methods described in CitationPage et al. (1982). Soil pH was determined using an electrode pH-meter in a soil : water ratio of 1:2. Cation exchange capacity was determined by replacement of exchangeable cations by ammonium acetate (pH 7). Organic matter was determined using the Walkley–Black method.
Since available Fe is determined by a complex combination of soil, plant, and environmental variables, we assessed the availability of Fe in the present study by the DTPA (diethylene thiamine pentacetic acid) procedure, which was designed for calcareous soils (CitationLindsay and Norvell 1978) and has been widely used for several decades. Twenty mL of 0.005 mol L−1 DTPA + 0.1 mol L−1 TEA (trietanolamine) + 0.01 mol L−1 CaCl2 (pH 7.3) were added to 10 g air-dry soil (<1.00 mm). Then, the suspension was shaken for 2 h at 25°C, filtered through a filter paper and stored in a polyethylene bottle at 4°C for analysis. Total Fe content was measured by a tri-acid digestion method (CitationShuman 1985). Half a gram of <0.25 mm soil was placed into a teflon beaker on a hot plate, and digested with a mixture of HNO3–HClO4–HF. After completion, the solution and suspended solids was transferred into a 100-mL flask and stored in a polyethylene bottle at 4°C for analysis.
The sequential extraction scheme used in the study was a modification of that of CitationTessier et al. (1979). During the fractionation, 5.0 g of sieved soil were used in 80-mL polypropylene centrifuge tubes. Each of the chemical fractions was extracted as follows:
| 1. | Exchangeable Fe (Ex-Fe): Soil samples were shaken at 25°C for 2 h with 25 mL of 1 mol L−1 NH4NO3 at pH 7.0 and centrifuged at 1776 g for 10 min. The supernatant was filtered with Whatman No. 5 filter paper. | ||||
| 2. | Carbonate bound Fe (Carb-Fe): The residue from (1) was shaken at 25°C for 5 h with 25 mL of 1 mol L−1 NaOAc adjusted to pH 5.0 with HOAc. The suspension was then centrifuged and filtered as done in step (1). | ||||
| 3. | Iron/manganese oxides bound Fe (Ox-Fe): Fifty mL of 0.04 M NH2OHHCl in 25% (v/v) HOAc were added to the residue from (2). The mixture was placed into a water bath at 95 ± 3°C with occasional agitation for 5 h, and the suspension was then centrifuged and filtered as in step (1). | ||||
| 4. | Organic matter bound Fe (Om-Fe): Three mL of 0.02 mol L−1 HNO3 and 5 mL of 30% H2O2 adjusted to pH 2.0 with HNO3 were added to the residue from (3) and the mixture was placed in a water bath at 85 ± 2°C for 2 h with intermittent agitation. An additional 5 mL of 30% H2O2 adjusted to pH 2.0 with HNO3 were then added and the mixture was kept in the water bath at 85 ± 2°C for another 3 h with intermittent agitation. After cooling, 50 mL of 1 mol L−1 NH4NO3 at pH 7.0 was added, and the suspension was shaken for 2 h before centrifugation and filtering. | ||||
| 5. | Minerals bound Fe (Min-Fe): The residue from Step (4) was heated to dryness at 180°C, then 0.5000 g residue (<0.25 mm) was placed in a Teflon beaker on a hot plate, and digested with a mixture of HNO3–HClO4–HF. The digest was transferred into a 100-mL flask. | ||||
Between each successive extraction, the supernatant solution was removed and stored in a polyethylene bottle at 4°C. The residue was washed once with 10 mL of deionized water before proceeding to the next step in the extraction procedure. All glassware was soaked in 14% HNO3 (v/v) and rinsed with deionized water prior to use. Reagents used in the extraction were analytical grade. All soil extracts and digests were analyzed for Fe using atomic absorption spectrometry (SpectrAA-220 Zeeman; Varian Inc., Palo Alto, CA, USA).
