Abstract
Beta‐thalassemia/HbE (beta‐thal/HbE) is a thalassemia intermedia (TI) which encompasses a broad spectrum of severity. Here, we used deferiprone (DFP) as an iron chelating agent in TI patients receiving intermittent blood transfusion who are asymptomatic for cardiovascular disease in order to evaluate the effectiveness in iron overload and reduce the possibility of cardiovascular complications. Thirty transfusion‐independent beta‐thal/HbE patients with iron overload were treated with DFP for 1 year. Hematological, biochemical, oxidative stress and echocardiographic parameters were determined. Serum ferritin, non‐transferrin‐bound iron, and malondialdehyde decreased significantly (P<0·05) after 1‐year treatment with DFP. For echocardiographic results, mean pulmonary arterial pressure and pulmonary vascular resistance were diminished significantly (P<0·05). All those parameters were still improved after subgroup analysis was done for the high ferritin group (>2500 ng/ml). DFP therapy alone improved iron overload and oxidative stress and compliance was good. We propose that prevention of pulmonary hypertension is also possible for TI undergoing intermittent blood transfusion.
Introduction
Thalassemia is the most common inherited hemolytic anemia in the world and is a significant health problem in many Middle East and Asian countries including Thailand.Citation1 One percent of the Thai population is born with thalassemia and up to 30% are carriers for this disease.Citation2 Beta‐thalassemia/HbE (beta‐thal/HbE) is the most common form of beta‐thalassemia in Thailand. Fifty to seventy percent of the Thai population resides in Northeastern part where a lack of health care resource is still the key problem.Citation3 Although a partner screening program has been developed over 30 years, the number of new cases has not decreased significantly. Epidemiologic data confirm that thalassemia disease is a continuing chronic health care problem.
The leading cause of death for thalassemia major (TM) is cardiac diseases from myocardial iron deposition.Citation4 For thalassemia intermedia (TI), only 5·4% develop congestive heart failure, but up to 23–60% of TI patients have pulmonary hypertension (PHT) which may lead to right sided heart failure.Citation5,Citation6 In contrast to TM, there is no consensus guideline provided for TI patients. Nowadays, clinical practice focuses on occasional symptomatic treatment with blood transfusion, but there is increasing evidence that early adequate iron chelator therapy associated with blood transfusion, regardless of ferritin level, prevents the detrimental sequalae of disease, especially PHT.Citation5
Although the ideal therapeutic option for curing this disease is stem cell transplantation, high cost of treatment, limited availability of HLA‐matched donors and morbidity/mortality from treatment are still important obstacles for thalassemia patients achieving access to such treatment option. Therefore, the standard treatment still consists of regular blood transfusion every 3–4 weeks, with iron chelation therapy to prevent secondary hemochromatosis. Standard chelation therapy is deferioxamine (DFO). However, some drawbacks of DFO either preclude many thalassemia patients from accessing this standard iron chelator or cause poor compliance. Those include the inconvenience and discomfort of subcutaneous infusion for 8–12 hours/day, 3–5 days/week.Citation7 Cianciulli et al. had shown that 50% of thalassemia patients who have poor adherence to DFO chelation regimens die before the age of 35 years.Citation8 It was estimated that compliance of DFO therapy ranged 59–78%, while that of deferiprone (DFP) was 79–98% with lower incidence of cardiac disease and endocrinopathy.Citation9 Hence, over the past 20 years, DFP has been recognized as an adjunctive iron overload treatment.
DFP (1,2‐dimethyl‐3‐hydroxypyrid‐4‐one) is a bidentate oral iron chelator not approved by the US Food and Drug Administration.Citation10 From retrospective studies, DFP appears superior to DFO in cardiac iron removal.Citation5,Citation11,Citation12 Evidence from magnetic resonance imaging (MRI) displayed faster myocardial iron unloading and a dramatic reduction of cardiac‐related mortality in the DFP treatment group compared with DFO.Citation4 Although, in terms of excreted iron, there is no significant difference between DFP and DFO.Citation13 Although DFP’s adverse effectsCitation14–Citation16 are embryotoxicity, arthritis (15%), gastrointestinal disturbance (6%), zinc deficiency (1%) and neutropenia (0·6%) including life threatening conditions like agranulocytosis (0·6%), the benefits from this drug may outweigh these risks.
Considering that there are limited resources in most of Asian developing countries for supporting the high financial burden inherent in the standard treatment of thalassemia disease, a great deal of attention should now focus on providing cheap, effective and good safety profile oral chelating agents for this group of patients. Although deferaxirox is another option of oral chelating agent, its cost is still a great barrier for patients in some developing countries.Citation17 DFP is now commonly used with DFO as a combination in order to get additive and synergistic effects of iron removal from particular organs.Citation18,Citation19 However, possible increased incidence of agranulocytosis and discomfort from the use of DFO are still a hindrance for this promising combination.Citation19,Citation20 For the sole DFP therapy trial, most are conducted in TM patientsCitation21,Citation22 or transfusion‐dependent patients.Citation23 In studies in which patients received intermittent blood transfusions, DFP was prescribed for seven beta‐thal/HbE and two homozygous beta‐thalassemia patients in the study by Pootrakul et al.Citation24 in which both effectiveness and good tolerance were observed. Thus, when considering better adherence to therapy and quality of life, along with easy availability with the lowest cost and superior benefit for the heart, DFP might be the appropriate choice for some groups of thalassemia patients.
