Abstract
This study aimed to evaluate oxidative stress and apoptosis in childhood acute lymphoblastic leukemia (ALL) at diagnosis and their impact on outcome at the end of the induction phase. Our study included 50 newly diagnosed children with ALL. Evaluation of oxidative stresses (malondialdehyde and total anti‐oxidant capacity) was made at diagnosis and at the end of the induction phase. Apoptosis level was determined by fluorometric terminal deoxynucleotidyl transferase dUTP nick end labeling system for patients at diagnosis and after 1 week of treatment. Our study showed that there was increased oxidative stress at diagnosis and after treatment with chemotherapy. Apoptosis index was higher after 1 week of treatment with chemotherapy when compared to its level at diagnosis.
Introduction
Acute lymphoblastic leukemia (ALL) is the most common cancer in the pediatric population as it represents about 50% of the hematopoietic malignancies.Citation1 ALL is a disease characterized by uncontrolled proliferation and maturation arrest of lymphoid progenitor cells in bone marrow resulting in an excess of malignant cells.Citation2
Reactive oxygen species (ROS) are known to play a dual role in biological systems, since they may be either harmful or beneficial to living systems.Citation3 Beneficial effects of ROS occur at low/moderate concentrations and involve physiological roles in cellular defense against infectious agents and in the function of a number of cellular signaling systems.Citation4 In contrast, at high concentrations, ROS can be important mediators of damage to cell structures, including lipids and membranes, proteins and nucleic acids.Citation3 A major aldehyde product of lipid peroxidation is malondialdehyde (MDA) which is a physiologic ketoaldehyde, produced by peroxidative decomposition of unsaturated lipids.Citation5
The harmful effect of free radicals causing potential biological damage is termed oxidative stress.Citation6 Oxidative stress represents a disturbance in the equilibrium status of pro‐oxidant/anti‐oxidant reactions in living organisms.Citation7 The serum contains many different anti‐oxidants. These include ascorbic acid, alpha‐tocopherol, beta‐carotene, uric acid, bilirubin, and albumin. In addition, trace amounts of anti‐oxidant enzymes such as glutathione peroxidase and superoxide dismutase (SOD) are found in serum to a lesser extent.Citation8 The measure of total anti‐oxidant capacity (TAC) considers the cumulative action of all the anti‐oxidants present in plasma and body fluids. Measuring plasma TAC may be helpful in the evaluation of physiological, environmental, and nutritional factors of the redox status in humans.Citation9 Several mechanisms may lead to oxidative stress in cancer patients such as altered energy metabolism due to malnutrition, an excessive production of pro‐inflammatory cytokines, which in turn may increase the ROS production, and the use of anti‐neoplastic drugs.Citation10
Apoptosis, or active cell death, occurs in physiological conditions as a regulatory mechanism of tissue growth to balance cell proliferation.Citation11 The human body has to get rid of precancerous and cancerous cells by means of ROS‐induced apoptosis.Citation12 Acquisition of mechanisms to evade apoptosis is a hallmark of cancer, with both the loss of function of pro‐apoptotic signals and gain of function of anti‐apoptotic mechanisms contributing to tumorigenesis. Defective apoptotic mechanisms allow genetically unstable cancer cells to avoid elimination and confer resistance to chemotherapy.Citation13
Our study aimed to evaluate oxidative stress (MDA and TAC) and apoptosis level in childhood ALL at diagnosis and their impact on outcome at the end of induction of remission phase.
Patients and methods
This study was a prospective observational study including newly diagnosed children with ALL selected consecutively from children admitted in Pediatric Department of Oncology Center of Mansoura University from September 2007 to December 2008. They were 29 males and 21 females with age ranging from 1·5 to 12 years [mean±standard deviation (SD) = 6·84±3·73 years]. All patients received the same treatment [modified Berlin–Frankfurt–Münster 76/79]. Healthy controls of matched age and sex (10 children) were randomly selected from outpatient clinic of general pediatrics surgery. Informed consent by the parents was obtained before enrollment. The study was approved by the local Ethical Committee of Mansoura University.
