Abstract
Mediator is a large, multisubunit complex that is required for essentially all mRNA transcription in eukaryotes. In spite of the importance of Mediator, the range of its targets and how it is recruited to these is not well understood. Previous work showed that in Saccharomyces cerevisiae, Mediator contributes to transcriptional activation by two distinct mechanisms, one depending on the tail module triad and favoring SAGA-regulated genes, and the second occurring independently of the tail module and favoring TFIID-regulated genes. Here, we use chromatin immunoprecipitation sequencing (ChIP-seq) to show that dependence on tail module subunits for Mediator recruitment and polymerase II (Pol II) association occurs preferentially at SAGA-regulated over TFIID-regulated genes on a genome-wide scale. We also show that recruitment of tail module subunits to active gene promoters continues genome-wide when Mediator integrity is compromised in med17 temperature-sensitive (ts) yeast, demonstrating the modular nature of the Mediator complex in vivo. In addition, our data indicate that promoters exhibiting strong and stable occupancy by Mediator have a wide range of activity and are enriched for targets of the Tup1-Cyc8 repressor complex. We also identify a number of strong Mediator occupancy peaks that overlap dubious open reading frames (ORFs) and are likely to include previously unrecognized upstream activator sequences.
INTRODUCTION
The Mediator complex occupies a unique and central role in transcription by RNA polymerase II (Pol II) in eukaryotic cells. Like the coactivator complexes SAGA and TFIID, Mediator is a large, multisubunit assemblage that is conserved across eukaryotes and is broadly required for transcription. Structural and genetic studies reveal Mediator to have a modular structure, consisting of head, middle, and tail modules, along with a cyclin-dependent kinase (CDK) module that associates dynamically with the core complex () (Citation1, Citation2). Early studies in yeast pointed to the tail module as an important target used by activator proteins such as Gal4 and Gcn4 to recruit Mediator upon gene induction and indicated that Mediator was recruited to upstream activating sequences (UASs) independently of preinitiation complex (PIC) components (Citation3, Citation4). However, deletion of tail module subunits was found to affect transcription of only a relatively small fraction (5 to 10%) of genes in exponentially growing yeast (Citation5, Citation6). Consistent with these findings, Mediator association in tail module deletion mutants was reduced at tested promoters that depend on the tail module for transcriptional activity but not at unaffected promoters, which comprise the large majority (Citation5). The effect of tail module subunit deletions, or indeed of any Mediator mutations, on Mediator recruitment genome-wide has not been reported.
FIG 1 Gene-specific Mediator association. (A) Schematic diagram of Mediator subunit organization. Underlined subunits (with their original nomenclature [Citation43]) were used as ChIP targets in this study. The darker-green ovals represent the components of the tail module triad Med2-Med3-Med15. (B to D) Normalized occupancy profiles for Pol II and the indicated Mediator subunits in wild-type yeast. (E) Mediator signal, averaged over promoters (left) and ORFs (right) for Med17 from the head module and Med2 from the tail module, in reads per million (RPM), is plotted against log2(expression level) (Citation26). Genes were sorted first on the basis of expression level and second by Mediator signal and binned into 20-gene groups for clarity.
![FIG 1 Gene-specific Mediator association. (A) Schematic diagram of Mediator subunit organization. Underlined subunits (with their original nomenclature [Citation43]) were used as ChIP targets in this study. The darker-green ovals represent the components of the tail module triad Med2-Med3-Med15. (B to D) Normalized occupancy profiles for Pol II and the indicated Mediator subunits in wild-type yeast. (E) Mediator signal, averaged over promoters (left) and ORFs (right) for Med17 from the head module and Med2 from the tail module, in reads per million (RPM), is plotted against log2(expression level) (Citation26). Genes were sorted first on the basis of expression level and second by Mediator signal and binned into 20-gene groups for clarity.](/cms/asset/50d7e49e-aa49-4839-8f01-8effbd31246e/tmcb_a_12275488_f0001_oc.jpg)
Three early studies on genome-wide localization of Mediator in budding yeast (Saccharomyces cerevisiae) and fission yeast (Saccharomyces pombe) were conducted at low resolution using microarray analysis following chromatin immunoprecipitation with microarray technology (ChIP-chip) against Mediator subunits and came to disparate conclusions (Citation7Citation–Citation9). Two very recently published studies of genome-wide localization of Mediator at higher resolution report Mediator occupancy at regions upstream of transcription start sites (TSSs) likely corresponding to upstream activating sequences (UASs) in yeast, while association with proximal promoters of active genes was found to be generally low or undetectable (Citation10, Citation11). Occupancy at proximal promoters was greatly increased in yeast mutants defective in phosphorylation of Ser5 in the C-terminal domain of Pol II, suggesting rapid, Kin28-dependent release of Mediator from proximal promoter regions in wild-type (WT) cells (Citation10, Citation11).
To obtain new insight into Mediator association with its target sites in vivo, we have performed chromatin immunoprecipitation sequencing (ChIP-seq) against Mediator subunits from head, middle/tail, tail, and CDK modules in budding yeast. To allow better distinction of low levels of association from experimental noise or artifacts accompanying ChIP or library amplification prior to sequencing (Citation12, Citation13), we compared ChIP-seq profiles from wild-type yeast to med17 temperature-sensitive (ts) yeast after 45 min at 37°C. In yeast harboring this mutation, the head module is disrupted at 37°C and mRNA transcription is greatly reduced genome-wide within 30 min (Citation14Citation–Citation16). Furthermore, comparison of ChIP against Mediator subunits and Pol II in wild-type and med17 ts yeast allowed detection of decreased association of Mediator and Pol II even at constitutively active promoters having relatively small amounts of Mediator association, while the relatively short temperature shift mitigates against the likelihood of indirect effects (Citation17). We also compare association of Mediator subunits and Pol II in wild-type and med3Δ med15Δ yeast, which lack two of the three subunits from the tail module triad of Med2-Med3-Med15, thus providing insight into the genome-wide function of the tail module in Mediator recruitment.
MATERIALS AND METHODS
Yeast strains and growth.
Yeast strains are listed in . Yeast cells were grown in rich medium, yeast extract-peptone-dextrose (YPD; 1% Bacto yeast extract [10 g/liter], 2% Bacto peptone extract [20 g/liter], 2% glucose [20 g/liter] in distilled water [dH2O]). For temperature shift experiments, cultures were grown to mid-log phase (optical density at 600 nm [OD600] = 0.6 to 1.2), diluted to an OD600 of 0.3 to 0.5, and allowed to grow for 2 h at the permissive temperature (25°C). Culture density was measured to ensure doubling, and cultures were shifted to the restrictive temperature (37°C) by adding medium heated to 65°C that amounted to 30% of the final volume and incubated at 37°C for 45 min. All other cultures were grown to mid-log phase (OD600 = 0.6 to 1.2), diluted to an OD600 of 0.3 to 0.5, and allowed to grow for 2 h at 30°C.