In this study, we aimed to use DFP as the sole iron chelator in transfusion‐independent TI patients who had no symptomatic heart disease in order to evaluate its effectiveness in the reduction in iron overload and its possible role in the prevention of cardiopulmonary complications.
Materials and Methods
Study population
Beta‐thal/HbE patients (age 18–50 years) who were diagnosed with iron overload (ferritin level above 1000 ng/ml) and unable to use deferoxamine were enrolled in the trial. Patients were transfused only when they developed anemic symptoms or had a hemoglobin level of less than 6 g/dl. Those with a history of any dysfunction of the heart, kidney, liver, lungs, hematologic disease or having gastrointestinal disease which could interfere with drug absorption, HIV positivity, pregnancy or in lactation period, or with a high blood transfusion needed (>3500 ml/year) and a ferritin level above 10 000 ng/ml which needed vigorous chelation therapy of deferoxamine, were excluded from the study. DFP was prescribed to every patient without blinding or placebo. The patients were scheduled to visit the OPD every month until the twelfth month of DFP treatment and follow‐up was continued to monitor clinical symptoms following DFP withdrawal, (both for those who completed the study or dropped out), for at least 3 months. A total daily dose of 50 mg/kg/day, taken orally every 8 hours, was started in every patient and dose adjustment was done according to serum ferritin level change. The dose was titrated up until a greater than 15% reduction serum ferritin concentration between each visit was achieved.
The study and protocol were approved by the Siriraj Hospital’s Ethics Committee. A written informed consent was obtained from each patient directly. DFP was manufactured and prepared by Thai Government Pharmaceutical Organization.
Compliance
Compliance was assessed with a monitoring system in which the numbers of DFP tablets will be counted at each visit to calculate the amount of drugs that patients forgot to take. In addition, the history of compliance taken by the physician was compared with the amount of missed dose to recheck the accuracy. Patients in this study were also instructed to add on the missed dose, not to exceed one dose, to the subsequent dose and record the times of such addition.
Efficacy
To determine the efficacy of DFP in decreasing iron burden, we choose the simple marker that is expected to be available in local hospitals in Thailand, serum ferritin. We also further evaluated the oxidative stress marker which indirectly represented the detrimental effect from iron overload. These markers included non‐transferrin‐bound iron (NTBI), malondialdehyde (MDA), and labile plasma iron (LPI). All those markers were measured every 3 months.
Since cardiac complications were leading causes of death in iron overloaded thalassemia patients, we assessed cardiac function by echocardiography at the baseline screening and at the twelfth month of DFP therapy.
Adverse effects and safety
Adverse effects and safety were determined by regularly monitoring the patients for both the adverse effects which had been reported in previous studies,Citation7,Citation14,Citation16 by clinical symptoms (anorexia, nausea/vomiting, arthralgia/arthritis, rash, dizziness) and by laboratory parameters (complete blood count, serum aminotransferase, albumin, globulin, total bilirubin, direct bilirubin, serum alkaline phosphatase, serum gamma glutamyl transpeptidase, urea nitrogen, creatinine).
Management of adverse effects from DFP included permanent drug withdrawal, temporary discontinuation, supportive/symptomatic treatment with or without concomitantly taken DFP. The patients had the right to stop treatment at anytime during the study.
Echocardiography
Transthoracic echocardiography [two‐dimensional, M‐mode, Doppler, and tissue Doppler imaging (TDI)] was performed using a Hewlett Packard Sonos 7500 echocardiograph interfaced with a multifrequency transducer with a standard VHS videotape recording. Each echocardiographic parameter was measured on 3–5 consecutive cardiac cycles and the average was used for the analysis.
The left ventricular (LV) end‐systolic and end‐diastolic dimensions were measured using the M‐mode study. The LV ejection fraction was determined using the modified Simpson’s rule (biplane) and Teicholtz’s method. The LV diastolic function was evaluated by Doppler echocardiography of transmitral inflow and TDI of medial mitral annulus. Peak early (E) and late (A) diastolic velocities of mitral inflow and deceleration time of E were measured using the pulsed wave Doppler study with the sample volume at the tip of mitral valve. The TDI determination of diastolic function was performed in apical four‐chamber view with the sample volume at the septal aspect of mitral annulus. Longitudinal early (Ea) and late (Aa) diastolic myocardial velocities were measured. Left atrial volume, indicating the chronicity of diastolic dysfunction, was also measured using the area‐length biplane method.
Peak tricuspid regurgitation velocity (TRV) (m/second) was obtained by using continuous wave Doppler at tricuspid valve. The highest velocity was used. The right ventricular outflow tract time‐velocity integral (RVOT‐TVI) (cm) was obtained by placing a 1‐ to 2‐mm pulsed wave Doppler sample volume in the proximal right ventricular outflow tract just within the pulmonary valve when imaged from the parasternal short‐axis view. Then, the pulmonary vascular resistance (PVR) was calculated using the previously described method as the following formula: PVR (Wood unit) = 0·1618+(10·006×TRV/RVOT‐TVI).