Detailed history with thorough clinical examination was done to all patients. Blood samples were collected at diagnosis and after 5 weeks of treatment (end of induction therapy) for evaluation of complete blood count, liver function test, serum creatinine and serum uric acid. Evaluation of oxidative stresses (MDA and TAC) was done at diagnosis and after the end of induction phase. Measurement of apoptosis was done by fluorometric terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) system for patients at diagnosis and after 1 week of treatment.
Bone marrow aspiration with French–American–British classification was done at diagnosis and after the end of induction phase for evaluation of blast percentage.
Measurement of serum TAC levels
Serum TAC levels were measured according to the method described by Koracevic et al.,Citation14 in which the determination of anti‐oxidative capacity is based on the reaction of anti‐oxidants in the sample with a defined amount of exogenously provided hydrogen peroxide. The anti‐oxidants in the sample eliminate a certain amount of the provided hydrogen peroxide, and the residual hydrogen peroxide is determined calorimetrically by an enzymatic reaction that involves the conversion of 3,5‐dichloro‐2‐hydroxybenzene sulfonate to a colored product.
Measurement of serum MDA
The colorimetric method described by SatohCitation15 and Ohkawa et al.Citation16 was used to measure serum MDA levels. Thiobarbituric acid reacts with MDA in an acidic medium at 95°C for 30 minutes to form a thiobarbituric acid reactive product. The absorbance of the resultant pink product can be measured at 534 nm.
Evaluation of apoptosis
Apoptosis was evaluated by using the DeadEndTM Fluorometric TUNEL System (Promega Corp., Madison, WI, USA). The fluorescein‐12‐dUTP labeled DNA was visualized by fluorescence microscopy for peripheral blood lymphocytes.
Evaluation of TAC and MDA was done for 50 patients at diagnosis and for only 38 patients after therapy (eight patients died before completion of induction phase and another four patients thought medical service in other centers).
Detection of apoptosis was done for 24 patients at diagnosis and 22 patients after 1 week of therapy. Included patients were divided according to risk to high risk and standard risk patients, depending on criteria for high risk.Citation17 Included patients were also divided into two groups at the end of 1 month of treatment, according to achievement of complete remission.Citation17 Forty‐three patients achieved complete remission (remission group) while only four patients were resistant to treatment (resistant group).
Statistical analysis
The SPSS software program, version 12 (SPSS, Chicago, IL, USA) was used to analyze the data. Data on the clinical and laboratory characteristics of patients and controls were presented as mean±SD. Statistical analysis was performed by using the Student’s t‐test. P values ⩽0·05 were considered of significance.
Results
Clinical and demographic data of our patients are shown in . Different levels of MDA and TAC for patients and controls at diagnosis are shown in . There was a significant difference (P<0·001) between patients and controls for MDA and TAC [MDA for patients (16·61±6·55 nmol/ml) and controls (6·42±1·52 nmol/ml); TAC for patients (0·88±0·33 mM/l) and controls (1·57±0·36 mM/l)]. Also, a significant increase in oxidative stress (P<0·001) was detected as MDA increased and TAC decreased when comparing the results for same patients at diagnosis and after 1 month of treatment (before MDA: 14·69±4·39 nmol/ml and after MDA: 22·71±5·70 nmol/ml; before TAC: 0·944±0·26 mM/l and after TAC: 0·45±0·20 mM/l).
Figure 1. Different levels of MDA and TAC for patients at diagnosis and after 5 weeks of treatment in comparison to healthy controls: levels of apoptosis of patients at diagnosis and after 1 week of treatment are also shown.
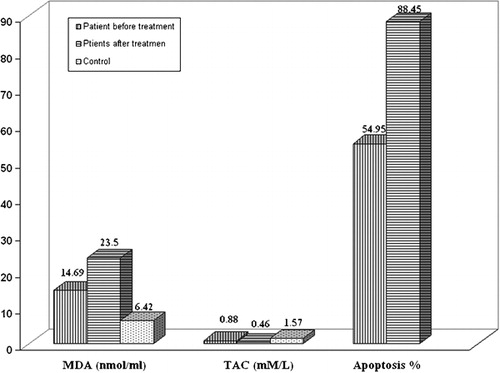
Table 1. Clinical and laboratory data of patients at diagnosis
On the other hand, apoptosis was increased significantly after 1 week of induction phase of chemotherapy in comparison to its level at diagnosis (P<0·001) (before: 26·58±14·06% and after 1 week: 88·45±12·11%; ).