TABLE 1 Yeast strains used in this study
Chromatin immunoprecipitation and library preparation and amplification.
Whole-cell extracts (WCE) were prepared from 50 ml of yeast cells as described previously (Citation5). Conventional ChIP was performed as described previously, using 180 μl of WCE with the following antibodies: anti-Cdk8 (12.5 μg; Santa Cruz, Santa Cruz, CA), anti-protein A for Med2-TAP and Med14-TAP (2 μg; Sigma, St. Louis, MO), anti-Myc for Med15-myc (2 μg; Roche, Indianapolis, IN), anti-Med17 (5 μl; Abcam, Cambridge, MA), and anti-Rpb1 (5 μl; clone 8WG16; Covance, Princeton, NJ).
ChIP-seq analysis of wild-type and med17 ts yeast was done by using wild-type strain RMY513 and med17 ts strain RMY514 for immunoprecipitation of Med14, Med17, Cdk8, Pol II, and the no-antibody control and by using wild-type strain RMY511 and med17 ts strain RMY512 for immunoprecipitation of Med15, using a temperature shift as described above. Two independent replicates were done for wild-type and med17 ts ChIP experiments using antibodies against Med15-myc, Med17 (in RMY511 and RMY512 in the second experiment), Med14-TAP, and the no-antibody control, and mapped reads were pooled for analyses. ChIP-seq analysis of wild-type and med3Δ med15Δ yeast was done using wild-type strain Med2-TAP and deletion strain RMY516 grown at 30°C. To prepare samples for sequencing (ChIP-seq), a modified protocol was used that includes adaptor ligation to samples bound to antibody-bound beads (Citation18), as described below. Immunoprecipitation was carried out using the total WCE (800 μl) from one 50-ml culture for one IP sample with 2 to 10 μg of antibody or without antibody for no-antibody control samples, at 4°C with gentle agitation. Immunoprecipitated samples were incubated with 30 μl of protein A-coated washed Sepharose beads for 90 min at 4°C with gentle agitation. After incubation, samples were transferred to Spin-X columns and washed twice with FA lysis buffer (50 mM HEPES, 140 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1% sodium deoxycholate) for 3 min each with rotation at room temperature. Samples were spun for 1 min in a microcentrifuge at 4,000 rpm (1,500 × g) and the flowthrough was discarded, and then the samples were washed twice with 10 mM Tris-HCl (pH 7.5). DNA ends in the IP samples were blunted and repaired while still retained on antibody-bound beads using the New England BioLabs (NEB) quick blunting kit by resuspending the beads in the following: 10 μl of 10× quick blunting buffer, 10 μl deoxynucleoside triphosphate (dNTP) mix (kit supplied), 80 μl dH2O, and 2 μl blunt enzyme mix. Samples were incubated at room temperature for 30 min with gentle mixing (75 rpm). After incubation, samples were washed twice with FA lysis buffer and then washed twice with 10 mM Tris-HCl (pH 8.0) for 3 min each. To add A-tails to the samples, beads were resuspended in the following mixture: 10 μl 10× NEB buffer, 22 μl 100 mM dATP, 88 μl dH2O, and 2 μl NEB exo-Klenow fragment. Samples were incubated at 37°C for 30 min with gentle mixing (75 rpm). After incubation, samples were washed twice with FA lysis buffer and then washed twice with 10 mM Tris-HCl (pH 7.5) for 3 min each. To ligate the adapters to the IP samples using the NEB quick ligation kit, the beads were resuspended in the following mixture: 100 μl 1× ligase buffer (50 μl 2× buffer and 50 μl dH2O), 1 μl or 3 μl 0.6 μM NEXTflex adapters (NEXTflex; Bioo Scientific, Austin, TX), and 4 μl quick ligase. Samples were incubated at room temperature for 15 min with gentle mixing (75 rpm). After incubation, samples were washed twice with FA lysis buffer and once each with FA lysis buffer-high salt (50 mM HEPES, 500 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1% sodium deoxycholate), LiCl wash solution (10 mM Tris-HCl, 250 mM LiCl, 0.5% NP-40, 1 mM EDTA, 0.5% sodium deoxycholate), and TE (10 mM Tris-HCl, 1 mM EDTA) for 3 min each. Spin-X columns with washed beads were transferred to a new microcentrifuge (dolphin-nosed) tube, and 100 μl of ChIP elution buffer (50 mM Tris-HCl [pH 7.5], 10 mM EDTA, 1% SDS) was added. Samples were incubated in an Eppendorf thermomixer at 65°C for 10 min with shaking at 600 rpm and then eluted by spinning for 1 min at 4,000 rpm. Cross-links on eluted DNA were reversed by incubating in the Eppendorf thermomixer at 100°C for 10 min with shaking at 600 rpm. DNA was purified using phenol-chloroform extraction and ethanol precipitated with 3 M sodium acetate and glycogen for 1 h at −80°C. After precipitation, samples were spun at 13,000 rpm for 15 min at 4°C to pellet DNA, washed with 70% ethanol to remove salt, and spun at 13,000 rpm for 5 min at 4°C. Bar-coded IP samples were resuspended in 11 μl of dH2O. To determine the number of PCR cycles needed for amplification, 1 μl of IP DNA was used as the template DNA in a quantitative real-time PCR (qRT-PCR) with the NEXTflex primer mix (diluted 1:2). Real-time PCR was performed with the cycle threshold (CT) set to 0.1. Samples were then amplified for x + 6 cycles, where x is the number of cycles each sample took to cross the set threshold. The amplification was carried out using a 50-μl PCR mixture composed of 5 μl 10× Taq buffer, 8 μl DNA with ligated adapters, 1.5 μl NEXTflex primer mix, 0.4 μl dNTP mix (25 mM each), 1.5 μl Taq polymerase, and 33.6 μl dH2O. Samples were subjected to 10 min at 95°C to activate the enzyme and then cycled at 95°C for 30 s, 60°C for 30 s, and 72°C for 30 s for x + 6 cycles. Amplified, adapter-ligated DNA was examined on a 2% EX-Gel on the Invitrogen E-Gel system, and a smear of DNA fragments was seen from 200 to 600 bp, with an intense band seen at 120 bp (amplified ligated adapters). DNA fragments from 200 to 500 bp were excised from the gel and purified using the Qiagen (Valencia, CA) gel purification kit. The purification was performed with no heat but twice the recommended amount of QG buffer and constant rotation at room temperature. Samples were eluted with 30 μl of water, which is the minimal volume for elution on the QIA columns. The eluate was run over the column twice to ensure maximal recovery of DNA. Samples were stored at −80°C until submitted and sequenced on an Illumina HiSeq 2500 at the University of Buffalo Next-Generation Sequencing and Expression Analysis Core.
qRT-PCR.
qRT-PCR was conducted as described previously using a StepOnePlus real-time PCR system (Applied Biosystems/Life Technologies, Green Island, NY) (Citation5). Technical replicates of individual 12.5-μl reaction mixtures were averaged prior to averaging biological replicates and calculating standard deviations. Reactions were run on Fast protocol (20 s at 95°C, followed by 40 cycles of 1 s at 95°C and 20 s at 60°C); data capture was at the end of the extension period. Primers () are for areas indicated in figures.