Statistical analysis
The trend of oxidative stress markers and ferritin were plotted by using Student’s t‐test (paired t‐test), comparing each visit with the baseline screening and presented as means±SE. For other parameters (i.e. hematologic parameters, echocardiographic parameters), paired t‐test was also used to determine the significant change between the twelfth month of DFP treatment and baseline screening. Wilcoxon Signed Ranks test was used in subgroup analysis of ‘low ferritin group’, baseline ferritin level lower than 2500 ng/ml, because of non‐Student’s t‐distribution. All tests were two‐tailed and a P value of 0·05 was considered to indicate statistical significance.
Results
Baseline characteristics
No beta‐thal/HbE patient in this study had signs or symptoms of congestive heart failure throughout the study. Our subjects mainly represent the adult group (mean age = 28±2·42 years, ) of non‐regular transfusion dependent beta‐thal/HbE patient who were liable to develop cardiac disease according to serum ferritin concentration (17/22 had serum ferritin concentration above 2500 ng/ml).Citation25,Citation26 Twelve patients had had splenectomy. The hematologic and biochemical parameters () at the end of study were not significantly different compared with baseline screening except for the alanine aminotransferase and serum ferritin level; P = 0·026 and 0·005, respectively). Heart rate and mean systolic blood pressure were not significantly changed during the study (P⩾0·05).
Table 1. Baseline clinical characteristics of beta‐thalassemia/HbE patients (total = 30)
Table 2. Blood and biochemical parameters at baseline and the end of deferiprone treatment in beta‐thalassemia/HbE patients
Follow‐up
Ferritin
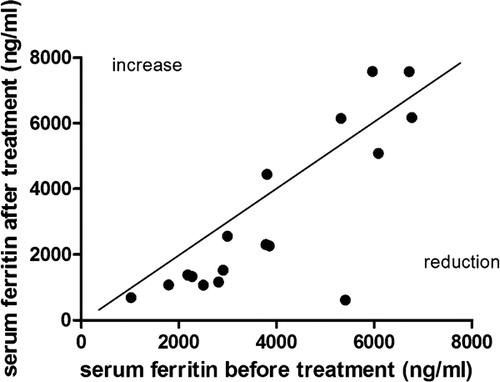
Mean serum ferritin concentration trend in the study is presented in . The significant decrease of mean ferritin level from baseline screening was observed after DFP administration for 6 months (means±SEM = −602·38±257·53 ng/ml,P = 0·03). Although the mean serum ferritin seemed to rise back up at the ninth month of treatment, its value at the twelfth month was decreased significantly compared with baseline after the dose of DFP was adjusted as per protocol (means±SEM = −844·68±272·63 ng/ml,P = 0·005). The individual trend of response (before–after) to single agent DFP therapy was calculated on a scatter plot and expressed as a linear curve (). As shown, most of the cases (18/22) were categorized in the reduction of ferritin group. Furthermore, the ferritin level was stable at least 3 months after stopping the treatment.
Figure 1. Levels of A) ferritin, B) non‐transferrin‐bound iron (NTBI,) C) Malondialdehyde (MDA), and D) labile plasma iron (LPI) (mean±SEM) in β‐thalassemia/HbE patients receiving deferiprone for 12 months. aCompared with baseline value at screening phase, P<0·05. Reproduced with permission.
Figure 2. Comparing ferritin values between baseline and after treatment phase; The diagonal line represents equivalence values. Values above the line denote increase of ferritin, the ones below denote reduction of ferritin after 1‐year deferiprone treatment. Reproduced with permission.
In the subgroup analysis, we classified the subjects in the study into two groups according to the level of serum ferritin concentration (). It was found that the mean ferritin concentrations of thalassemic patients in both groups were significantly decreased after 12 months of DFP treatment (P = 0·043 and 0·026 for group A and B respectively).
Table 3. Comparison of the treatment efficacy between the beta‐thalassemia/HbE patients with baseline ferritin <2500 ng/ml (low ferritin group – A) and those with the level ⩾2500 ng/ml (high ferritin group – B)
Oxidative stress marker
For the oxidative stress amelioration effect of DFP, the oxidative stress markers, NTBI and MDA showed a statistically significant decrease (P<0·01, paired t‐test) after treatment of oral DFP for 12 months, except for the LPI. NTBI was decreased significantly after only 3 months of treatment (−5·9±3·93 μM, P<0·001) and the lowest point of decrement was observed at the sixth month of treatment (3·21±0·42 μM; mean±SEM) (). Showing the same trend, the level of MDA markedly lessened (P = 0·027, paired t‐test) after DFP being continued in the patients for 3 months.
In the subgroup analysis, we found significant improvement of NTBI in both high and low ferritin groups (), but MDA was diminished significantly only in the high ferritin group (P = 0·0002).
Echocardiography
We found that early (E) and late (A) peak diastolic flow velocities were diminished significantly after 12 months of sole DFP treatment (P = 0·001 and 0·031, respectively; ). For E/A ratio, this changing value also reached statistical significance (1·70±0·39 versus 1·57±0·38, P = 0·019). Using criteria for diastolic dysfunction evaluation,Citation27 we found that there was neither a restrictive filling nor an impaired relaxation pattern in our study at the baseline screening. Two out of five cases of pseudonormalization pattern changed to normal pattern after 12 months of therapy.