As regards outcome after 5 weeks of induction therapy, eight patients died during induction phase [uric acid nephropathy (two patients), complicated febrile neutropenia (two patients developed bronchopneumonia and respiratory failure and two patients developed sepsis), one patient developed intracranial hemorrhage due to thrombocytopenia, and the last patient developed thromboembolism].
Comparing the patients when divided as regards risk, there was no significant difference between standard and high risk groups as regards MDA, TAC or apoptosis at diagnosis and after treatment (P = 0·957, 0·328, and 0·54 respectively).
However, after treatment, TAC was significantly lower (P = 0·024) for high risk (0·34±0·11 mM/l) versus standard risk (0·5±0·22 mM/l) while apoptosis was significantly higher in high risk group (97·66±4·8%) versus standard risk group (85±12·38%; P = 0·027). While, there was no significant difference as regards MDA [24·28±5·80 nmol/ml for standard risk group versus 21·67±5·44 nmol/ml for high risk group (P = 0·97)].
As regards remission status and evaluation of oxidative stress, there was no significant difference for MDA or TAC at diagnosis or after 1 month of treatment (P = 0·47 and P = 0·28 respectively). But as regards apoptosis at diagnosis, it was significantly higher among remission group (P = 0·016) which was of non‐significance after 1 week of treatment ().
Table 2. Comparison between patients who achieve remission and those who were resistant as regards MDA, TAC, and apoptosis at diagnosis and after therapy (Student’s t‐test)
We observed that oxidative stress was higher at diagnosis among patients who died during the induction phase in comparison to those who survived (), while apoptosis at diagnosis was of no significant difference.
Table 3. Comparison between patients who lived and died (at the end of 5 weeks of treatment) as regards MDA, TAC, and apoptosis at diagnosis (Student’s t‐test)
There was a significant negative correlation between TAC and MDA at diagnosis (r = −0·461, P = 0·001) and after treatment (r = −0·518, P = 0·001). While there was a significant correlation with apoptosis after treatment for MDA (r = 0·858, P<0·001) and for TAC (r = −0·439, P = 0·041) which was not found at diagnosis.
Discussion
In our study, there was a higher oxidative stress in children with ALL (significant higher levels of MDA and lower levels of TAC) at diagnosis when compared to healthy controls. Both decrease in anti‐oxidants [enzymatic (SOD or CAT) or thiol, vitamin E, vitamin C, beta‐carotene or zinc] and/or increases in the production of ROS have been reported in leukemia patients.Citation18–Citation26 However, Devi et al.Citation27 found high superoxide generation and a normal lipid peroxide levels in patients with different types of leukemias before therapy. This might be due to the increased activities of anti‐oxidant enzymes as an adaptive protective response which may be indicative of mild oxidative stress in ALL. Also, Nishiura et al.Citation28 reported elevated anti‐oxidants and serum SOD activity in acute leukemia and found that regression of the leukemia was accompanied by a decrease in the serum level of SOD. Papageorgiou et al.Citation29 found similar values for TAC between cancer free children and children with malignancy at diagnosis.
