Abstract
Renal failure is a common manifestation of multiple myeloma (MM). Bortezomib is primarily metabolized by cytochrome p450 isoforms. It also has a cytochrome-independent metabolism by excretion through the bile and kidney. Based on our observations, we aimed to explore the efficacy and toxicity profiles of bortezomib in 56 patients with MM, 24 of which had moderate to severe renal failure. Overall response and complete response, as well as very good partial response rates, were comparable between patients with normal renal functions and renal impairment. The median overall survivals for patients with estimated glomerular filtration rates of <60 and ⩾60 ml/minute were similar. Although there was a tendency for shorter overall survival along lower estimated glomerular filtration rates, this difference did not reach a statistical significance. Overall and severe adverse events, and dose modification and treatment discontinuation rates were higher in patients with renal impairment. Patients with renal failure had more thrombocytopenia and diarrhea. While thrombocytopenia was mild to moderate and manageable, diarrhea, which led to serious adverse events, was more severe in patients with renal failure who received bortezomib as monotherapy. Bortezomib appears to be active; however, when used alone, it may cause more frequent and severe adverse events in patients with MM and renal failure.
Introduction
Renal failure is a common manifestation and end-organ damage of multiple myeloma (MM). It may complicate the treatment and management of the disease. At the time of diagnosis, 20–50% of patients present with renal failure which is one of the main direct causes of initial mortality. An additional 25% experience this complication during the course of the disease.Citation1–Citation4 Cast nephropathy is the typical renal complication of MM. Hypercalcemia, dehydratation, several drugs, and contrast media used for imaging studies are the other causes of renal failure in MM.Citation5 The median survival with conventional chemotherapy in MM is about 2–3 years. High-dose therapy with hematopoietic stem cell transplantation extends this figure to 5–7 years.Citation6 Median survival in patients with renal failure has been reported to be <2 years.Citation7Citation7,8 Effects of novel drugs alone or in combination with the conventional agents on survival and time to progression are being investigated.Citation9Citation9,10
Bortezomib is a synthetic boronic acid dipeptide. It targets the proteasome by blocking the degradation of nuclear factor kappa B (NF-kappaB) inhibitor and hence the activation of NF-kappaB. This mechanism leads to the cell cycle to arrest, anti-angiogenic effects, activation of oxidative stress mediators, and apoptosis of MM cells.Citation11 Bortezomib exerts activity in the treatment of both relapsed/refractory and newly diagnosed MM.Citation12Citation12,13 Most common side-effects are peripheral neuropathy, transient thrombocytopenia, fatigue, and gastrointestinal disorders (constipation, nausea, and diarrhea).Citation14 Bortezomib is primarily metabolized by oxidative deboronation in several cytochrome p450 isoforms. It has also a cytochrome-independent metabolism by excretion through the bile and kidney.Citation15 Pharmacological dose escalating studies of bortezomib have shown that the pharmacokinetics of bortezomib is independent of renal clearance and not influenced by the degree of renal impairment.Citation16
Almost all studies concluded that bortezomib had similar efficacy and safety profiles in MM patients with renal impairment and normal renal functions.Citation17–Citation22 However, bortezomib combinations, response rates, median survivals, and toxicity profiles were somewhat heterogeneous between the studies and the numbers of patients with renal impairment included into those studies were mostly <20. The cumulative number of patients with renal failure included into those studies is still too small to reflect a true safety profile of bortezomib in this patient population.
The reversal rate of renal impairment in patients with MM has been shown to be about 30–44% in patients using bortezomib-based treatments which had been better than that achieved by combinations not including bortezomib.Citation17–Citation19 However, bortezomib may worsen the outcome of renal ischemic reperfusion injury by leading to increased apoptosis of tubular cells in mice despite its potency of limiting the inflammatory response. Thus, the improvement in renal functions with the use of bortezomib results from the prevention of cast nephropathy by its activity on clonal cells which secret light chains instead of its suggested beneficial effect on harmed tubular cells.Citation23 These indirect beneficial effects on renal functions cannot exclude the possible detrimental effects of bortezomib in those studies consisting of a limited number of patients with renal impairment.