TABLE 2 Primers used in this study
Data analysis.
Unfiltered sequencing reads were aligned to the S. cerevisiae reference genome (SacCer3) by using bwa (Citation19). Up to 1 mismatch was allowed for each aligned read. Reads mapping to multiple sites were retained to allow evaluation of associations with nonunique sequences (Citation19), and duplicate reads were also retained because of the high coverage. Binding regions were identified using SICER (Citation20) with the following parameters: effective genome size of 0.97 (97% of the yeast genome is mappable), window size of 100 bp, and gap size of 100 bp. Calculation of coverage, comparisons between different data sets, and identification of overlapping binding regions were preceded by library size normalization and were performed with the “chipseq” and “GenomicRanges” packages in BioConductor (Citation21). Occupancy profiles were generated using the Integrative Genomics Viewer (IGV) (Citation22). For occupancy profiles in , Fastq files from reference Citation23 were downloaded from E-MTAB-1595 at Array Express and processed using the Galaxy server (Citation24); reads were mapped using bwa and visualized using IGV (Citation19, Citation22); ChIP-chip data from reference Citation10 was downloaded from accession no. GSE55402 at the Genome Expression Omnibus website and visualized using the UCSC Genome Browser.
Microarray data accession number.
ChIP-seq data have been deposited in the NCBI Sequence Read Archive under accession number SRP047524.
RESULTS
Mediator occupancy at promoters does not correlate simply with gene expression.
We examined occupancy of Mediator subunits genome-wide in S. cerevisiae by performing ChIP-seq in wild-type, med17 ts, and med3Δ med15Δ yeast. ChIP was carried out against subunits from the tail, tail/middle, head, and CDK modules (), along with the Rpb1 subunit of Pol II and a no-antibody control, in wild-type and med17 ts yeast after 45 min of growth at 37°C, and from wild-type and med3Δ med15Δ yeast grown at 30°C (). We obtained 4 to 14 million uniquely mapped reads for the wild-type and med17 ts yeast samples, and 17 to 39 million uniquely mapped reads for the med3Δ med15Δ yeast samples ().
TABLE 3 Summary of ChIP-seq experiments
Occupancy profiles obtained by plotting read density against chromosomal coordinates revealed gene-specific patterns of Mediator subunit occupancy ( to ). ChIP profiles were validated for several loci by standard ChIP followed by qPCR (see Fig. S1 in the supplemental material). We expected to observe peaks of occupancy for Mediator core complex subunits upstream of active genes, corresponding to sites of recruitment by transcriptional activators, and possibly to find association with transcribed open reading frames (ORFs) (Citation3, Citation7Citation–Citation9). At some genes, such as UTR2, MIT1, and YBL029W, upstream peaks of Mediator occupancy likely corresponding to sites of recruitment were readily visible ( and ). However, at other transcribed genes, such as RPL19B, such upstream peaks were much less evident (), in spite of Mediator being required for recruitment of Pol II and the PIC (Citation10, Citation17). Mediator peaks were sometimes also found upstream of poorly transcribed genes, such as FLO9 (which is repressed by the Cyc8-Tup1 complex [see below]) (). Correspondingly, Mediator association with promoters (including upstream regions) was only modestly correlated with expression ().
ChIP signal for Mediator subunits was also apparent across ORFs of transcribed genes and coincident with that for Pol II ( and ). This signal showed a moderate correlation with expression level () and was generally stronger for subunits from the core Mediator complex than for Cdk8, consistent with association of the core Mediator complex with Pol II being incompatible with association of the CDK module (Citation25). However, this observation must be interpreted cautiously, as highly transcribed ORFs have been found to give rise to signals in ChIP experiments against transcriptionally irrelevant targets and in controls using yeast strains lacking the epitopes targeted for ChIP (Citation10, Citation12, Citation13, Citation23).
Recruitment of Mediator tail module subunits, including Med14, can occur independently of the head module.
To obtain a global perspective on Mediator association with active genes, we computed heat maps and average gene occupancy profiles for Mediator core subunits Med17 (head), Med14 (tail/middle), and Med15 (tail) and for Pol II over the 300 most highly expressed ORFs and flanking regions in wild-type and med17 ts yeast (Citation26), corrected for signal present in the negative control (). ChIP signals were weaker and generally not informative for more weakly transcribed genes (see Fig. S2 in the supplemental material), although exceptions to this have already been noted (). Consistent with previous reports, peaks of Mediator occupancy are apparent 200 to 500 bp upstream of many of these genes (Citation3, Citation10, Citation11, Citation17, Citation23). In med17 ts yeast, Med17 upstream occupancy is largely abolished, as we previously showed for Med18 (head) at select active genes (Citation17). Pol II occupancy is also greatly reduced, consistent with previous results (Citation10, Citation17). In contrast, upstream occupancy by Med14 and Med15 is retained in med17 ts yeast, demonstrating that the tail module of Mediator can be recruited in the absence of intact Mediator on a genome-wide scale. Furthermore, the similar behavior of Med14 and Med15 supports a close connection of Med14 with the tail module triad Med2-Med3-Med15 (Citation1) and is consistent with the continued Med14 association with UAS regions but not with core promoter regions, observed at a few specific promoters in med17 ts yeast (Citation17). Upstream occupancy by tail module subunits in both wild-type and med17 ts yeast can also be seen at some individual loci, such as for Med15 at PMA1 (), UTR2, and RPL19B (see Fig. S3 in the supplemental material).
FIG 2 Gene occupancy by Mediator subunits and Pol II in wild-type and med17 ts yeast. Heat maps and occupancy profiles are shown for the indicated Mediator subunits and Pol II, normalized and corrected for no-antibody control, for the 300 most highly transcribed genes (Citation26). Occupancy profiles show normalized reads per million; the x axis shows “stretched” genic regions, normalized for length, flanked by regions ±3 kb.