In our study, we found that higher levels of oxidative stress were associated with poor prognosis. There was a statistically significant higher level of MDA and lower levels of TAC at diagnosis among patients who died than those who lived after 5 weeks of treatment (P = 0·002 and P = 0·044 respectively). In agreement with that finding, Kennedy et al.Citation30 concluded that higher 8‐oxo‐7,8‐dihydro‐2′‐deoxyguanosine levels (one of the mutagenic base modifications produced in DNA by the reaction of reactive oxygen species) in children undergoing treatment for ALL was associated with an increase in chemotherapy‐related side effects, and dietary intake of anti‐oxidants would be inversely correlated with levels of 8‐oxo‐7,8‐dihydro‐2′‐deoxyguanosine. Also, evidence is growing that anti‐oxidants may provide some benefit when combined with certain types of chemotherapy.Citation31 It was also demonstrated that supplementations with anti‐oxidants like vitamin E and N‐acetyl cysteine with chemotherapy for children with ALL were associated with less occurrence of toxic hepatitis, hematological complications, need for blood and platelet transfusions, decreased level of MDA, and higher level of glutathione peroxidase.Citation32 Although anti‐oxidants may be useful in the reduction of adverse effects of chemotherapy, there is a theoretical concern that anti‐oxidant therapies interfere with chemotherapy and radiation by lowering oxidative damage.Citation31
Under chemotherapy, there is an increase in lipid peroxidation products and a marked reduction in plasma levels of anti‐oxidants.Citation33 Lower levels of anti‐oxidants are associated with increases in the adverse side effects of chemotherapy.Citation30
We also found higher levels of oxidative stress and lower levels of TAC after the end of induction phase of chemotherapy when compared to their levels at diagnosis. Similar results were reported for ALL patients,Citation25,Citation29,Citation34 bone marrow transplant recipients,Citation35 and chronic leukemia.Citation36 Contrary to the results of the present study, Singh et al.Citation21 found that serum MDA levels were increased in leukemia and were higher in the active phase as compared to remission, suggesting that serum MDA may predict relapse. While, Battisti et al.Citation18 suggested that increased oxidative stress may be involved with the pathogenesis of leukemia and not as a result of the treatment with chemotherapy.
In this study, apoptotic index at diagnosis showed a wide variability ranging from 3 to 48%. While, a lower level was found by Liu et al.,Citation37 who estimated baseline apoptosis in blasts from children with leukemia between 3 and 29%. There was an increase in apoptotic index in blast cells after 1 week of chemotherapy treatment compared to the level at diagnosis (P<0·001). Apoptosis is the primary mechanism through which most chemotherapeutic agents induce tumor cell death.Citation38 Classical chemotherapeutic agents induce cell death mainly through the mitochondrial apoptotic pathway.Citation39 An acute generation of ROS, or transient ROS burst, is frequently observed in apoptosis induced by various agents.Citation40
Treatment failure of leukemia represents a failure of the malignant clone to undergo apoptosis in response to chemotherapeutic agents. The molecular basis of apoptosis resistance and treatment failure in leukemia is only partially understood.Citation41 In our study, apoptosis was lower in patients with no remission after treatment than those who achieved remission. We found that patients which were resistant to chemotherapy had low apoptotic index at diagnosis. There were two patients, a 2‐year‐old and a 8‐year‐old males, whose apoptotic index at diagnosis were 3 and 10% respectively. Unfortunately, the number of patients was too low (two patients) to give a moderately significant statistics. Therefore, we believe that higher apoptotic index at presentation may be associated with better response to chemotherapy. In agreement with our observation, Meyer et al.Citation42 suggested that intact apoptosis signaling is a characteristic for favorable outcome as reported intact apoptosis signaling in only ALL patients who had a good response and patients in continuous remission with pediatric ALL. Also, Aref et al.Citation38 found lower Bcl‐2 and higher Fas expression in ALL responding to induction chemotherapy. Hafez et al.Citation43 found that markers of apoptosis in children with ALL were significantly lower in cases than controls, and significantly lower in cases who did not achieve remission. Moreover, Zandieh et al.Citation11 concluded that low expression of Fas antigen and increase in Fas antigen expression (above 20%) after treatment, was a favorable prognostic outcome. Moreover, Brajusković et al.Citation44 showed that both the levels of spontaneous and therapy‐induced apoptosis are in correlation with the clinical response of the patients to therapy and of prognostic significance. Besides, there was decreased activation of apoptotic parameters in resistant cases of pediatric ALL.Citation45 It was reported that the rapidity and structure of the apoptotic response within the first 24–48 hours correlates with ultimate outcome.Citation37
Apoptosis and cancer are opposed phenomena, but ROS may play a key role in both.Citation46 Induction or inhibition of cell proliferation seems to be dependent on levels of oxidants/anti‐oxidants in the cell.Citation3 Apoptosis can be induced by ROS as mediators of apoptosis, induce intracellular production of ROS and are inhibited by the addition of anti‐oxidants.Citation46
In this study, we observed a positive correlation between apoptotic percentage in peripheral lymphocytes after 1 week and MDA level (P<0·001), and a negative correlation between apoptotic percentage and TAC level (P = 0·041) after induction phase of chemotherapy. While previous parameters at diagnosis were of no significance. In a similar study, Nakazato et al.Citation47 observed that highly toxic ROS may be the direct mediators of apoptosis in MPO‐positive leukemic cells. Also, Sharara and AdawyCitation19 found that increased oxidative stress in ALL patients at diagnosis (elevated MDA and reduced SOD) correlated positively with the increased levels of the anti‐apoptotic factors sFas‐L and Bcl‐2 which may cause initiation and promotion of ALL.