Statistical analysis
Two-way analysis of variance (anova) was conducted to test landscape position and soil depth main effects and interaction effects on soil properties, DTPA-Fe and Fe fractions. Correlation analysis, path analysis, and factor analysis were performed to identify the relationships between DTPA-Fe and soil properties and among Fe fractions. Path analysis has advantages in partitioning correlations into direct and indirect effects with an attempt to differentiate correlation and causation compared with a multiple regression analysis. This technique also features multiple linear regressions and generates standardized partial regression coefficients (path coefficients) (CitationKnoke and Bohrnsted 1994). Path analysis was used to partition the correlation coefficients between DTPA-Fe and soil properties and between DTPA-Fe and Fe fractions into direct and indirect coefficients. The factor analysis technique, which allows a considerable reduction in the number of variables and the detection of structure in the relationships between variables has been successfully used for evaluation of heavy metals and their relations with metal fractions by CitationMaiz et al. (2000). In the present study, factor analysis was used to group Fe fractions and DTPA-Fe into a few factors. These factors, although statistically constructed, can be assessed with respect to the availability of each Fe fraction. Factor analysis was performed by evaluating principal components and computing the eingenvectors. Afterwards, the rotation of the principal components was carried out by the varimax normalized method. The results were presented as factor loadings of the rotated matrix. The factor plot was therefore portrayed according to the factor loadings of factor 1 and 2. All statistical analyses were performed using SAS software (CitationSAS Institute Inc 2000).
Results
Profile distribution of soil properties and DTPA-Fe
Soil organic matter, pH, and CEC varied significantly with soil depth at the selected landscape positions (, ). As expected, organic matter content decreased with soil depth, whereas soil pH and CEC only slightly changed. The decrease in organic matter content with soil depth was more pronounced at the gully bottom than at the other three landscape positions. Plateau soil was higher in organic matter and CEC but lower in pH compared with soils at the other landscape positions. Conversely, compared with other positions, the soils in the gully bottom had higher CEC and lower pH and organic matter.
Table 1 The analysis of variance (anova) results of soil properties, diethylene thiamine pentacetic acid (DTPA)-Fe and Fe fractions at different landscape positions and soil depths
Figure 2 Profile distribution of soil pH, organic matter, and cation exchange capacity at selected landscape positions.
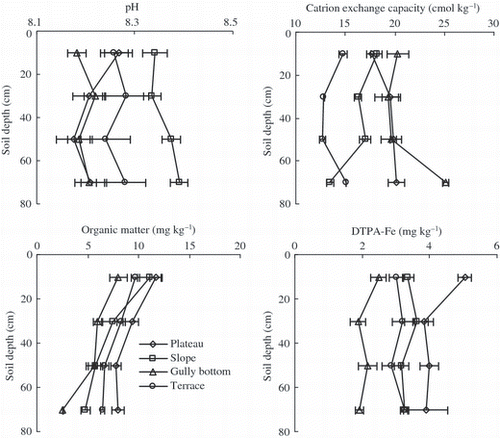
The distribution of DTPA-Fe in the soil profiles varied significantly with landscape positions (, ). The largest DTPA-Fe was observed in the plateau soils, and the smallest in the gully bottom soils. The mean values of DTPA-Fe in plateau soils were about two to three times larger than in the gully bottom soils. In addition, the DTPA-Fe varied significantly with depth in the plateau and the gully bottom soils but only slightly in the slope and the terrace soils. In the plateau and the gully bottom, the DTPA-Fe was significantly larger in the 0–20 cm layer than in the deeper soil.