Table 4. Echocardiographic parameters at baseline and the end of DFP treatment in beta‐thalassemia/HbE patients
To determine left atrial pressure, spectral tissue Doppler‐derived index; E/E′ ratio was used for overcoming ventricular relaxation influence on peak E velocity and differentiating pseudonormal filling pattern from normal one.Citation26 The average mitral valve E/E′ ratio was significantly lessened from 11·71±3·31 at baseline to 9·93±3·06 at the end of study (P = 0·003).
One of the main pathophysiologies in thalassemia is vascular involvement which is attributed to PVR and systemic vascular stiffness.Citation28 In this study, six patients (6/22) reached the criteria of PHT.Citation29 Four out of these six patients had undergone splenectomy. After 1 year of DFP administration, significant decreases in mean pulmonary artery pressure and PVR (PVR) were found in whole study analysis (P = 0·021 and 0·003, respectively; ). Other parameters representing pulmonary pressure such as right atrial pressure, right ventricular systolic pressure and pulmonary artery end‐diastolic pressure were also diminished, though no statistical significance was found. Interestingly, after analyzing a subgroup of six PHT patients, we found that five had significant decreases in all those parameters (P<0·05).
In subgroup analysis, comparing the twelfth month and baseline screening, we also found dramatic changes in LV diastolic parameters (mitral valve E, A, E/A ratio and E/E′ ratio, P<0·05; ) in the high ferritin group which were not observed in the low ferritin group. Furthermore, PVR was significantly lessened in both groups after subgroup analysis (P<0·05).
Adverse effect
In this present study, we monitored and recorded the adverse effect data in every visit (). After excluding five subjects who could not be followed up regularly, agranulocytosis was observed in one patient (1/25). The patient was a splenectomized beta‐thal/HbE who started with 50 mg/kg/day of DFP and titrated up gradually 5–10 mg/kg/day in each visit to 80 mg/kg/day at the fifth visit when the episode of agranulocytosis was found. Absolute neutrophil count (ANC) was 0·47×109/l and dropped further to 0·28×109/l within 5 days after drug discontinuation. The signs and clinical symptoms of sepsis were not observed. This patient’s ANC had risen back above 1·5×109/l within 2 weeks without administration of granulocyte colony stimulating factor and was stable after 3 months of follow‐up after drop‐out from the project. One further patient dropped their ANC to 1·17×109/l at the fifth visit (on DFP 75 mg/kg/day). Again, the patient was clinically stable without any complaint and ANC was stable at approximately 2·3×109/l after rechallenging with the drug until the end of the study without recurrent neutropenic episode.
Table 5. Adverse effects and outcomes occurring in this study (total no. of drop out = 8/30)
In regard to other common adverse effects of DFP, the nausea/vomiting (3/25), arthralgia/arthritis (4/25), and rash (1/25) were observed. All episodes of nausea/vomiting occurred within the first 2 weeks of DFP initiation. One patient did not response to temporary drug discontinuation/symptomatic treatment with metoclopramide and decided to quit the study at the fourth visit. The others responded well and the symptom disappeared after 1 month of DFP therapy. Patients with arthralgia/arthritis responded to non‐steroidal anti‐inflammatory drugs (NSAIDs). The symptoms subsided and the patients did not require symptomatic treatment after the sixth month of the study. One patient developed maculopapular rash which was self‐limited and disappeared after the second month of DFP initiation without symptomatic treatment. Anaphylaxis was found in second month of treatment in one patient. She presented at emergency room of a district hospital with the symptoms of chest discomfort and dyspnea with skin involvement and unstable vital signs. After, epinephrine administration with antihistamine, the patient recovered within 24 hours and had no recurrence of such symptoms. On retrospective history review, she denied concomitant drug use or any other exposure and decided to withdraw from the study. Of the other five cases who withdrew all had transport problems after participating for 2 months. Thus, data from 22 patients were analyzed after those eight patients withdrew.
Discussion
Serum ferritin concentration was used as a mean of body iron burden assessments. Although this acute phase reactant can be influenced with many factors like liver status, inflammation or ascorbate deficiency,Citation30 some studies have shown that there was significant correlation between liver iron concentration and ferritin level (r = 0·62, P<0·001).Citation31 So with a combination of oxidative stress markers measurement, we used ferritin to monitor the efficacy of DFP treatment. At the average dose of 67·05 mg/kg/day of DFP and a compliance rate of 89·5% in this study, this chelating agent reduced serum ferritin effectively. Considering that the patients with a serum ferritin concentration equal to or above 2500 ng/ml would have a significantly increased risk of cardiac siderosis from iron overload,Citation32 DFP still showed favorable efficacy of serum ferritin reduction in both high and low risk groups for cardiac siderosis after subgroup analysis was done. Because subjects in this study were mainly comprised of high ferritin (⩾2500 ng/ml) patients (17/22), we can assume that its potency in serum ferritin reduction is promising in the cardiac siderosis high risk group.
