Abstract
Transplantation with cryopreserved allogeneic peripheral blood stem cells (PBSCs) from related donors is widely conducted in Japan. To freeze PBSCs, a solution containing dimethyl sulfoxide (DMSO), which can have various adverse effects, is added. DMSO-depleted allogeneic PBSCs were transplanted into 21 patients. The cryoprotectant was manually removed from thawed PBSCs and the cells were mixed with a solution containing citrate dextrose as an anticoagulant and RPMI-1640 medium. DMSO-depleted PBSCs were immediately infused into patients subjected to conditioning. Infusion-related adverse effects were only observed in three patients. The median neutrophil recovery (⩾0·5×109/l) and platelet recovery (⩾20×109/l) were 13·0 and 14·0 days, respectively. Only one patient with mixed-lineage leukemia in non-complete remission did not show engraftment, likely due to a second transplantation and a two-antigen disparity in human leukocyte antigen system A. The results suggest the removal of DMSO from thawed PBSCs to be safe and useful for transplantation.
Introduction
Peripheral blood stem cells (PBSCs) have become increasingly popular for allogeneic hematopoietic stem cell transplantations.Citation1 PBSCs collected from donors are infused into recipients either that same day or the next; however, the cells can be also cryopreserved.Citation2 Most centers in Europe and the United States take the former approach based on concerns that the cryopreservation and thawing of PBSCs may worsen clinical outcomes. Recently, Kim et al. reported similar outcomes of peripheral blood stem cell transplantation (PBSCT) between the infusion of freshly isolated allografts and cryopreserved allografts.Citation3 The advantages of cryopreserving PBSCs are that the cells can be collected from donors independently of recipients and the recipients can receive transplants at the most appropriate times.
The efficacy of cryopreserved hematopoietic progenitor cells for autologous blood and marrow transplantations is well established.Citation4–Citation7 The freezing process requires a cryoprotectant to prevent cell injury caused by low temperature. Dimethyl sulfoxide (DMSO), the most commonly used cryoprotectant, is added to reduce the formation of intracellular ice and effects of the solution during freezing. However, the infusion of DMSO can cause a range of dose-dependent toxic effects such as nausea, vomiting, coughing, flushing, rash, chest tightness, chills, abdominal pain, hypotension, renal failure, and neurologic complications.Citation8–Citation12 These adverse effects can be reduced by removing the DMSO from thawed cells prior to infusion.Citation13–Citation15 To our knowledge, there have been no reports on allogeneic PBSCT with cryoprotectant-depleted cells. We report here the outcomes of such transplantations.
Materials and Methods
Patients and donors
During the period from March 1998 to September 2008, 21 patients with hematological malignancies received cryoprotectant-depleted PBSCs for allogeneic PBSCT in our hospital (). Detailed information on PBSCT and PBSC collection was offered to patients and donors, respectively, prior to the procedures. Informed consent was obtained from all the subjects. In each case, donor was related to the patient. PBSCs were mobilized with subcutaneous injections of granulocyte colony-stimulating factor (G-CSF) at a dose of 400 μg/m2/day (filgrastim; Kirin Co. Ltd, Tokyo, Japan) or 10 μg/kg/day (lenograstim; Chugai Pharmaceutical Inc., Tokyo, Japan) for 5 days. On day 4 or 5 of G-CSF treatment, the collection of PBSCs was started using an automated continuous-flow blood cell separator (Spectra; Cobe, Englewood, CO, USA). The number of times for which apheresis was performed was set according to the collected cell number or collected CD34-positive cell number with a target of more than 2×108 and 2×106 per recipient body weight kilogram, respectively.
Table 1. Patient characteristics
Cell viability was measured using the trypan blue dye exclusion test. CD34-positive cell numbers were determined using flow cytometry.Citation16 Colony-forming unit-granulocyte-macrophages (CFU-GM) were counted using the methylcellulose culture technique.Citation17
Cryopreservation
Sixty milliliters of a collected PBSC solution was mixed with 40 ml of RPMI-1640 medium (Sigma Co., St Louis, MO, USA) supplemented with 2000 units of heparin. Thirty-two milliliters of 25% human albumin (Kaketsuken, Kumamoto, Japan) and 8 ml of saline were added to the cryoprotectant solution, CP-1 (Kyokuto Pharmaceutical Industrial Co., Ltd, Tokyo, Japan), which contains 50 ml of 24% hydroxyethylstarch and 10 ml of 99% DMSO.Citation18 One hundred milliliters of the harvested PBSC solution was mixed with an equivalent volume of the CP-1 solution containing albumin and saline on ice. The final concentration of DMSO in the mixture was 5%. Then, bags containing the cell mixture were stored at −80 or −120°C in a deep freezer for up to 1 year.Citation19
Removal of cryoprotectant and cell infusion
To reduce the toxicity of DMSO, the cryoprotectant including DMSO in the bags was removed as described below. Cryopreserved bags were rapidly thawed in a 37°C water bath. Bags were centrifuged at 2000 rpm for 10 minutes and the supernatant was removed. Cells were resuspended with ACD-RPMI medium, containing 30 ml of anticoagulant citrate dextrose solution A (ACD-A; Terumo Co., Tokyo, Japan) and 170 ml of RPMI-1640 medium. PBSCs in the prepared bags were immediately infused into patients through a central vein catheter without a filter. All patients received 100 mg of hydrocortisone to prevent adverse effects associated with the infusion. Patients’ symptoms and vital signs were carefully monitored by doctors and nurses during infusions. From day 1, G-CSF was administered intravenously.