Exposure of cancer cells to ROS generating anti‐cancer agents exhausts the cellular anti‐oxidant capacity, and a ROS level beyond a threshold leads to apoptosis.Citation48 In the absence of adequate anti‐oxidant defenses, the damage produced by oxidative stress leads to the activation of genes (stress‐responsive transcription factors) responsible for apoptosis.Citation49 It was shown that higher oxidant concentrations not only depress proliferation rates, but also increase apoptotic cells. Also, the mode of apoptosis depends on the severity of the oxidative damage.Citation50
In conclusion, the present work provides evidence for the increased levels of oxidative damage and decreased levels of the anti‐oxidant system in ALL patients, suggesting that oxidative stress may participate in leukemia pathogenesis. Chemotherapeutic agents may further increase oxidative stress and apoptosis in ALL. Apoptotic index may serve as a predictor for response to chemotherapy. Level of apoptosis after therapy may be correlated with level of oxidative stress although higher oxidative stress may be associated with more side effects and bad prognosis. Modulation of ROS by anti‐oxidants therapy is a matter of debate, also cutoff levels for oxidative stress should be evaluated by further studies to define beneficial or harmful levels which might be useful to decide anti‐oxidants therapy usage for good of the patients.
References
- Downing JR, Shannon KM. Acute leukemia: a pediatric perspective. Cancer Cell 2002; 2: 437–445.
- Plasschaert SL, Kamps WA, Vellenga E, de Vries EG, de Bont ES. Prognosis in childhood and adult acute lymphoblastic leukaemia: a question of maturation? Cancer Treat Rev 2004; 30: 37–51.
- Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact 2006; 160: 1–40.
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007; 39: 44–84.
- Stocker R, Keaney JF. Role of oxidative modifications in atherosclerosis. Physiol Rev 2004; 84: 1381–1478.
- Ridnour LA, Isenberg JS, Espey MG, Thomas DD, Roberts DD, Wink DA. Nitric oxide regulates angiogenesis through a functional switch involving thrombospondin-1. Proc Natl Acad Sci USA 2005; 102: 13147–13152.
- Dröge W. Free radicals in the physiological control of cell function. Physiol Rev 2002; 82: 47–95.
- Chapple IL. Reactive oxygen species and antioxidants in inflammatory diseases. J Clin Periodontol 1997; 24: 287–296.
- Ghiselli A, Serafini M, Natella F, Scaccini C. Total antioxidant capacity as a tool to assess redox status: critical view and experimental data. Free Radic Biol Med 2000; 29: 1106–1114.
- Mantovani G, Macciò A, Madeddu C, et al.. Antioxidant agents are effective in inducing lymphocyte progression through cell cycle in advanced cancer patients: assessment of the most important laboratory indexes of cachexia and oxidative stress. J Mol Med 2003; 81: 664–673.