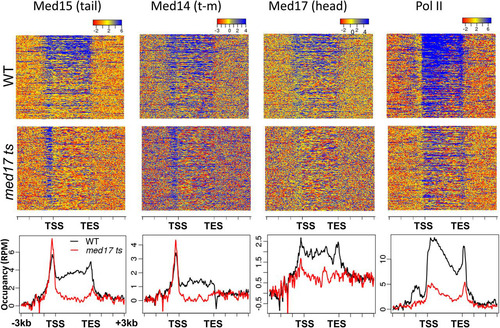
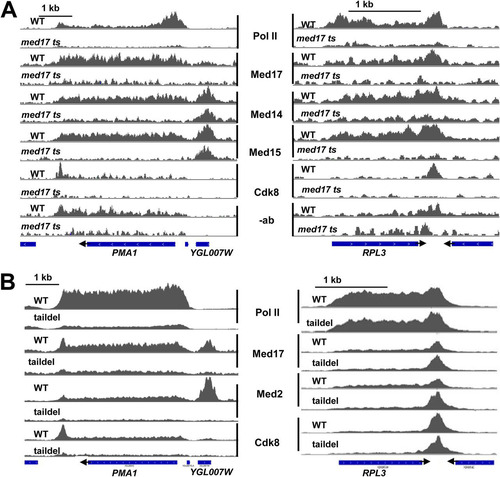
FIG 3 Dependence of Mediator and Pol II occupancy on Med17 (A) and on the tail module triad (B). Normalized occupancy profiles are shown for Pol II or Mediator subunits, as indicated, in wild-type and med17 ts yeast (A) or wild-type and med3Δ med15Δ yeast (B), at the PMA1 and RPL3 loci. (A) Control no-antibody occupancy profiles are shown from wild-type and med17 ts yeast as well. Occupancy profiles are normalized between each wild type and corresponding mutant to allow direct comparison, and the no-antibody control samples are normalized to the same scale as the Med17 profiles in panel A, which displays the lowest signal among the Mediator subunit ChIP targets. Blue bars indicate ORFs, and arrowheads indicate the direction of the transcripts. YGL007W is a dubious ORF that contains a Rap1 binding site required for PMA1 activation (Citation44).
ChIP signals for Med17, Med15, and Med14 were apparent across ORFs of highly transcribed genes, albeit at lower levels than observed for Pol II, and their association with coding sequences decreases to baseline levels in med17 ts yeast ( and ; see also Fig. S3 in the supplemental material). However, as mentioned earlier, highly transcribed regions have been found to yield artifactual ChIP signals, for example, from irrelevant proteins such as ectopically expressed green fluorescent protein (GFP) (Citation13). Furthermore, the “chippability” of these regions is likely to be a consequence of their transcriptional competence (Citation12, Citation13). For this reason, decreased signal that is seen under conditions that cause decreased transcription (e.g., in med17 ts yeast) cannot be used to distinguish artifactual signal from that arising from transcriptionally relevant targets. Thus, although our results do not exclude Mediator occupancy across ORFs, additional studies will be needed to address this issue conclusively.
Differential behavior of Mediator at SAGA-regulated and TFIID-regulated genes.
Yeast promoters can be divided into two principal types: about 15% have consensus TATA elements, are regulated by the SAGA complex, and are enriched for stress-regulated genes, while 80 to 85% lack consensus TATA elements, are regulated by TFIID, and are enriched for housekeeping genes (Citation27, Citation28). We recently reported that, on a genome-wide basis, SAGA-regulated genes show preferential dependence, relative to TFIID-regulated genes, on the tail module triad, Med2-Med3-Med15, for transcriptional activation (Citation5). Based on ChIP analysis at several promoters, this preferential dependence for transcriptional activation was shown to be due at least in part to a stronger dependence for recruitment of Mediator on tail module subunits at SAGA-regulated genes than at TFIID-regulated genes (Citation5). This distinction is supported and extended by the global analysis reported here.
First, Pol II occupancy is more substantially reduced across gene bodies of SAGA-regulated than TFIID-regulated genes in med3Δ med15Δ yeast, consistent with previous transcriptome analysis () (Citation5). In contrast, both SAGA- and TFIID-regulated genes show greatly reduced Pol II occupancy in med17 ts compared to wild-type yeast, although SAGA-regulated genes show more Pol II retention near the TSS than TFIID-regulated genes do (). The effect of the med3Δ med15Δ mutation appears strongest for those genes having highest Pol II occupancy (), consistent with SAGA-regulated genes exhibiting highest average Pol II occupancy levels among the top 1,000 transcribed genes (). Differences between SAGA- and TFIID-regulated genes can also be seen in profiles of Mediator and Pol II occupancy at individual genes. For example, PMA1, which is SAGA regulated and depends strongly on the Mediator tail module (Citation5, Citation28), shows strongly reduced Pol II occupancy and upstream Mediator peaks in med3Δ med15Δ yeast, while occupancy is nearly unchanged in this mutant at the TFIID-dependent RPL3 and RPL19B loci (although upstream occupancy by Mediator is low at these latter two loci) (; see also Fig. S3B in the supplemental material). UTR2, which shows mixed dependence on TFIID and SAGA (Citation28), correspondingly exhibits a partial reduction in Pol II and Mediator occupancy in med3Δ med15Δ yeast (see Fig. S3B). Additional examples, validated by conventional ChIP followed by qPCR, are shown in Fig. S1 in the supplemental material.
FIG 4 Distinct Mediator occupancy patterns at SAGA-regulated and TFIID-regulated (TF) genes. (A) Average Pol II occupancy profiles for the 1,000 genes showing highest expression, normalized for length of the transcribed sequence, and divided into SAGA-regulated (195 genes) and TFIID-regulated (781 genes) genes, as indicated. Top, profiles are shown for wild-type and med3Δ med15Δ yeast; bottom, profiles are shown for wild-type and med17 ts yeast. We do not know the cause for the enrichments seen at the 5′ end of ORFs in the bottom panel and not in the upper panel, but note that these profiles are from yeast shifted to 37°C for 1 h, whereas the profiles in the upper panel are from yeast grown at 30°C. (B) Pol II occupancy, given as the average intensity in reads per million (RPM) over the gene body, plotted for all genes in med3Δ med15Δ yeast against wild-type yeast; (C) average Med2 occupancy profiles, as described for panel A, in wild-type and med3Δ med15Δ yeast for the 1,000 genes showing highest and lowest expression; (D) average Med15 occupancy profiles in wild-type and med17 ts yeast for the 1,000 genes showing highest expression; (E) ChIP signal for GFP (using data from reference Citation13) expressed in yeast grown in galactose medium, plotted for the 1,000 most highly expressed genes, normalized for length, and separated into SAGA-regulated and TFIID-regulated (TF) genes.
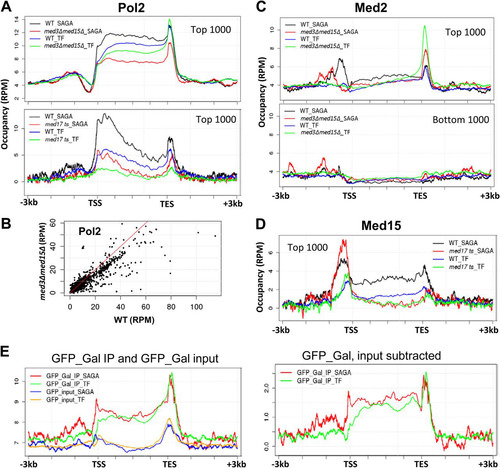
Another distinction between SAGA- and TFIID-regulated genes is the presence of conspicuous peaks of Mediator occupancy upstream of the TSS of many SAGA-regulated genes, while such peaks are typically less evident for TFIID-regulated genes ( and ; see also Fig. S4 in the supplemental material). No appreciable upstream occupancy peaks within 1 kb of the TSS are seen for the lowest expressed genes (). Med2 occupancy upstream of the TSS decreases substantially at SAGA-regulated genes in med3Δ med15Δ yeast, whereas occupancy of TFIID-regulated genes is constitutively low (). In sum, the genome-wide trend of greater dependence on the Mediator tail module of SAGA-regulated over TFIID-regulated genes seen for transcriptional activity is also reflected in a greater dependence for recruitment, as seen previously for a select few examples (Citation5).