Definitions
Clinical data including engraftment of neutrophils, engraftment of platelets, acute graft-versus-host disease (aGVHD), chronic GVHD, and overall survival (OS) were collected. The day of infusion was defined as day 0. Neutrophil engraftment was defined as an absolute neutrophil count greater than 0·5×109/l for three consecutive days. Platelet engraftment was defined as a platelet count greater than 20×109/l for three consecutive days without platelet transfusion. aGVHD and chronic GVHD were diagnosed and graded according to established criteria.Citation20Citation20,21 OS was defined as the time from transplantation until death from any cause. Disease risk was categorized as low, intermediate, or high according to risks for mortality after allogeneic hematopoietic stem cell transplantation.Citation22 Adverse effects were identified according to Common Terminology Criteria for Adverse Events v2·0 by a search of medical charts.
Statistical analysis
A paired t-test was used to investigate the mean differences between two dependent populations. OS was calculated by the Kaplan–Meier method. Analyses were performed using R.Citation23
Results
shows the characteristics of the patients. The diseases of the patients were as follows: 5 patients, acute myeloblastic leukemia; 2 patients, acute lymphoblastic leukemia patients; 1 patient, acute mixed-lineage leukemia; 4 patients, myelodysplastic syndrome; 1 patient, chronic myelogenous leukemia; 6 patients, non-Hodgkin lymphoma; and 1 patient, Hodgkin lymphoma. Only one patient was in their first complete remission (CR) and the others were beyond a second CR or in non-remission. Seven and 14 patients were classified into the intermediate-risk and high-risk disease categories, respectively. A disparity in human leukocyte antigen system A (HLA) between patients and donors was found in four cases. The apheresis number was two times higher in 11 patients and three times higher in 10 patients. Ex vivo cell manipulation including T-cell depletion was not conducted in any patients. Fifteen patients and six patients underwent myeloablative conditioning and reduced intensity conditioning, respectively. GVHD prophylaxis involved short-term treatment with methotrexate and cyclosporine.
Freshly isolated nucleated cells, CD34+ cells and CFU-GM numbered 7·97×108, 3·46×106 and 68·78×105/kg, respectively (). The median storage duration of bags was 32 days (range: 8–73 days). Cell viability remained good (86·8%) even after the cryoprotectant was removed (). However, the nucleated cell number and CFU-GM number significantly decreased to 83·7 and 96·7%, respectively (). The CD34+ cell number after the procedure was not measured.
Table 2. Characteristics of DMSO-depleted allografts
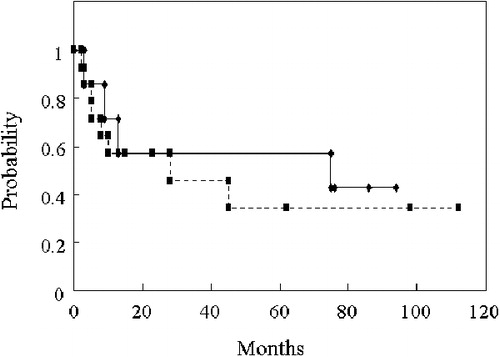
Three patients showed mild adverse effects during the infusion of PBSCs: two patients, grade 1 gastrointestinal symptoms and one, grade 1 headache. No serious immediate adverse effects were observed. Engraftment of the infused PBSCs was rapid; the median times of neutrophil and platelet engraftment were 13·0 and 14·0 days, respectively. One patient did not achieve engraftment. This patient had acute mixed-lineage leukemia in non-CR. She first received a cord blood transplant for her leukemia in non-CR after a myeloablative-conditioning regimen. Three months after the transplantation, her leukemia relapsed. She immediately received a PBSC transplant again from her HLA 2-antigen mismatched mother after a reduced intensity-conditioning regimen. However, blasts persisted in the bone marrow and a normal blood cell recovery did not occur. The total CD34+ cell number at PBSC collection was 1·5×106/kg. The incidence of aGVHD grades II–IV was 55% (11/20). The cumulative incidence of chronic GVHD amounted to 69·2% (9/13). The OS rates for all recipients at 1 and 2 years were 61·9 and 57·1%, respectively. The OS rate at 1 year for patients categorized as being intermediate risk was 71·4%, as compared to 57·1% for those at high risk ().