- Zandieh T, Safarifard A, Nikogoftar M. In vitro evaluation of cytarabin induced apoptosis in leukemic blasts. Iran J Allergy Asthma Immunol 2005; 4: 173–178.
- Howes RM. [Internet]. Cancer, apoptosis and reactive oxygen species: a new paradigm. 2007 [cited 2010 Jan 15]. Available from: http://philica.com/display_article.php?article_id=86
- Ziegler DS, Kung AL. Therapeutic targeting of apoptosis pathways in cancer. Curr Opin Oncol 2008; 20: 97–103.
- Koracevic D, Koracevic G, Djordjevic V, Andrejevic S, Cosic V. Method for the measurement of antioxidant activity in human fluids. J Clin Pathol 2001; 54: 356–361.
- Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta 1978; 90: 37–43.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979; 95: 351–358.
- Lanzkowsky P. Leukemias. In: , Lanzkowsky P, (ed) Manual of pediatric hematology and oncology. 3rd ed. San Diego, CA: Academic Press, 2005; 359–411.
- Battisti V, Maders LD, Bagatini MD, et al.. Measurement of oxidative stress and antioxidant status in acute lymphoblastic leukemia patients. Clin Biochem 2008; 41: 511–518.
- Sharara GM, Adawy NM. Study of oxidative stress, antioxidant status and their relation to apoptosis in acute lymphoblastic leukemia. Bull Alex Fac Med 2005; 41: 729–736.
- McEligot AJ, Yang S, Meyskens FL. Redox regulation by intrinsic species and extrinsic nutrients in normal and cancer cells. Annu Rev Nutr 2005; 25: 261–295.
- Singh V, Ghalaut PS, Kharb S, Singh GP. Plasma concentrations of lipid peroxidation products in children with acute leukaemia. Indian J Med Sci 2001; 55: 215–217.
- Sentürker S, Karahalil B, Inal M, et al.. Oxidative DNA base damage and antioxidant enzyme levels in childhood acute lymphoblastic leukemia. FEBS Lett 1997; 27: 286–290.
- Mazor D, Abucoider A, Meyerstein N, Kapelushnik J. Antioxidant status in pediatric acute lymphocytic leukemia (ALL) and solid tumors: the impact of oxidative stress. Pediatr Blood Cancer 2008; 51: 613–615.
- Malvy DJ, Arnaud J, Burtschy B, et al.. Antioxidant micronutrients and childhood malignancy during oncological treatment. Med Pediatr Oncol 1997; 29: 213–217.
- Kennedy DD, Ladas EJ, Rheingold SR, Blumberg J, Kelly KM. Antioxidant status decreases in children with acute lymphoblastic leukemia during the first six months of chemotherapy treatment. Pediatr Blood Cancer 2005; 44: 378–385.
- Małyszczak K, Tomasz W, Mazur G, et al.. ‘Anxiety and depressive symptoms in patients treated due to haematologic malignancies’. Psychiatr Pol 2005; 39: 33–40.
- Devi GS, Prasad MH, Saraswathi I, Raghu D, Rao DN, Reddy PP. Free radicals antioxidant enzymes and lipid peroxidation in different types of leukemias. Clin Chim Acta 2000; 293: 53–62.
- Nishiura T, Suzuki K, Kawaguchi T, et al.. Elevated serum manganese superoxide dismutase in acute leukemias. Cancer Lett 1992; 62: 211–215.
- Papageorgiou M, Stiakaki E, Dimitriou H, et al.. Cancer chemotherapy reduces plasma total antioxidant capacity in children with malignancies. Leuk Res 2005; 29: 11–16.
- Kennedy DD, Santella RM, Wang Q, Ladas EJ, Kelly KM. 8-oxo-dG elevated in children during leukemia treatment. Integr Cancer Ther 2004; 3: 301–309.
- Drisko JA, Chapman J, Hunter VJ. The use of antioxidant therapies during chemotherapy. Gynecol Oncol 2003; 88: 434–439.
- Al-Tonbary Y, Al-Haggar M, El-Ashry R, El-Dakroory S, Azzam H, Fouda A. Vitamin E and N-acetylcysteine as antioxidant adjuvant therapy in children with acute lymphoblastic leukemia. Adv Hematol 2009; 2009: 689639.