NTBI plays a pivotal role in free radicals production leading to oxidative stress found in thalassemic pathophysiology. This low molecular weight form of iron had been proven previously as a good index of iron overload in both TM and TI.Citation31,Citation33–Citation37 It showed positive correlation with serum ferritin, splenectomy, amount of blood transfusionCitation2 and a negative correlation with reduced glutathione (GSH).Citation33 Also, as a marker of early iron overload when the ferritin level is normalCitation31 and being associated with cardiac siderosis,Citation37 it is very impressive that oral DFP therapy alone in this study could decrease this marker significantly as early as after 3 months of treatment and constantly maintain this effect until the end of the study. Thus, DFP can be considered as a potential reducer of oxidative damage, via NTBI chelation, that occurs continuously in beta‐thal/HbE patients. Furthermore, Breuer et al. have proven that the NTBI chelating effect of DFO was only transient, as it reappeared after stopping DFO infusion for 1 hour in 55% of the cases.Citation38 Therefore, DFP with longer half‐life might be superior to DFO in chelating this free form iron.
Labile plasma iron is the fraction of NTBI that is absent from serum of a healthy individualCitation39 and can move across cell membranes in a non‐regulated manner leading to the iron overload in visceral organs.Citation36 To analyze this redox‐active component of NTBI that is chelatable, we measured the LPI level in parallel with other oxidative stress parameters. The result showed that there was no significant change in LPI level at the third, sixth, ninth and twelfth month of DFP treatment compared to baseline. However, these data do not reflect the lack of effect of the iron chelating role of DFP. The data from Cabantchik et al. showed that the decreased LPI level from DFP will rise back up after DFP washout periods longer than 2 hours.Citation36 So, the blood sampling for LPI level in the morning of each visit did not represent the efficacy of DFP in LPI chelation. In addition, it was found that mean LPI level in four daily doses regimen of DFP treatment (75 mg/kg/day) did not exceed the 0·6 μM (threshold level) until midnight, compared with the three daily doses regimen in which LPI exceeded that level by evening.Citation36 This suggests that patients on the three daily doses regimen will have a longer time of LPI exposure and will be put to the risk of more iron overload in visceral organs. Ideally, these data suggest that it might be more appropriate to prescribe DFP in four daily doses rather than three doses as in this study for the beta‐thal/HbE patients who plan to use this drug as sole therapy. However, the suitable regimen has to balance the efficacy of high LPI exposure time decrement and the compliance of patients.
MDA is the highly toxic product of polyunsaturated fatty acid peroxidation. It is involved in many detrimental pathways such as atherosclerosis,Citation40 mutagenesisCitation41 and apoptosis.Citation42 From our results, the decreased MDA level was observed from the third month of DFP treatment. This effect of DFP to MDA was still observed at the twelfth month (; P = 0·002, paired t‐test). As the MDA level correlated significantly (r = 0·39–0·88, P<0·05) with the iron content of liver, spleen, and heart,Citation43 correlation of decreased MDA after DFP treatment and decreased deposited iron level in those vital organs should be tested further.
Beta‐thal/HbE is a TI which encompasses a broad spectrum of severity ranging from mild anemia to severe transfusion dependent anemia.Citation25,Citation44 For patients requiring intermittent transfusions, morbidity and mortality from iron overload still occur through dysregulation of hepcidin resulting in increased gastrointestinal iron absorption.Citation5,Citation45 This pathology brings about the cardiopulmonary disease which is the leading cause of death in thalassemia patients. Evidence from previous studies point out many responsible pathological steps in cardiovascular physiology, i.e., high output state from chronic hypoxia,Citation5 abnormal LV relaxation or decreased compliance of left ventricle,Citation29 and vasculopathy from nitrite disturbance leading to PHT.Citation29,Citation46 Cardiac MRI T2* has been claimed as the most accurate and reliable tool in cardiac iron burden assessment;Citation26 however, this technique is not available in most of hospitals in many Southeast Asian countries where there is high prevalence of thalassemia disease. It is mainly used in the research field and its cost is at a level that most thalassemia patients cannot get access to this investigational tool. Taking into account such limitations, the basic and easily available tools such as EKG and echocardiography are more appropriate. In this study, we used echocardiography to approach cardiovascular pathology in thalassemia patients.
From prior echocardiographic studies, the abnormal LV diastolic function in early stage of TM has been discussed.Citation47–Citation49 However, this issue has never been in consensus for TI patient group. In this study, we selected asymptomatic TI patients to observe how structural and functional parameters of the heart changed after DFP administration for 1 year. At baseline, we did not find any impaired relaxation or restrictive filling case. Thus, the significantly decreased early (E) and late (A) diastolic peak including E/A ratio should be cautiously interpreted. All pseudonormalized filling patients (five cases) had levels of serum ferritin above 2500 ng/ml (mean±SEM = 3474·40±243·20 ng/ml), but the rest 17 normal diastolic function patients consisted of up to 12 high ferritin level patients. Consistent with the previous studies,Citation50,Citation51 high serum ferritin alone could not be considered an isolated risk factor for abnormal diastolic filling in TI patient. In contrast to the study of Spirito et al.Citation48 in which TM patients received optimal chelation with DFO, we demonstrated an improvement of early and late diastolic peak including E/A and E/E′ ratios after 1‐year DFP treatment. This result and the fact that the heart might be the last organ to be loaded with excess ironCitation52 suggest further studies regarding efficacy of DFP in preventing the diastolic abnormality.