The 3′ ends of highly active genes are strongly “chippable.”
We were surprised to see peaks in our ChIP-seq data for Pol II and Mediator subunits at the 3′ end of many genes, both SAGA and TFIID regulated ( to ; see also Fig. S4 in the supplemental material). Similarly, previous reports indicated possible enrichment of Pol II (Citation29) and of Sin4/Med16 but not other Mediator subunits (Citation10) at 3′ regions of active genes. To test whether this apparent 3′ enrichment could be related to “chippability” artifacts (Citation12, Citation13), we plotted ChIP-seq data for GFP expressed in yeast cells grown in glucose or galactose medium (Citation13) over highly transcribed genes normalized for length (). Marked enrichment was observed at the 3′ ends of highly transcribed genes in both ChIP and input samples; these peaks were not eliminated from the ChIP samples upon subtraction of input, as was also found for ChIP signals over coding sequences (Citation12, Citation13). Based on these observations, the ChIP signals observed in our data at 3′ ends of active genes cannot be interpreted as representing true peaks of Mediator occupancy.
Mediator peaks are associated with promoters spanning a broad range of activities and enriched for genes regulated by the Tup1-Cyc8 repressor complex.
To identify discrete sites of Mediator association with the yeast genome, we screened for Mediator subunit peaks using SICER (Citation20). As expected, substantial overlap was found among occupancy peaks for Mediator subunits from the tail (Med2), head (Med17), and CDK (Cdk8) modules (). The presence of Mediator occupancy peaks upstream of many active genes ( to ) suggested that we might be able to use occupancy peaks to identify sites of activator-dependent recruitment of Mediator. To test this idea, we focused on the 1,113 occupancy peaks for the tail module subunit Med2, as tail module subunits are frequently direct targets of activator proteins in yeast (Citation30). These peaks can be divided into three principal classes (). The first class comprises about 670 Med2 islands that overlap Pol II peaks, which almost entirely (96%) overlap ORFs. The second class comprises 179 peaks that overlap 176 tRNA genes and the Pol III transcribed snR6, snR52, and SCR1 genes, and the third consists of 228 peaks that did not overlap Pol II peaks or Pol III transcripts (see Data set S1 in the supplemental material). Because both Pol II and Pol III transcribed regions are subject to ChIP artifacts (Citation12, Citation13), we excluded these peaks from further analysis.
FIG 5 Peaks of Med2 occupancy are found primarily in intergenic regions and are associated with genes varying in expression. (A) Overlap among occupancy peaks for Med2, Med17, and Cdk8. Because more than one peak for a given subunit can overlap a peak from another, the numbers in the overlap regions do not add up exactly to the number of peaks for each subunit. (B) Med2 peaks of <2 kb in width were sorted into those overlapping and not overlapping Pol II peaks or Pol III transcripts. (C) Normalized occupancy profiles for Mediator subunits and Pol II showing examples of Med2 peaks located between divergent, tandemly oriented, or convergent genes or overlapping dubious ORFs. (D) Normalized Med15, Med14, and Med17 average occupancy in RMY511 and RMY513 wild-type yeast centered on the 146 Med2 peaks as indicated in panel B. (E) Med2 and Med17 occupancy from reference Citation23 for the 228 Med2 peaks indicated in panel B, divided into low (med2cov < 5) and high (med2cov > 5) Med2 peaks. (F) Genes having promoters associated with Med2 peaks (med2cov > 5) were identified and plotted according to their ranking for expression level (Citation14), from highest (quintile 1) to lowest (quintile 5).
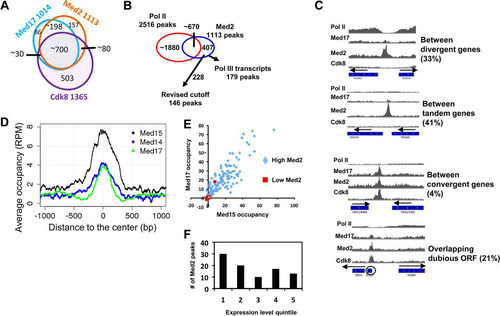
We examined these peaks more closely by sorting them according to whether or not they overlapped with annotated ORFs and by the nature of the overlapping or nearest ORF. Manual inspection of peaks using the IGV browser revealed a higher proportion of broad and likely spurious “peaks” at the lower end of the range of integrated peak intensities. Based on these observations, we revised our cutoff (using “med2 coverage” of >5) (see Data set S1 in the supplemental material), leaving a total of 146 Med2 peaks as defined above. These peaks seem likely to represent sites of Mediator recruitment. First, they are preferentially located in intergenic regions (115 of 146 sites; P < 10−16 after accounting for removal of Pol II peaks and Pol III transcribed genes) (), and intergenic peaks were seldom found between convergent genes (6/112; P < 10−4, hypergeometric distribution) (). Second, the 31 peaks that overlapped ORFs were enriched for dubious ORFs (18/31; P < 10−9, hypergeometric test) (). Some of these are likely to contain upstream activating sequences that are sites of Mediator recruitment, as with YGL007W (). Third, these strongly occupied Med2 peaks were also occupied by additional Mediator subunits and were corroborated as being occupied by Mediator subunits using data from another recent study (Citation23) ( and ).
Contrary to expectation, although the genes associated with Med2 peaks showed a modest enrichment for strongly transcribed genes, many were not expressed at high levels (). Using CERES (Citation31), we examined whether promoters associated with Med2 peaks were enriched for particular transcription factor (TF) binding sites and identified 20 TFs enriched in promoters associated with Med2 intergenic peaks (). Remarkably, 8 of these are among the approximately 12 TFs known to recruit the Tup1-Cyc8 corepressor complex (P < 2 × 10−4, hypergeometric distribution) (Citation32, Citation33). Consistent with this enrichment, genes associated with Med2 peaks that are intergenic or that overlap dubious ORFs were enriched for genes repressed by the Tup1-Cyc8 repressor complex (33/101 upregulated >2× in tup1Δ yeast [Citation34]; P < 10−13). These findings indicate that Mediator is recruited to promoters of genes that are repressed by Tup1-Cyc8. Nonetheless, Mediator still exerts an activating effect on many of the genes associated with Med2 intergenic peaks, as 29 out of 106 such genes are downregulated by >1.5× in med3Δ med15Δ yeast (Citation5) (P < 10−10, hypergeometric test).