Profile distribution of Fe fractions
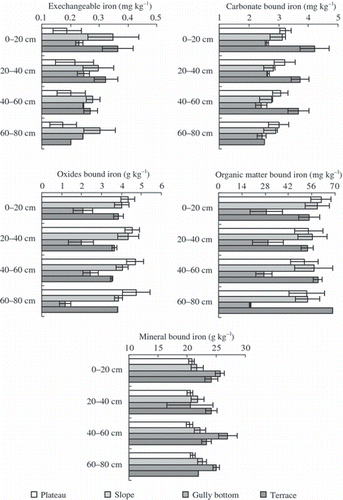
Concentrations of Fe fractions varied greatly with soil depth in the study area (). Average concentrations of Ex-Fe, Carb-Fe, and Om-Fe decreased with soil depth. The concentrations of Ex-Fe and Carb-Fe were <0.5 and 5 mg kg−1, respectively. These two fractions account for <0.05% of the total amount of Fe. The concentration of Om-Fe ranged from 14 to 102 mg kg−1 with a proportion of <0.5% of the total Fe. In addition, the contents of Ox-Fe varied from 0.89 to 7.92 g kg−1, corresponding to 3.5–27.3% of the total Fe. The Ox-Fe concentration was larger in the 20–60 cm soil layer than in the 0–20 and 60–80 cm soil layers. The fraction of Min-Fe ranged between 72.5 and 96.4% of the total Fe in the studied area.
Table 2 Fractional distribution of Fe in soils in Wangdonggou watershed
The profile distribution of Fe fractions was significantly influenced by landscape position, soil depth and their interactive effect (, ). Among the soil profiles, the lowest Ex-Fe was observed in plateau soils. In the 0–60 cm soil layer, Ex-Fe was also lower in gully bottom soils than that in slope and terrace soils. Besides, Ex-Fe changed slowly with soil depth in gully bottom and plateau soils, but varied markedly in slope and terrace soils. Carbonate bound Fe decreased rapidly with soil depth in terrace soils but slowly in soils at the other three landscape positions. In general, the Carb-Fe concentration followed the order of terrace > plateau > slope > gully bottom in the 0–60 cm layer, but had a slightly different order of plateau > slope > terrace > gully bottom in 60–80 cm layer. The lowest Ox-Fe was found in gully bottom soils where it varied markedly with soil depth. In gully bottom soils, Ox-Fe in the 0–60 cm layer was nearly twice that in the depth of 60–80 cm layer. Among the four landscape positions, the plateau soils had the largest Ox-Fe concentration, which increased gradually with soil depth. The Ox-Fe concentration was somewhat larger in slope than in terrace, and changed slightly with soil depth in both soils. Similarly, the lowest concentration of Om-Fe occurred in gully bottom in the 0–80 cm soil layer, while the largest concentration occurred at different soil depths, which varied with landscape positions. The Min-Fe concentration changed significantly with soil depths in gully bottom soils, whereas it changed slowly with soil depth in plateau, slope and terrace soils. Relatively, gully bottom soil contained more Min-Fe except for the 20–40 cm soil layer than the soils at other landscape positions, and plateau soils had the least Min-Fe.
Discussion
Relationships between DTPA-Fe and soil properties
The content of DTPA-Fe in soils is controlled by soil physicochemical properties (e.g. pH, organic matter and CEC) and is correlated to Fe fractions. The result of path analysis indicated that organic matter posed the largest positive direct influence on DTPA-Fe, while pH exerts a negative influence on the concentration of DTPA-Fe (). These results were in agreement with results reported in the literature (CitationLi et al. 2007; CitationMoreno-Caselles et al. 2005; CitationSharma et al. 2000, 2004; CitationWei et al. 2006). The relations shown in are also supported by the profile distribution of DTPA-Fe, pH, and organic matter at different landscape positions. In our study, the plateau soil with the largest organic matter and smallest pH had the highest DTPA-Fe. The gully bottom soil with small organic matter had the smallest DTPA-Fe concentration among all of the selected landscape positions, though the pH of gully bottom soil was also small. It is thus obvious that the influence of soil organic matter on DTPA-Fe concentration was greater than that of pH in the study area due likely to the narrow range of soil pH. This result is consistent with that observed in some Alfisols of Punjab by CitationSharma et al. (2005).