Discussion
Patients undergoing autologous stem cell transplantation by the infusion of cryopreserved marrow have a significantly increased incidence of infusion-associated adverse effects, when compared to allogeneic recipients of fresh marrow.Citation2 Such infusion-associated symptoms are, in large part, due to the presence of DMSO which is the most commonly used cryoprotectant and is traditionally present at final concentration of 10% in cryopreserved donor grafts.Citation8 Several studies showed that DMSO depletion from cryopreserved autologous PBSCs reduces adverse events with minimal effects on engraftment.Citation13–Citation15 Unlike in Europe and the United States, allogeneic PBSCs are usually frozen until infused into patients in Japan.Citation24Citation24,25 This is mainly due to the concerns about insufficient CD34+ cells due to poor mobilization. Very recently, the use of PBSCs from unrelated donors has been approved by the Ministry of Health, Labor and Welfare in Japan. The Japan Marrow Donor Program has stated that PBSCs collected from unrelated donors ought to not be frozen but be infused directly into patients. Therefore, fewer occasions will exist for freezing allogeneic PBSCs from related donors.
In our study, the reason for the removal of cryoprotectants from frozen PBSCs was to reduce the volume of cryoprotectant stored in more than two bags. We showed that the removal of cryoprotectant and replacement with ACD-RPMI medium reduced the adverse effects associated with DMSO. Importantly, such a procedure retains the engraftment ability of hematopoietic stem cells after PBSCT. In autologous PBSC collection, factors associated with poor CD34+ cell mobilization are well known.Citation26Citation26,27 In an allogeneic setting, female gender and older age are associated with a lower yield of CD34+ cells.Citation28Citation28,29 In a situation of poor CD34+ cell mobilization, PBSCs may be collected repeatedly and frozen in several bags. If such bags are thawed and the cells directly infused at transplantation, the toxicity of DMSO might be a concern. In this setting, our procedure seems to be important for reducing the toxic effects of DMSO with minimal loss of hematopoietic stem cell ability. To confirm our results, prospective studies are needed.
References
- Cutler C, Antin JH. Peripheral blood stem cells for allogeneic transplantation: a review. Stem Cells 2001;19:108–17.
- Frey NV, Lazarus HM, Goldstein SC. Has allogeneic stem cell cryopreservation been given the ‘clod shoulder’? An analysis of the pros and cons of using frozen versus fresh stem cell products in allogeneic stem cell transplantation. Bone Marrow Transplant 2006;38:399–405.
- Kim DH, Jamal N, Saragosa R, Loach D, Wright J, Gupta V, et al.. Similar outcome of cryopreserved allogeneic peripheral stem cell transplants (PBSCT) compared to fresh allografts. Biol Blood Marrow Transplant 2007;13:1233–43.
- Kessinger A, Schmit-Pokorny K, Smith D, Armitage J. Cryopreservation and infusion of autologous peripheral blood stem cells. Bone Marrow Transplant 1990;5(Suppl 1):25–7.
- Williams SF, Bitran JD, Richards JM, DeChristopher PJ, Barker E, Conant J, et al.. Peripheral blood-derived stem cell collections for use in autologous transplantation after high dose chemotherapy: an alternative approach. Bone Marrow Transplant 1990;5:129–33.
- Jacobs P, Wood L, Horak S. Collection and cryopreservation of human stem and progenitor cells for bone marrow transplantation. J Clin Apher 1991;6:54–8.
- Harada M, Taniguchi S, Teshima T, Makino S, Takamatsu Y, Akashi K, et al.. Cryopreservation, cytokine mobilization, and autotransplantation of peripheral blood stem cells. Prog Clin Biol Res 1992;377:297–308.
- Zambelli A, Poggi G, Da Prada GA, Pedrazzoli P, Cuomo A, Miotti D, et al.. Clinical toxicity of cryopreserved circulating progenitor cells infusion. Anticancer Res 1998;18:4705–8.
- Alessandrino EP, Bernasconi P, Caldera D, Colombo A, Bonfichi M, Malcovati L, et al.. Adverse events occurring during bone marrow or peripheral blood progenitor cell infusion: analysis of 126 cases. Bone Marrow Transplant 1999;23:533–7.
- Donmez A, Tombuloglu M, Gungor A, Soyer N, Saydam G, Cagirgan S. Clinical side effects during peripheral blood progenitor cell infusion. Transfus Apher Sci 2007;36:95–101.