TABLE 4 Transcription factors enriched at intergenic Med2 peaks from CERES (Citation31)Table Footnotea
Taken together, our results indicate that in yeast grown in YPD, sites stably and strongly occupied by the Mediator complex are enriched for sites of activator-dependent Mediator recruitment that occur in conjunction with a relatively small set of TFs. These recruitment sites are associated with genes spanning a broad range of activities and are enriched for promoters associated with genes regulated by the Mediator tail module and/or the Tup1-Cyc8 corepressor complex.
DISCUSSION
We report here comparison of genome-wide association of Mediator and Pol II in wild-type yeast with med17 ts and med3Δ med15Δ yeast. Our results partly support the canonical view of Mediator being recruited to upstream activating sites (UASs) and in turn recruiting Pol II and the general transcription machinery (Citation3, Citation30, Citation35), as we observe peaks of Mediator occupancy 200 to 500 bp upstream of many of the most highly transcribed ORFs, and we show that Pol II occupancy over these ORFs is greatly reduced in med17 ts yeast. However, our findings also reveal some aspects of Mediator recruitment that are not predicted by the canonical view.
First, although many highly transcribed genes are associated with upstream peaks of Mediator occupancy, at some genes such peaks are not readily apparent ( and ). In some cases, such as the PHO84 and CHA1 promoters, Mediator occupancy peaks can be seen at known UASs but are very low (). This variability in Mediator association upstream of active genes is also apparent in ChIP-seq and ChIP-chip data from two recently published studies ( and ) (Citation10, Citation23). Mediator occupancy peaks were found to increase upon inactivation of the Kin28 kinase or mutation of the Kin28 target, Ser5 from the Pol II carboxy-terminal domain () (Citation10, Citation11). However, this increased occupancy was associated with promoter-proximal regions rather than UASs. We found that Med2 peaks identified using the SICER algorithm are associated with a limited set of transcriptional activators (). Previous work has also shown much stronger Mediator association at genes induced by galactose and by heat shock than at strongly transcribed, constitutively active genes (Citation36). Why some UASs exhibit prominent Mediator peaks and others do not is unclear and could reflect altered recruitment or dynamics.
FIG 6 Variable Mediator occupancy at different UAS regions. (A) UAS regions of PHO84 and CHA1 show occupancy peaks for Med2 from the Mediator tail module that depend on the tail module triad and low occupancy for Pol II at these UASs. Screenshots from IGV for Med17 (head module), Med2 (tail module), and Pol II occupancy in wild-type and med3Δ med15Δ yeast, at the PHO84 and CHA1 loci and surrounding regions. Occupancy profiles are normalized between wild-type and mutant yeast but not between ChIP targets. The PHO5 and CHA1 UAS regions are indicated by the vertical red lines (Citation45, Citation46). (B) Occupancy profiles from IGV for Med17 and Med15 using data from reference Citation23. All profiles are set to the same scale. (C) Occupancy profiles from the UCSC Genome Browser for Med15 in wild-type yeast and under conditions of Kin28 inactivation, using data from reference Citation10; all profiles are set to the same scale.
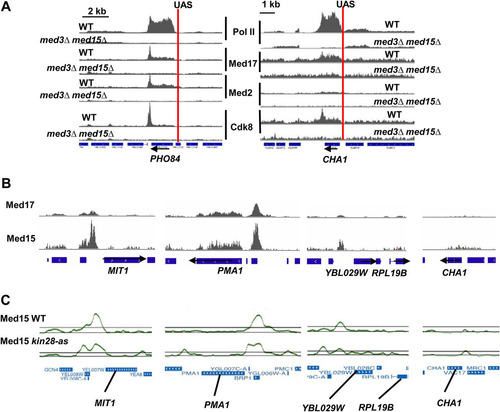
Conspicuous upstream peaks of Mediator occupancy are preferentially associated with SAGA-regulated over TFIID-regulated genes and are generally lost or greatly reduced in med3Δ med15Δ yeast, concomitant with strongly reduced Pol II association over the associated ORFs ( and ). This finding corroborates previous findings that SAGA-regulated genes exhibit greater dependence on tail module subunits for transcription than do TFIID-regulated genes (Citation5, Citation37). The absence of prominent upstream Mediator peaks at most TFIID-regulated, tail module-independent promoters, in spite of the presence of Mediator at proximal promoter regions of these genes (Citation10, Citation11, Citation17), underscores the possibility that Mediator is recruited to such targets by a distinct mechanism from that used at SAGA-regulated, tail module-dependent genes (Citation38). Mediator can evidently be recruited in part via interactions with the general transcription machinery (Citation39, Citation40); such a mechanism could be consistent with patterns of Mediator occupancy that we observe here at TFIID-regulated, tail module-independent genes (; see also Fig. S3 in the supplemental material).
Mediator has been reported to be associated with transcribed ORFs in both S. cerevisiae and S. pombe (Citation7, Citation9). However, recent reports of artifactual ChIP signals occurring at highly transcribed loci in yeast call these results into question (Citation10, Citation12, Citation13). Our results are not inconsistent with Mediator association with transcribed ORFs, but neither can they establish such association until a means is found to distinguish artifactual, transcription-dependent ChIP signals from functionally relevant association.
Finally, we note that this study assesses only Mediator localization in yeast grown in rich medium. Many studies have already examined the role of Mediator in gene regulation changes in response to environmental and developmental signals, but the genome-wide association patterns of Mediator have not been examined in such studies. Given the association of Mediator with developmental disorders and disease (Citation41, Citation42), it will also be important to examine the extent to which mechanisms of Mediator recruitment and association reported here are conserved in metazoan cells.
tmcb_a_12275488_sm0001.pdf
Download PDF (422.6 KB)tmcb_a_12275488_sm0002.pdf
Download PDF (1.1 MB)tmcb_a_12275488_sm0003.pdf
Download PDF (215.8 KB)tmcb_a_12275488_sm0004.pdf
Download PDF (794 KB)tmcb_a_12275488_sm0005.pdf
Download PDF (119.5 KB)tmcb_a_12275488_sm0006.xls
Download MS Excel (104 KB)ACKNOWLEDGMENTS
We thank Joe Wade and Suraiya Ansari for helpful discussions and acknowledge assistance from the Wadsworth Center Bioinformatics Core Facility.
This work was supported by funding from the NSF (MCB0949722 to R.H.M.), the Wadsworth Center, and the Intramural Research Program of the NIH, NLM, NCBI.
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.00991-14.
REFERENCES
- Tsai KL, Tomomori-Sato C, Sato S, Conaway RC, Conaway JW, Asturias FJ. 2014. Subunit architecture and functional modular rearrangements of the transcriptional mediator complex. Cell 157:1430–1444. http://dx.doi.org/10.1016/j.cell.2014.05.015.