Profile distribution of Fe fractions
The fractional distribution of Fe in the studied soils was similar to that in other Chinese soils. CitationShao et al. (1995) found that the ranges of Om-Fe, Ox-Fe, and Min-Fe were 7.4–29.6, 2.7–4.8, and 22.2–33.1 g kg−1, respectively in agricultural soils in Gansu Province, China. Similarly, CitationLüet al. (1993) reported that the amounts of Om-Fe, Ox-Fe, and Min-Fe in four calcareous soils were 3.3–41.4, 0.3–1.8 mg kg−1, and 2.6–31.2 g kg−1, respectively in Sichuan Province, China. CitationXing and Zhu (2003) studied Fe fractional distribution in 25 Chinese soils, including Cambisol, and found that 23 soils had Om-Fe concentrations <1.0% of the total Fe, whereas Min-Fe in nine soils accounted for more than 70% of the total soil Fe. In their investigation, the proportions of Om-Fe, Ox-Fe, and Min-Fe in Cambisol were 0.1, 34.1, and 85.7%, respectively, which were similar to our findings in this study.
Table 3 Path coefficient and correlation coefficients of soil properties to diethylene thiamine pentacetic acid (DTPA)-Fe
On the Loess Plateau, soil water regimes and soil physicochemical properties vary greatly with landscape positions (CitationWei and Shao 2007), resulting in large variation of soil Fe fractions (). Landscape position effects on the soil profile distributions of Carb-Fe can be attributed to carbonate distributions in the profiles because soil carbonate concentration is usually affected by the amount of leaching water and soil pH, which change with location and depth of soils in the landscape. In gully bottom, runoff water accumulates leading to larger soil water content and lower soil pH compared with other landscape positions (). The high water content favors dissolution of soil carbonate by changing the carbonic acid equilibrium (CitationMulder and Cresser 1994; CitationRogovska et al. 2007; CitationZuo et al. 2007), and eventually results in low concentrations of carbonate and Carb-Fe ().
The solubility of Fe in oxides is governed by soil reduction conditions, which are partly controlled by soil water regime (CitationFiedler and Sommer 2004; CitationTong et al. 1987; CitationZhang et al. 2003). The persistent wet soil condition may facilitate the formation of reducing conditions (CitationBohrerova et al. 2004; CitationClay et al. 1992; CitationHan et al. 2001; CitationPorter et al. 2004). As a result, dissolution and release of Fe from soil metal oxides increases as observed in gully bottom soil, which had lower Ox-Fe concentrations than other soils. After release, a part of the Fe may move deeper into the soil, while the remaining Fe can be transformed into other Fe fractions depending on the capacity of binding sites available in the different soil components. In contrast, soils in plateau, terrace and slope are oxidized and such a condition favors the formation and retention of Fe oxides.
Iron in soils can be strongly complexed by organic matter because Fe-organic matter complexes have a very high chemical stability constant (CitationMackowiak et al. 2001). Many researchers have reported that the content of Om-Fe is closely related with organic matter content (CitationAgbenin 2003; CitationDarke and Walbridge 2000; CitationYoung et al. 2006; CitationZhou et al. 2003). This relationship was also detected in our study (r = 0.654, P < 0.0001, n = 141), which explained the observations that Om-Fe and organic matter shared similar profile distributions at each landscape position.
Relationships between Fe fractions and DTPA-Fe and its indications to Fe availability
In order to further reveal the relationship between Fe fraction and its availability, we analyzed the contribution of each Fe fraction to Fe availability through correlation analysis and path analysis (). The results showed that Ex-Fe was positively related to DTPA-Fe concentration at P < 0.01. In view of the fact that Ex-Fe is the most available Fe in soils for plant absorption (CitationAgbenin 2003; CitationXue et al. 2006; CitationYoung et al. 2006; CitationZhou et al. 2003), we can safely conclude that Ex-Fe is the most direct source of available Fe in soils, which is in agreement with other findings (CitationAgbenin 2003; CitationXue et al. 2006; CitationYoung et al. 2006; CitationZhou et al. 2003). However, in our study, the content of Ex-Fe in soils was <0.7 mg kg−1, while the content of DTPA-Fe ranged from 1.3 to 5.2 mg kg−1 with an average value of 3.3 mg kg−1, nearly six times the Ex-Fe content. The great difference between Ex-Fe and DTPA-Fe suggests that Ex-Fe only contributes about one sixth of the available Fe and that other Fe fractions must also contribute. This assumption is supported by the relatively small direct path coefficient of Ex-Fe to DTPA-Fe (0.227).