- Jeanne A, Drisko JA, Chapman J, Hunter VJ. The use of antioxidant therapies during chemotherapy. Gynecol Oncol 2003; 88: 434–439.
- Drabko K, Bojarska-Junak A, Kowalczyk J. ‘Activity of superoxide dismutase and glutathione peroxidase and concentrations of malonyldialdehyde, vitamin E, total antioxidant status and extracellular cytokines concentrations in children with acute lymphoblastic leukaemia (ALL)’. Med Wieku Rozwoj 2006; 10: 861–868.
- Hunnisett A, Davies S, McLaren-Howard J, Gravett P, Finn M, Gueret-Wardle D. Lipoperoxides as an index of free radical activity in bone marrow transplant recipients. Preliminary observations. Biol Trace Elem Res 1995; 47: 125–132.
- Al-Gayyar MM, Eissa LA, Rabie AM, El-Gayar AM. Measurements of oxidative stress status and antioxidant activity in chronic leukaemia patients. J Pharm Pharmacol 2007; 59: 409–417.
- Liu T, Raetz E, Moos PJ, et al.. Diversity of the apoptotic response to chemotherapy in childhood leukemia. Leukemia 2002; 16: 223–232.
- Aref S, Salama O, Al-Tonbary Y, Mansour A. Assessment of bcl-2 expression as modulator of fas mediated apoptosis in acute leukemia. Hematology 2004; 9: 113–121.
- Klener PJr, Andera L, Klener P, Necas E, Zivný J. Cell death signalling pathways in the pathogenesis and therapy of haematologic malignancies: overview of apoptotic pathways. Folia Biol (Praha) 2006; 52: 34–44.
- Carmody RJ, Cotter TG. Signalling apoptosis: a radical approach. Redox Rep 2001; 6: 77–90.
- Suhara T, Kim HS, Kirshenbaum LA, Walsh K. Suppression of Akt signaling induces Fas ligand expression: involvement of caspase and Jun kinase activation in Akt-mediated Fas ligand regulation. Mol Cell Biol 2002; 22: 680–691.
- Meyer LH, Karawajew L, Schrappe M, Ludwig WD, Debatin KM, Stahnke K. Cytochrome c-related caspase-3 activation determines treatment response and relapse in childhood precursor B-cell ALL. Blood 2006; 107: 4524–4531.
- Hafez M, Al-Tonbary Y, El-Bayoumi MA, et al.. Markers of apoptosis and proliferation related gene products as predictors of treatment outcome in childhood acute lymphoblastic leukemia. Hematology 2007; 12: 209–218.
- Brajusković G, Milić AS, Vukosavić S, et al.. ‘Apoptosis in chronic lymphocytic leukemia’. Vojnosanit Pregl 2002; 59: 47–52.
- Holleman A, den Boer ML, Kazemier KM, Janka-Schaub GE, Pieters R. Resistance to different classes of drugs is associated with impaired apoptosis in childhood acute lymphoblastic leukemia. Blood 2003; 102: 4541–4546.
- Matés JM, Sánchez-Jiménez FM. Role of reactive oxygen species in apoptosis: implications for cancer therapy. Int J Biochem Cell Biol 2000; 32: 157–170.
- Nakazato T, Sagawa M, Yamato K, et al.. Myeloperoxidase is a key regulator of oxidative stress mediated apoptosis in myeloid leukemic cells. Clin Cancer Res 2007; 13: 5436–5445.
- Kong Q, Beel JA, Lillehei KO. A threshold concept for cancer therapy. Med Hypotheses 2000; 55: 29–35.
- Haddad JJ. Redox and oxidant-mediated regulation of apoptosis signaling pathways: immuno-pharmaco-redox conception of oxidative siege versus cell death commitment. Int Immunopharmacol 2004; 4: 475–493.
- Ozben T. Oxidative stress and apoptosis: impact on cancer therapy. J Pharm Sci 2007; 96: 2181–2196.