- Lariviere L, Seizl M, Cramer P. 2012. A structural perspective on Mediator function. Curr Opin Cell Biol 24:305–313. http://dx.doi.org/10.1016/j.ceb.2012.01.007.
- Kuras L, Borggrefe T, Kornberg RD. 2003. Association of the Mediator complex with enhancers of active genes. Proc Natl Acad Sci U S A 100:13887–13891. http://dx.doi.org/10.1073/pnas.2036346100.
- Myers LC, Gustafsson CM, Hayashibara KC, Brown PO, Kornberg RD. 1999. Mediator protein mutations that selectively abolish activated transcription [see comments]. Proc Natl Acad Sci U S A 96:67–72. http://dx.doi.org/10.1073/pnas.96.1.67.
- Ansari SA, Ganapathi M, Benschop JJ, Holstege FC, Wade JT, Morse RH. 2012. Distinct role of Mediator tail module in regulation of SAGA-dependent, TATA-containing genes in yeast. EMBO J 31:44–57. http://dx.doi.org/10.1038/emboj.2011.362.
- van de Peppel J, Kettelarij N, van Bakel H, Kockelkorn TT, van Leenen D, Holstege FC. 2005. Mediator expression profiling epistasis reveals a signal transduction pathway with antagonistic submodules and highly specific downstream targets. Mol Cell 19:511–522. http://dx.doi.org/10.1016/j.molcel.2005.06.033.
- Andrau JC, van de Pasch L, Lijnzaad P, Bijma T, Koerkamp MG, van de Peppel J, Werner M, Holstege FC. 2006. Genome-wide location of the coactivator mediator: binding without activation and transient Cdk8 interaction on DNA. Mol Cell 22:179–192. http://dx.doi.org/10.1016/j.molcel.2006.03.023.
- Venters BJ, Pugh BF. 2009. A canonical promoter organization of the transcription machinery and its regulators in the Saccharomyces genome. Genome Res 19:360–371. http://dx.doi.org/10.1101/gr.084970.108.
- Zhu X, Wiren M, Sinha I, Rasmussen NN, Linder T, Holmberg S, Ekwall K, Gustafsson CM. 2006. Genome-wide occupancy profile of mediator and the Srb8 to 11 module reveals interactions with coding regions. Mol Cell 22:169–178. http://dx.doi.org/10.1016/j.molcel.2006.03.032.
- Jeronimo C, Robert F. 2014. Kin28 regulates the transient association of Mediator with core promoters. Nat Struct Mol Biol 21:449–455. http://dx.doi.org/10.1038/nsmb.2810.
- Wong KH, Jin Y, Struhl K. 2014. TFIIH phosphorylation of the Pol II CTD stimulates mediator dissociation from the preinitiation complex and promoter escape. Mol Cell 54:601–612. http://dx.doi.org/10.1016/j.molcel.2014.03.024.
- Park D, Lee Y, Bhupindersingh G, Iyer VR. 2013. Widespread misinterpretable ChIP-seq bias in yeast. PLoS One 8:e83506. http://dx.doi.org/10.1371/journal.pone.0083506.
- Teytelman L, Thurtle DM, Rine J, van Oudenaarden A. 2013. Highly expressed loci are vulnerable to misleading ChIP localization of multiple unrelated proteins. Proc Natl Acad Sci U S A 110:18602–18607. http://dx.doi.org/10.1073/pnas.1316064110.
- Holstege FC, Jennings EG, Wyrick JJ, Lee TI, Hengartner CJ, Green MR, Golub TR, Lander ES, Young RA. 1998. Dissecting the regulatory circuitry of a eukaryotic genome. Cell 95:717–728. http://dx.doi.org/10.1016/S0092-8674(00)81641-4.
- Linder T, Zhu X, Baraznenok V, Gustafsson CM. 2006. The classical srb4-138 mutant allele causes dissociation of yeast Mediator. Biochem Biophys Res Commun 349:948–953. http://dx.doi.org/10.1016/j.bbrc.2006.08.099.
- Thompson CM, Young RA. 1995. General requirement for RNA polymerase II holoenzymes in vivo. Proc Natl Acad Sci U S A 92:4587–4590. http://dx.doi.org/10.1073/pnas.92.10.4587.
- Ansari SA, He Q, Morse RH. 2009. Mediator complex association with constitutively transcribed genes in yeast. Proc Natl Acad Sci U S A 106:16734–16739. http://dx.doi.org/10.1073/pnas.0905103106.
- Singh SS, Singh N, Bonocora RP, Fitzgerald DM, Wade JT, Grainger DC. 2014. Widespread suppression of intragenic transcription initiation by H-NS. Genes Dev 28:214–219. http://dx.doi.org/10.1101/gad.234336.113.
- Seoighe C, Wolfe KH. 1999. Updated map of duplicated regions in the yeast genome. Gene 238:253–261. http://dx.doi.org/10.1016/S0378-1119(99)00319-4.
- Zang C, Schones DE, Zeng C, Cui K, Zhao K, Peng W. 2009. A clustering approach for identification of enriched domains from histone modification ChIP-Seq data. Bioinformatics 25:1952–1958. http://dx.doi.org/10.1093/bioinformatics/btp340.
- Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JY, Zhang J. 2004. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5:R80. http://dx.doi.org/10.1186/gb-2004-5-10-r80.
- Robinson JT, Thorvaldsdottir H, Winckler W, Guttman M, Lander ES, Getz G, Mesirov JP. 2011. Integrative genomics viewer. Nat Biotechnol 29:24–26. http://dx.doi.org/10.1038/nbt.1754.
- Eyboulet F, Cibot C, Eychenne T, Neil H, Alibert O, Werner M, Soutourina J. 2013. Mediator links transcription and DNA repair by facilitating Rad2/XPG recruitment. Genes Dev 27:2549–2562. http://dx.doi.org/10.1101/gad.225813.113.
- Goecks J, Nekrutenko A, Taylor J. 2010. Galaxy: a comprehensive approach for supporting accessible, reproducible, and transparent computational research in the life sciences. Genome Biol 11:R86. http://dx.doi.org/10.1186/gb-2010-11-8-r86.
- Tsai KL, Sato S, Tomomori-Sato C, Conaway RC, Conaway JW, Asturias FJ. 2013. A conserved Mediator-CDK8 kinase module association regulates Mediator-RNA polymerase II interaction. Nat Struct Mol Biol 20:611–619. http://dx.doi.org/10.1038/nsmb.2549.
- Nagalakshmi U, Wang Z, Waern K, Shou C, Raha D, Gerstein M, Snyder M. 2008. The transcriptional landscape of the yeast genome defined by RNA sequencing. Science 320:1344–1349. http://dx.doi.org/10.1126/science.1158441.