Table 4 Path coefficient and correlation coefficients of Fe fractions to diethylene thiamine pentacetic acid (DTPA)-Fe
Carbonate bound Fe (Carb-Fe) represents the associated or the co-precipated Fe fraction that has been occluded into carbonates (CitationKabata-Pendias 2000; CitationXing and Zhu 2003). The presence of carbonate may decrease the Ex-Fe fraction because Ex-Fe is apt to be precipitated or occluded by carbonates in calcareous soils (CitationRogovska et al. 2007; CitationWei et al. 2005;CitationZuo et al. 2007), resulting in reduction of Fe availability in soil. In the present study, Carb-Fe was negatively related to DTPA-Fe (). Its very small direct path coefficient indicated that Carb-Fe had negligible contribution to soil Fe availability.
Organic matter bound-Fe (Om-Fe) was positively related with DTPA-Fe at a highly significant level (P < 0.01). This relationship is in line with the well-accepted conclusion that available Fe in soils is greatly determined by Om-Fe (CitationAgbenin 2003; CitationWei et al. 2005; CitationXing and Zhu 2003; CitationYoung et al. 2006; CitationZhou et al. 2003). In nature, the contribution of Om-Fe to soil Fe availability is subject to the interactions between organic matter and oxides. Because most Om-Fe can be absorbed by plants (CitationMackowiak et al. 2001), Om-Fe can be assumed to be a major direct source of available Fe. In our study, Ox-Fe was associated with the largest direct path coefficient and the largest positive correlation coefficient to DTPA-Fe (), suggesting that Ox-Fe was another source of available Fe in soils. The reason for this is probably that oxides in soils can be reduced, and Fe bound into this component will be released into soil solutions under proper soil conditions (such as low soil pH, high organic matter and CEC), providing available Fe into soils and increasing DTPA-Fe content (CitationXing and Zhu 2003; CitationXue et al. 2006). However, it is worth noting that Ox-Fe is an indirect rather than a direct source of available Fe in soils because the release of Ox-Fe is slow in well drained soils (CitationXue et al. 2006).
Iron bound into minerals (Min-Fe) cannot be absorbed by plants and is therefore not readily available for plant growth. The release and transformation of Min-Fe into other Fe fractions is very slow and can hardly occur in natural soil environments, meaning it contributes least to Fe availability, as indicated by its small direct and indirect path coefficients ().
Factor analysis was conducted to clarify the relationships between Fe fractions and DTPA-Fe. The results in show that Ex-Fe was solely classified into a group because it is the direct source of available Fe in soils. However, the low contents and small contributions of Ex-Fe to available Fe makes Ex-Fe have a relatively long distance from DTPA-Fe in , indicating its low contribution to Fe availability. As discussed above, Om-Fe is the major direct source of available Fe and Ox-Fe is an indirect source of available Fe. The influence of Om-Fe on DTPA-Fe is also influenced by Ox-Fe. These two Fe fractions are thus classified into the same group with DTPA-Fe. Since Min-Fe and Carb-Fe are not significantly correlated to DTPA-Fe, they plot further away from DTPA-Fe in .
Figure 4 Factor plot of diethylene thiamine pentacetic acid (DTPA) extractable iron and iron fractions.