- Nishihara G, Sakemi T, Ikeda Y, Baba N, Shimamoto Y. Multiple organ failure associated with dimethylsulfoxide and hydroxyethyl starch in autologous blood stem cell transplantation. Nephron 1996;72:356–7.
- Bauwens D, Hantson P, Laterre PF, Michaux L, Latinne D, De Tourtchaninoff M, et al.. Recurrent seizure and sustained encephalopathy associated with dimethylsulfoxide-preserved stem cell infusion. Leuk Lymphoma 2005;46:1671–4.
- Calmels B, Houzé P, Hengesse JC, Ducrot T, Malenfant C, Chabannon C. Preclinical evaluation of an automated closed fluid management device: Cytomate™, for washing out DMSO from hematopoietic stem cell graftsafter thawing. Bone Marrow Transplant 2003;31:823–8.
- Rodríguez L, Velasco B, García J, Martín-Henao GA. Evaluation of an automated cell processing device to reduce thedimethyl sulfoxide from hematopoietic grafts after thawing. Transfusion 2005;45:1391–7.
- Akkök ÇA, Holte MR, Tangen JM, Ostenstad B, Bruserud O. Hematopoietic engraftment of dimethyl sulfoxide-depleted autologous peripheral blood progenitor cells. Transfusion 2009;49:354–61.
- Kamata Y, Takahashi Y, Iwamoto M, Matsui K, Murakami Y, Muroi K, et al.. Local implantation of autologous mononuclear cells from bone marrow and peripheral blood for treatment of ischaemic digits in patients with connective tissue diseases. Rheumatology 2007;6:882–4.
- Muroi K, Handa K, Amemiya Y, Hakomori S, Ozawa K, Miura Y. Expression profiles of I and sialosyl-I antigens on blood cells: the sialosyl-I antigen is expressed along the monocytic differentiation. Leuk Res 1998;22:1029–36.
- Matsumoto N, Yoshizawa H, Kagamu H, Abe T, Fujita N, Watanabe S, et al.. Successful liquid storage of peripheral blood stem cells at subzero non-freezing temperature. Bone Marrow Transplant 2002;30:777–84.
- Ayello J, Semidei-Pomales M, Preti R, Hesdorffer C, Reiss RF. Effects of long-term storage at -90 degrees C of bone marrowand PBPC on cell recovery, viability, and clonogenic potential. J Hematother 1998;7:385–90.
- Korbling M, Huh YO, Durett A, Mirza N, Miller P, Engel H, et al.. Allogeneic blood stem cell transplantation: peripheralization and yield of donorderived primitive hematopoietic progenitor cells (CD34+Thy-1dim) and lymphoid subsets, and possible predictors of engraftment and graft-versus-host disease. Blood 1995;86:2842–8.
- Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, et al.. Chronic graftversus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med 1980;69:204–17.
- Parimon T, Au DH, Martin PJ, Chien JW. A risk score for mortality after allogeneic hematopoietic cell transplantation. Ann Intern Med 2006;144:407–14.
- http://www.r-project.org
- Watanabe T, Takaue Y, Kawano Y. Peripheral blood stem cell transplantation: an update. J Med Invest 1997;44:25–31.
- http://www.jmdp.or.jp/documents/file/07_about_us/chukantoshin.pdf#search = 'PB (in Japanese).
- Haas R, Mohle R, Fruhauf S, Goldschmidt H, Witt B, Flentje M, et al.. Patient characteristics associated with successful mobilizing and autografting of peripheral blood progenitor cells in malignant lymphoma. Blood 1994;83:3787–94.
- Carral A, de la Rubia J, Martín G, Mollá S, Martínez J, Sanz GF, et al.. Factors influencing the collection of peripheral blood stem cell collection in patients with acute myeloblastic leukemia and non-myloid malignancies. Leuk Res 2003;27:5–12.
- Kasparu H, Krieger O, Girschikofsky M, Kolb A, Bettelheim P, Lutz D. Factors influencing the timing of peripheral blood stem cell collection (PBSC). Transfus Apheresis Sci 1996;17:595–600.
- de la Rubia J, Arbona C, de Arriba F, del Cañizo C, Brunet S, Zamora C, et al.. Analysis of factors associated with low peripheral blood progenitor cell collection in normal donors. Transfusion 2002;42:4–9.
- Stockerl-Goldstein KE, Reddy SA, Horning SF, Blume KG, Chao NF, Hu WW, et al.. Favorable treatment outcome in non-Hodgkin’s lymphoma patients with ‘poor’ mobilization of peripheral blood progenitor cells. Biol Blood Marrow Transplant 2000;6:506–12.