Recently, the body of evidence about PHT notably found in TI patient has been growing. Aessopos et al. have demonstrated that an intermittently transfused TI patient is more likely to develop PHT compared with a TM patient who gets adequate transfusion and chelation therapy.Citation27,Citation28,Citation53 Chronic hemolysis, tissue hypoxia, and oxidative damage cause depletion of nitrite which is the key reservoir of nitric oxide for a free hemoglobin scavenging effect. This negative effect of nitric oxide availability leads to endothelial dysfunction that promotes hypercoagulability and compromises pulmonary vascular dilatation which is essential for the compensatory mechanism of the increased cardiac output condition commonly found in thalassemia disease.Citation40,Citation54,Citation55 All those pathologies support the natural history of PHT in the irregularly transfused TI patient. However, there is no clinical study regarding PHT prevention in the TI group so far.
Our preliminary result regarding efficacy of DFP in the improvement in pulmonary vasculopathy is encouraging. This finding may be explained by previous evidence regarding DFP effect. DFP has been shown to be effective and safe in the reversal of accelerating oxidative stress related to tissue damage in iron related cardiomyopathy in thalassaemia. In vitro, in vivo and clinical data suggest that L1 is one of the most potent antioxidants because of its ability to reach extracellular and intracellular compartments of many tissues and inhibit iron catalyzed free radical reactions.Citation56 Hence, DFP may retrieve nitric oxide availability via such an antioxidative effect and iron deposition attenuation in pulmonary and cardiac system.Citation40 Unfortunately, this study was not designed for evaluation of the benefit of DFP on PHT. Thus, the inclusion criteria did not allow us to select adequately known PHT cases into this study. From a total of 30 patients, eight cases dropped out from the trial because of intolerable or life threatening adverse effects. Hence, we had only six cases (6/22) diagnosed with PHT which is insufficient to draw a conclusion about the beneficial effect from DFP in PHT treatment. To sum up, the result from this study primarily provided confirmation of a plausible and promising role for DFP as the sole therapy in PHT prevention and treatment in TI patients.
Consequently, in the cases when MRI is not available, choosing which parameter would be appropriately used for monitoring DFP treatment is an important question to be answered. Here we propose the combination of ferritin, oxidative stress markers and some of echocardiographic parameters as a solution. Though there are some limitations in this combination, it will probably aid physicians make better decisions in DFP dose adjustment, and in starting or stopping DFP therapy. To achieve this ultimate goal, further study regarding this issue should be conducted.
Limitations and Further Study
The limitations of this study are derived from the cross‐sectional echocardiographic evaluation, before and after treatment, which cannot represent a trend of improved parameters. There is also the lack of more reliable and precise tools like MRI T2* or cardiac catheterization to compare with and confirm our findings. Moreover, how better values of biochemical and cardiologic parameters contribute to clinical symptoms and morbidity/mortality is the issue which merits further study. Another key limitation is that study results from 1 year might not be convincing enough in drawing a definite conclusion about some DFP’s advantages in the long term and too short for beneficial hematologic or cardiologic improvement to be observed. Large‐scale trials, longer periods of observation, well‐selected TI cases and good methodology for each particular purpose are needed in any further study.
Conclusion
Our primary objective in this study focused on using DFP as the sole chelating agent in amelioration of iron overload in beta‐thal/HbE patient, a type of TI commonly found in Thailand. From the preliminary results, we observed the benefits of DFP in iron excess and oxidative stress reduction and some significant improvement of echocardiographic parameters. Our findings suggest the possibility of using oral DFP alone for the improvement of cardiovascular complications in TI. We also postulate further studies of the remaining questions that must be answered before general adoption of such therapy.
This study was funded by Faculty of Medicine Siriraj hospital, Mahidol University, Thailand. We would like to thank the staff and patients of Thalassemia clinic at Siriraj hospital, Bangkok, Thailand for their cooperation and give an appreciation to Dr Chulaluk Komontri for statistics opinion.
References
- Viprakasit V, Lee‐Lee C, Chong QT, Lin KH, Khuhapinant A. Iron chelation therapy in the management of thalassemia: the Asian perspectives. Int J Hematol 2009;90:435–45.
- Fucharoen S, Ketvichit P, Pootrakul P, Siritanaratkul N, Piankijagum A, Wasi P. Clinical manifestation of beta‐thalassemia/hemoglobin E disease. J Pediatr Hematol Oncol 2000;22:552–7.
- Nuntakarn L, Fucharoen S, Fucharoen G, Sanchaisuriya K, Jetsrisuparb A, Wiangnon S. Molecular, hematological and clinical aspects of thalassemia major and thalassemia intermedia associated with Hb E‐beta‐thalassemia in Northeast Thailand. Blood Cells Mol Dis 2009;42:32–5.
- Neufeld EJ. Oral chelators deferasirox and deferiprone for transfusional iron overload in thalassemia major: new data, new questions. Blood 2006;107:3436–41.
- Taher A, Hershko C, Cappellini MD. Iron overload in thalassaemia intermedia: reassessment of iron chelation strategies. Br J Haematol 2009;147:634–40.
- Aessopos A, Farmakis D, Karagiorga M, Voskaridou E, Loutradi A, Hatziliami A, et al.. Cardiac involvement in thalassemia intermedia: a multicenter study. Blood 2001;97:3411–6.
- Cappellini MD, Pattoneri P. Oral iron chelators. Annu Rev Med 2009;60:25–38.
- Cianciulli P. Treatment of iron overload in thalassemia. Pediatr Endocrinol Rev 2008;6(Suppl 1):208–13.