The profile distribution of DTPA-Fe in soils of various landscape positions were related with the profile distributions of Fe fractions. Since Ox-Fe and Om-Fe are the major sources of available Fe in soils, a relatively high content of these two fractions results in a relatively high content of DTPA-Fe in plateau soils, whereas the lower contents of the two fractions lead to a lower DTPA-Fe in gully bottom soils. Although Ex-Fe is the direct source of available Fe in soils, the low content causes a weak relationship between Ex-Fe and DTPA-Fe. The higher content of Min-Fe often indicates high amounts of primary and secondary minerals in soils. As a result, the strong association of Fe onto minerals decreases concentrations of DTPA-Fe. In the present study, Min-Fe is highest in gully bottom soils and lowest in plateau soils, corresponding to the lowest DTPA-Fe in gully bottom soils and highest in plateau soils.
Conclusions
The soil profile distributions of DTPA-Fe varied greatly at selected landscape positions in the loessial gully region. The highest and lowest DTPA-Fe contents were observed in the plateau and gully bottom soils, respectively. The mineral bound Fe accounted for about 73 to 96% of the total Fe in this study area. The proportions of Ex-Fe, Carb-Fe, Ox-Fe, and Om-Fe were <28%. Among the five Fe fractions, Ex-Fe and Min-Fe were higher in slope, terrace, and gully bottom soils than in plateau soils, and Carb-Fe, Ox-Fe, and Om-Fe contents were higher in plateau, slope, and terrace soils than in gully bottom soils. Exchangeable Fe is the direct source of available Fe, but has limited contribution to Fe availability due to its low content. Oxide bound Fe is the largest indirect source of available Fe, thereby contributing most to Fe availability in soils. Organic matter bound Fe is the major direct source of available Fe. The different profile distributions of available Fe at the selected landscape positions are affected by Fe fractional distribution and organic matter.
Acknowledgment
This study was supported by the National Key Basic Research Special Foundation Project (2007CB106803), National Natural Science Foundation of China (40801111), Program for Youthful Talents in Northwest A & F University, Chinese Academy of Sciences Visiting Professorship for Senior International Scientists (2009Z2-37), West Light Foundation of the Chinese Academy of Sciences, and Japan Society for the Promotion of Science-Chinese Academy of Sciences (JSPS-CAS) Core-University Program “Researches on Combating Desertification and Developmental Utilization in Inland China”. The authors thank Dr Isaac Shainberg of the Institute of Soil, Water and Environmental Sciences, the Agricultural Research Organization, Israel for help in improving the quality of the manuscript.
References
- Abadia , J . 1995 . Iron Nutrition in Soils and Plants , Dordrecht : Kluwer Academic Publishers .
- Agbenin , JO . 2003 . The distribution and transformation of iron and manganese in soil fractions in a savanna Alfisol under continuous cultivation . Nutr. Cycl. Agroecosys. , 66 : 259 – 270 .
- Barton , LL and Abadia , J . 2006 . Iron Nutrition in Plants and Rhizospheric Microorganisms , Dordrecht : Kluwer Academic Publishers .
- Bohrerova , Z , Stralkova , R , Podesvova , J , Bohrer , G and Pokorny , E . 2004 . The relationship between redox potential and nitrification under different sequences of crop rotations . Soil Till. Res. , 77 : 25 – 33 .
- Clay , DE , Clapp , CE , Linden , DR and Molina , JAE . 1992 . Tillage influence on redox potential following rainfall . Soil Till. Res. , 22 : 211 – 219 .
- Connolly , EL and Guerinot , ML . 2002 . Iron stress in plants . Genome Biol. , 3 : 1024.1 – 1024.4 .
- Darke , AK and Walbridge , MR . 2000 . Al and Fe biogeochemistry in a floodplain forest: implications for P retention . Biogeochemistry , 51 : 1 – 32 .
- FAO/ISRIC/ISSS . 1998 . World Reference Base for Soil Resources , Rome : World soil resources reports .
- Fiedler , S and Sommer , M . 2004 . Water and redox conditions in wetland soils-their influence on pedogenic oxides and morphology . Soil Sci. Soc. Am. J. , 68 : 326 – 335 .
- Han , FX , Banin , A and Triplett , GB . 2001 . Redistribution of heavy metals in arid-zone soils under a wetting-drying cycle soil moisture regime . Soil Sci. , 166 : 18 – 28 .