- Basehoar AD, Zanton SJ, Pugh BF. 2004. Identification and distinct regulation of yeast TATA box-containing genes. Cell 116:699–709. http://dx.doi.org/10.1016/S0092-8674(04)00205-3.
- Huisinga KL, Pugh BF. 2004. A genome-wide housekeeping role for TFIID and a highly regulated stress-related role for SAGA in Saccharomyces cerevisiae. Mol Cell 13:573–585. http://dx.doi.org/10.1016/S1097-2765(04)00087-5.
- Rhee HS, Pugh BF. 2012. Genome-wide structure and organization of eukaryotic pre-initiation complexes. Nature 483:295–301. http://dx.doi.org/10.1038/nature10799.
- Ansari SA, Morse RH. 2013. Mechanisms of Mediator complex action in transcriptional activation. Cell Mol Life Sci 70:2743–2756. http://dx.doi.org/10.1007/s00018-013-1265-9.
- Morris RT, O'Connor TR, Wyrick JJ. 2009. Ceres: software for the integrated analysis of transcription factor binding sites and nucleosome positions in Saccharomyces cerevisiae. Bioinformatics 26:168–174. http://dx.doi.org/10.1093/bioinformatics/btp657.
- Boorsma A, Lu XJ, Zakrzewska A, Klis FM, Bussemaker HJ. 2008. Inferring condition-specific modulation of transcription factor activity in yeast through regulon-based analysis of genomewide expression. PLoS One 3:e3112. http://dx.doi.org/10.1371/journal.pone.0003112.
- Hanlon SE, Rizzo JM, Tatomer DC, Lieb JD, Buck MJ. 2011. The stress response factors Yap6, Cin5, Phd1, and Skn7 direct targeting of the conserved co-repressor Tup1-Ssn6 in S. cerevisiae. PLoS One 6:e19060. http://dx.doi.org/10.1371/journal.pone.0019060.
- Hughes TR, Marton MJ, Jones AR, Roberts CJ, Stoughton R, Armour CD, Bennett HA, Coffey E, Dai H, He YD, Kidd MJ, King AM, Meyer MR, Slade D, Lum PY, Stepaniants SB, Shoemaker DD, Gachotte D, Chakraburtty K, Simon J, Bard M, Friend SH. 2000. Functional discovery via a compendium of expression profiles. Cell 102:109–126. http://dx.doi.org/10.1016/S0092-8674(00)00015-5.
- Poss ZC, Ebmeier CC, Taatjes DJ. 2013. The Mediator complex and transcription regulation. Crit Rev Biochem Mol Biol 48:575–608. http://dx.doi.org/10.3109/10409238.2013.840259.
- Fan X, Chou DM, Struhl K. 2006. Activator-specific recruitment of Mediator in vivo. Nat Struct Mol Biol 13:117–120. http://dx.doi.org/10.1038/nsmb1049.
- Miller C, Matic I, Maier K, Schwalb B, Roether S, Straesser K, Tresch A, Mann M, Cramer P. 2012. Mediator phosphorylation prevents stress response transcription during non-stress conditions. J Biol Chem 287:44017–44026. http://dx.doi.org/10.1074/jbc.M112.430140.
- Ansari SA, Morse RH. 2012. Selective role of Mediator tail module in the transcription of highly regulated genes in yeast. Transcription 3:110–114. http://dx.doi.org/10.4161/trns.19840.
- Ansari SA, Paul E, Sommer S, Lieleg C, He Q, Daly AZ, Rode KA, Barber WT, Ellis LC, Laporta E, Orzechowski AM, Taylor E, Reeb T, Wong J, Korber P, Morse RH. 2014. Mediator, TATA-binding protein, and RNA polymerase II contribute to low histone occupancy at active gene promoters in yeast. J Biol Chem 289:14981–14995. http://dx.doi.org/10.1074/jbc.M113.529354.
- Lacombe T, Poh SL, Barbey R, Kuras L. 2013. Mediator is an intrinsic component of the basal RNA polymerase II machinery in vivo. Nucleic Acids Res 41:9651–9662. http://dx.doi.org/10.1093/nar/gkt701.
- Spaeth JM, Kim NH, Boyer TG. 2011. Mediator and human disease. Semin Cell Dev Biol 22:776–787. http://dx.doi.org/10.1016/j.semcdb.2011.07.024.
- Hashimoto S, Boissel S, Zarhrate M, Rio M, Munnich A, Egly JM, Colleaux L. 2011. MED23 mutation links intellectual disability to dysregulation of immediate early gene expression. Science 333:1161–1163. http://dx.doi.org/10.1126/science.1206638.
- Bourbon HM, Aguilera A, Ansari AZ, Asturias FJ, Berk AJ, Bjorklund S, Blackwell TK, Borggrefe T, Carey M, Carlson M, Conaway JW, Conaway RC, Emmons SW, Fondell JD, Freedman LP, Fukasawa T, Gustafsson CM, Han M, He X, Herman PK, Hinnebusch AG, Holmberg S, Holstege FC, Jaehning JA, Kim YJ, Kuras L, Leutz A, Lis JT, Meisterernest M, Naar AM, Nasmyth K, Parvin JD, Ptashne M, Reinberg D, Ronne H, Sadowski I, Sakurai H, Sipiczki M, Sternberg PW, Stillman DJ, Strich R, Struhl K, Svejstrup JQ, Tuck S, Winston F, Roeder RG, Kornberg RD. 2004. A unified nomenclature for protein subunits of mediator complexes linking transcriptional regulators to RNA polymerase II. Mol Cell 14:553–557. http://dx.doi.org/10.1016/j.molcel.2004.05.011.
- Capieaux E, Vignais ML, Sentenac A, Goffeau A. 1989. The yeast H+-ATPase gene is controlled by the promoter binding factor TUF. J Biol Chem 264:7437–7446.
- Wippo CJ, Krstulovic BS, Ertel F, Musladin S, Blaschke D, Sturzl S, Yuan GC, Horz W, Korber P, Barbaric S. 2009. Differential cofactor requirements for histone eviction from two nucleosomes at the yeast PHO84 promoter are determined by intrinsic nucleosome stability. Mol Cell Biol 29:2960–2981. http://dx.doi.org/10.1128/MCB.01054-08.
- Bornaes C, Ignjatovic MW, Schjerling P, Kielland-Brandt MC, Holmberg S. 1993. A regulatory element in the CHA1 promoter which confers inducibility by serine and threonine on Saccharomyces cerevisiae genes. Mol Cell Biol 13:7604–7611.
- He Q, Battistella L, Morse RH. 2008. Mediator requirement downstream of chromatin remodeling during transcriptional activation of CHA1 in yeast. J Biol Chem 283:5276–5286. http://dx.doi.org/10.1074/jbc.M708266200.
- Longtine MS, McKenzie A, III, Demarini DJ, Shah NG, Wach A, Brachat A, Philippsen P, Pringle JR. 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14:953–961. http://dx.doi.org/10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U.