- Delea TE, Edelsberg J, Sofrygin O, Thomas SK, Baladi JF, Phatak PD, et al.. Consequences and costs of noncompliance with iron chelation therapy in patients with transfusion‐dependent thalassemia: a literature review. Transfusion 2007;47:1919–29.
- Taher A, Chamoun FM, Koussa S, Saad MA, Khoriaty AI, Neeman R, et al.. Efficacy and side effects of deferiprone (L1) in thalassemia patients not compliant with desferrioxamine. Acta Haematol 1999;101:173–7.
- Peng CT, Tsai CH, Wu KH. Effects of chelation therapy on cardiac function improvement in thalassemia patients: literature review and the Taiwanese experience. Hemoglobin 2008;32:49–62.
- Huang YC, Chang JS, Wu KH, Peng CT. Regression of myocardial dysfunction after switching from desferrioxamine to deferiprone therapy in beta‐thalassemia major patients. Hemoglobin 2006;30:229–38.
- Mamtani M, Kulkarni H. Influence of iron chelators on myocardial iron and cardiac function in transfusion‐dependent thalassaemia: a systematic review and meta‐analysis. Br J Haematol 2008;141:882–90.
- Vlachaki E, Tselios K, Perifanis V, Tsatra I, Tsayas I. Deferiprone‐related arthropathy of the knee in a thalassemic patient: report of a case and review of the literature. Clin Rheumatol 2008;27:1459–61.
- Kwiatkowski JL. Oral iron chelators. Pediatr Clin North Am 2008;55:461–482, x.
- Kontoghiorghes GJ, Neocleous K, Kolnagou A. Benefits and risks of deferiprone in iron overload in Thalassaemia and other conditions: comparison of epidemiological and therapeutic aspects with deferoxamine. Drug Saf 2003;26:553–84.
- Kontoghiorghes GJ. Ethical issues and risk/benefit assessment of iron chelation therapy: advances with deferiprone/deferoxamine combinations and concerns about the safety, efficacy and costs of deferasirox. Hemoglobin 2008;32:1–15.
- Cario H, Janka‐Schaub G, Janssen G, Jarisch A, Strauss G, Kohne E. Recent developments in iron chelation therapy. Klin Padiatr 2007;219:158–65.
- Kattamis A. Combined therapy with deferoxamine and deferiprone. Ann NY Acad Sci 2005;1054:175–82.
- Victor Hoffbrand A. Deferiprone therapy for transfusional iron overload. Best Pract Res Clin Haematol 2005;18:299–317.
- Olivieri NF, Brittenham GM, Matsui D, Berkovitch M, Blendis LM, Cameron RG, et al.. Iron‐chelation therapy with oral deferipronein patients with thalassemia major. N Engl J Med 1995;332:918–22.
- Olivieri NF, Brittenham GM, McLaren CE, Templeton DM, Cameron RG, McClelland RA, et al.. Long‐term safety and effectiveness of iron‐chelation therapy with deferiprone for thalassemia major. N Engl J Med 1998;339:417–23.
- Hoffbrand AV, AL‐Refaie F, Davis B, Siritanakatkul N, Jackson BF, Cochrane J, et al. Long‐term trial of deferiprone in 51 transfusion‐dependent iron overloaded patients. Blood 1998;91:295–300.
- Pootrakul P, Sirankapracha P, Sankote J, Kachintorn U, Maungsub W, Sriphen K, et al.. Clinical trial of deferiprone iron chelation therapy in beta‐thalassaemia/haemoglobin E patients in Thailand. Br J Haematol 2003;122:305–10.
- Davis BA, O’Sullivan C, Jarritt PH, Porter JB. Value of sequential monitoring of left ventricular ejection fraction in the management of thalassemia major. Blood 2004;104:263–9.
- Arques S, Roux E, Luccioni R. Current clinical applications of spectral tissue Doppler echocardiography (E/E′ ratio) as a noninvasive surrogate for left ventricular diastolic pressures in the diagnosis of heart failure with preserved left ventricular systolic function. Cardiovasc Ultrasound 2007;5:16.
- Aessopos A, Farmakis D, Deftereos S, Tsironi M, Tassiopoulos S, Moyssakis I, et al.. Thalassemia heart disease: a comparative evaluation of thalassemia major and thalassemia intermedia. Chest 2005;127:1523–30.
- Aessopos A, Kati M, Farmakis D. Heart disease in thalassemia intermedia: a review of the underlying pathophysiology. Haematologica 2007;92:658–65.
- McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, et al.. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation 2009;119:2250–94.
- Wang W, Knovich MA, Coffman LG, Torti FM, Torti SV. Serum ferritin: Past, present and future. Biochim Biophys Acta 2010;1800:760–9.
- Taher A, El Rassi F, Isma’eel H, Koussa S, Inati A, Cappellini MD. Correlation of liver iron concentration determined by R2 magnetic resonance imaging with serum ferritin in patients with thalassemia intermedia. Haematologica 2008;93:1584–6.
- Olivieri NF, Nathan DG, MacMillan JH, Wayne AS, Liu PP, McGee A, et al.. Survival in medically treated patients with homozygous beta‐thalassemia. N Engl J Med 1994;331:574–8.
- Taher A, Musallam KM, El Rassi F, Duca L, Inati A, Koussa S, et al.. Levels of non‐transferrin‐bound iron as an index of iron overload in patients with thalassaemia intermedia. Br J Haematol 2009;146:569–72.