- Kabata-Pendias , A . 2000 . Trace Elements in Soils and Plants , 3rd edn , Boca Raton : CRC Press .
- Knoke , D and Bohrnsted , GW . 1994 . Statistics for Social Data Analysis , 3rd edn , Itaska : F. E. Peacock Publishers .
- Li , H , Futch , SH , Stuart , RJ , Syvertsen , JP and McCoy , CW . 2007 . Associations of soil iron with citrus tree decline and variability of sand, soil water, pH, magnesium and Diaprepes abbreviatus root weevil: two-site study . Environ. Exp. Bot. , 59 : 321 – 333 .
- Lindsay , WL and Norvell , WA . 1978 . Development of a DTPA soil test for zinc, iron, manganese and copper . Soil Sci. Soc. Am. J. , 42 : 421 – 428 .
- Lü , SH , Liu , BH , Hu , S , Xiang , ZJ and Zhou , ZY . 1993 . “ Studies on Fe forms in calcareous soils as affected by different tillage measurements ” . In Role of Sulphur, Magnesium and Micronutrients in Balanced Plant Nutrition , Edited by: Hu , S . 518 – 522 . Sichuan, , China : Publishing House of Sichuan Scientific University . in Chinese
- Mackowiak , CL , Grossl , PR and Bugbee , BG . 2001 . Beneficial effects of humic acid on micronutrient availability to wheat . Soil Sci. Soc. Am. J. , 65 : 1744 – 1750 .
- Maiz , I , Arambarri , I , Garcia , R and Millán , E . 2000 . Evaluation of heavy metal availability in polluted soils by two sequential extraction procedures using factor analysis . Environ. Pollut. , 110 : 3 – 9 .
- Moreno-Caselles , J , Moral , R , Perez-Murcia , MD , Perez-Espinosa , A , Paredes , C and Agulló , E . 2005 . Fe, Cu, Mn, and Zn input and availability in calcareous soils amended with the solid phase of pig slurry . Commun. Soil Sci. Plant Anal. , 36 : 525 – 534 .
- Mulder , J and Cresser , MS . 1994 . “ Soil and soil solution chemistry ” . In Biogeochemistry of Small Catchments: A Tool for Environmental Research , Edited by: Moldan , B and Cerny , J . 107 – 131 . John Wiley & Sons Ltd., Chichester, UK .
- Page , AL , Miller , RH and Kenney , DR . 1982 . Methods of Soil Analysis, Part 2. Agronomy Monographs, vol. 9 , Madison : American Society of Agronomy .
- Plekhanova , IO . 2007 . Transformation of Fe, Mn, Co, and Ni compounds in humic podzols at different moisture . Biol. Bull. , 34 : 67 – 75 .
- Porter , GS , Bajita-Locke , JB , Hue , NV and Strand , D . 2004 . Manganese solubility and phytotoxicity affected by soil moisture, oxygen levels, and green manure additions . Commun. Soil Sci. Plant Anal. , 35 : 99 – 116 .
- Rogovska , NP , Blackmer , AM and Mallarino , AP . 2007 . Relationships between soybean yield, soil pH, and soil carbonate concentration . Soil Sci. Soc. Am. J. , 71 : 1251 – 1256 .
- SAS Institute Inc . 2000 . SAS Procedures Guide. Version 8 , Cary, NC : SAS Inst. Inc .
- Shao , YT , Zhen , QX and Liu , SD . 1995 . Study on forms and availability of Cu, Zn, Mn and Fe of principal agricultural soils in Gansu Province . Acta Pedologica Sin. , 32 : 423 – 429 . in Chinese
- Sharma , BD , Arora , H , Kumar , R and Nayyar , VK . 2004 . Relationships between soil characteristics and total and DTPA-extractable micronutrients in Inceptisols of Punjab . Commun. Soil Sci. Plant Anal. , 35 : 799 – 818 .