- Domenica Cappellini M, Tavazzi D, Duca L, Marelli S, Fiorelli G. Non‐transferrin‐bound iron, iron‐related oxidative stress and lipid peroxidation in beta‐thalassemia intermedia. Transfus Sci 2000;23:245–6.
- Al‐Refaie FN, Wickens DG, Wonke B, Kontoghiorghes GJ, Hoffbrand AV. Serum non‐transferrin‐bound iron in beta‐thalassaemia major patients treated with desferrioxamine and L1. Br J Haematol 1992;82:431–6.
- Cabantchik ZI, Breuer W, Zanninelli G, Cianciulli P. LPI‐labile plasma iron in iron overload. Best Pract Res Clin Haematol 2005;18:277–87.
- Piga A, Longo F, Duca L, Roggero S, Vinciguerra T, Calabrese R, et al.. High nontransferrin bound iron levels and heart disease in thalassemia major. Am J Hematol 2009;84:29–33.
- Breuer W, Ronson A, Slotki IN, Abramov A, Hershko C, Cabantchik ZI. The assessment of serum nontransferrin‐bound iron in chelation therapy and iron supplementation. Blood 2000;95:2975–82.
- Esposito BP, Breuer W, Sirankapracha P, Pootrakul P, Hershko C, Cabantchik ZI. Labile plasma iron in iron overload: redox activity and susceptibility to chelation. Blood 2003;102:2670–7.
- Hill GE, Miller JA, Baxter BT, Klassen LW, Duryee MJ, Tuma DJ, et al.. Association of malondialdehyde‐acetaldehyde (MAA) adducted proteins with atherosclerotic‐induced vascular inflammatory injury. Atherosclerosis 1998;141:107–16.
- Chuang CH, Huang CE, Chen HL. DNA strand breakage and lipid peroxidation after exposure to welding fumes in vivo. Mutagenesis 2010;25:71–6.
- Willis MS, Klassen LW, Tuma DJ, Thiele GM. Malondialdehyde‐acetaldehyde‐haptenated protein induces cell death by induction of necrosis and apoptosis in immune cells. Int Immunopharmacol 2002;2:519–35.
- Linpisarn S, Satoh K, Mikami T, Orimo H, Shinjo S, Yoshino Y. Effects of iron on lipid peroxidation. Int J Hematol 1991;54:181–8.
- Fucharoen S, Winichagoon P. Clinical and hematologic aspects of hemoglobin E beta‐thalassemia. Curr Opin Hematol 2000;7:106–12.
- Tanno T, Bhanu NV, Oneal PA, Goh SH, Staker P, Lee YT, et al.. High levels of GDF15 in thalassemia suppress expression of the iron regulatory protein hepcidin. Nat Med 2007;13:1096–101.
- Morris CR, Gladwin MT, Kato GJ. Nitric oxide and arginine dysregulation: a novel pathway to pulmonary hypertension in hemolytic disorders. Curr Mol Med 2008;8:620–32.
- Bosi G, Crepaz R, Gamberini MR, Fortini M, Scarcia S, Bonsante E, et al.. Left ventricular remodelling, and systolic and diastolic function in young adults with beta thalassaemia major: a Doppler echocardiographic assessment and correlation with haematological data. Heart 2003;89:762–6.
- Spirito P, Lupi G, Melevendi C, Vecchio C. Restrictive diastolic abnormalities identified by Doppler echocardiography in patients with thalassemia major. Circulation 1990;82:88–94.
- Kremastinos DT, Tsiapras DP, Tsetsos GA, Rentoukas EI, Vretou HP, Toutouzas PK. Left ventricular diastolic Doppler characteristics in beta‐thalassemia major. Circulation 1993;88:1127–35.
- Isma’eel H, Chafic AH, Rassi FE, Inati A, Koussa S, Daher R, et al.. Relation between iron‐overload indices, cardiac echo‐Doppler, and biochemical markers in thalassemia intermedia. Am J Cardiol 2008;102:363–7.
- Leon MB, Borer JS, Bacharach SL, Green MV, Benz EJ, Griffith P, et al. Detection of early cardiac dysfunction in patients with severe beta‐thalassemia and chronic iron overload. N Engl J Med 1979;301:1143–8.
- Buja LM, Roberts WC. Iron in the heart. Etiology and clinical significance. Am J Med 1971;51:209–21.
- Aessopos A, Farmakis D. Pulmonary hypertension in beta‐thalassemia. Ann NY Acad Sci 2005;1054:342–9.
- Grubina R, Huang Z, Shiva S, Joshi MS, Azarov I, Basu S, et al.. Concerted nitric oxide formation and release from the simultaneous reactions of nitrite with deoxy‐ and oxyhemoglobin. J Biol Chem 2007;282:12916–27.
- Villagra J, Shiva S, Hunter LA, Machado RF, Gladwin MT, Kato GJ. Platelet activation in patients with sickle disease, hemolysis‐associated pulmonary hypertension, and nitric oxide scavenging by cell‐free hemoglobin. Blood 2007;110:2166–72.
- Kontoghiorghes GJ. Prospects for introducing deferiprone as potent pharmaceutical antioxidant. Front Biosci (Elite Ed) 2009;1:161–78.