- Sharma , BD , Mukhopadhyay , SS and Arora , H . 2005 . Total and DTPA-extractable micronutrients in relation to pedogenesis in some Alfisols of Punjab, India . Soil Sci. , 170 : 559 – 572 .
- Sharma , BD , Mukhopadhyay , SS , Sidhu , PS and Katyal , JC . 2000 . Pedospheric attributes in distribution of total and DTPA-extractable Zn, Cu, Mn and Fe in Indo-Gangetic plains . Geoderma , 96 : 131 – 151 .
- Shuman , LM . 1985 . Fractionation method for soil micronutrients . Soil Sci. , 140 : 11 – 22 .
- Tessier , A , Campbell , PGC and Bisson , M . 1979 . Sequential extraction procedure for the speciation of particulate trace metals . Anal. Chem. , 51 : 844 – 851 .
- Tong , YA , Chaney , RL , Korcak , RF , Fan , F and Faust , M . 1987 . Influence of soil moisture level on apple iron chlorosis development in a calcareous soil . Plant Soil , 104 : 85 – 92 .
- Wei , XR , Hao , MD and Shao , MA . 2005 . Effects of long-term cropping on the forms and the availability of micronutrients in dryland soils on the Loess Plateau . Acta Ecologica Sinica , 27 : 3196 – 3203 . in Chinese
- Wei , XR , Hao , MD , Shao , MA and Gale , WJ . 2006 . Changes in soil properties and the availability of soil micronutrients after 18 years of cropping and fertilization . Soil Till. Res. , 91 : 120 – 130 .
- Wei , XR and Shao , MA . 2007 . Distribution of soil properties as affected by landforms in watershed of loessial gully region . J. Nat. Resour. , 22 : 946 – 953 . in Chinese
- Xing , GX and Zhu , JG . 2003 . Soil Chemistry of Trace Elements and Rare Earth Elements , Beijing : Science Press . in Chinese
- Xue , N , Seip , HM , Guo , J , Liao , B and Zeng , Q . 2006 . Distribution of Al-, Fe- and Mn-pools and their correlation in soils from two acid deposition small catchments in Hunan, China . Chemosphere , 65 : 2468 – 2476 .
- Young , KC , Maurice , PA and Hersman , LE . 2006 . Acquisition of Fe from various natural organic matter isolates by aerobic pseudomonad bacteria . Geomicrobiol. J. , 23 : 183 – 188 .
- Yu , CZ , Peng , L , Liu , YH , Dai , MJ and Peng , XL . 1991 . Content and distribution of trace elements and fertilizer efficiency in soils of loessial region . Acta Pedologica Sin. , 28 : 317 – 326 . in Chinese
- Zhang , LY , Zhang , XF and Zhai , H . 2002 . Effects of soil factors on plant chlorosis due to iron deficit . Chin. J. Soil Sci. , 33 : 74 – 77 . in Chinese
- Zhang , CE and Zheng , FL . 2002 . Temporal and spatial change characteristics of elements in reclaimed slope forest land . Chin. J. Appl. Ecol. , 13 : 672 – 674 . in Chinese
- Zhang , FS , Zheng , Y and Li , L . 2003 . Iron availability as affected by soil moisture in intercropped peanut and maize . J. Plant Nutr. , 26 : 2425 – 2437 .
- Zhou , QX , Gibson , CE and Stewart , BM . 2003 . Chemical forms and extractability of iron in sediments of three contrasting lakes of China and UK . J. Environ. Sci. (China) , 15 : 728 – 733 .
- Zhou , XM , Guo , JX and Zhao , J . 2004 . Dynamics of nutrient element iron in soil-plant ecosystem of the Songnen Plain Leymus chinensisgrassland . Chin. J. Appl. Ecol. , 15 : 2250 – 2254 . in Chinese
- Zuo , Y , Ren , L , Zhang , F and Jiang , RF . 2007 . Bicarbonate concentration as affected by soil water content controls iron nutrition of peanut plants in a calcareous soil . Plant Physiol. Biochem. , 45 : 357 – 364 .