We observed that some of our patients receiving bortezomib for MM had protracted courses of diarrhea during the bortezomib treatment. Considering the issues mentioned above, we believe that existing data are not enough to make a consistent conclusion about the safety and efficacy of bortezomib. Based on our observations, we aimed to explore the efficacy and toxicity profiles of bortezomib in patients with moderate to severe renal impairment.
Design and Methods
Patients
Data from a total of 56 patients with MM who received bortezomib-based treatments in Marmara University Hospital, Hematology Clinic, Istanbul, Turkey between May 2005 and December 2008 were retrospectively collected from the records. All patients have received at least one line of therapy before bortezomib treatment. Bortezomib was initiated as a single agent in an half of the patients. Remaining patients received bortezomib with dexamethasone in combination except one patient in whom bortezomib was combined with melphalan, prednisone, and thalidomide. Forty-three percent (24 patients) and 25% (14 patients) of the study population had estimated glomerular filtration rates (eGFRs) of <60 and 30 ml/minute, respectively. None of the patients was on hemodialysis when bortezomib treatment was given. The study was approved by local ethics committee. All the authors vouch for the accuracy and completeness of the data and the analyses.
Treatment
Bortezomib was given at a dose of 1·3 mg/m2 on days 1, 4, 8, 11, every 21 days. Patients who received dexamethasone combined with bortezomib received 20 mg dexamethasone on days 1, 2, 4, 5, 8, 9, 11, and 12. Bortezomib, melphalan, thalidomide, and prednisone combination was scheduled in one patient as previously described.Citation24
Evaluation of response and toxicity
Response data were evaluated according to the International Myeloma Working Group criteria by using serum protein electrophoresis and serum/urine immunofixation electrophoresis.Citation25 Overall survival (OS) was defined as the time between the first dose of bortezomib and death from any cause. Grades of toxicities were determined by using National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0. Adverse events were reported as adverse event rate per person and proportions in specified renal subgroups.
Renal subgroups
Renal subgroups were stratified according to a modified staging system based on the National Kidney Foundation Practice Guidelines for Chronic Kidney Disease.Citation26Citation26,27 We preferred to use this standard classification of renal impairment instead of its arbitrary modification in which a more strict cut-off point for eGFR has been used to define renal impairment by previous studies.Citation18–Citation22 Cockroft–Gault formula was used to estimate eGFR. Cockroft–Gault formula was calculated as follows: eGFR (ml/minute) = [(140−age)/72×serum creatinine (mg/dl)]×0·85 (if female). Patients with an eGFR of <60 ml/minute were defined to have renal impairment. eGFRs between 30–59 and <30 ml/minute were moderate and severe renal impairment, respectively. Although the revised staging system for chronic kidney failure has eliminated it, patients with an eGFR between 60 and 89 ml/minute which was previously defined as mild renal impairment were also estimated.
Statistical analysis
Differences among the groups were calculated by the chi-square test for categoric variables using Fisher’s exact test when appropriate. Skewed continuous variables were compared by using the Mann–Whitney test. OS was estimated with median survival time and 95% confidential intervals. Survival curves were plotted with the method of Kaplan and Meier, and log-rank test was used for comparisons among groups. Patients were censored for OS when they were alive as of last contact. Comparisons were made for both <60 versus ⩾60 ml/minute and <30 versus 30–59 versus 60–89 versus ⩾90 ml/minute. A two-sided P value of ⩽0·05 was considered to be statistically significant.
Results
Characteristics of the study patients
The mean age of the patients was 55 years and 58·9% were men. The median number of bortezomib cycles was five. Despite the retrospective nature of the study, groups were well balanced with respect to baseline characteristics for groups with eGFRs of ⩾60 and <60 ml/minute except creatinine and beta2-microglobulin levels (mean 0·82 versus 2·79 mg/dl, P = 0·0001, and median 3494 versus 10 093 ng/ml, P = 0·0001, respectively). Although the group with an eGFR of <60 ml/minute had higher patients with light chain disease and lower hemoglobin levels as expected, these differences were not significant (19·4% versus 45·8%, P = 0·1 and median 11·1 versus 8·7 g/dl, P = 0·053, respectively). Patients with an eGFR of <60 ml/minute had a higher proportion of ISS stage III disease (90·9% versus 16·7%, P = 0·0001). The proportions of patients who received bortezomib in combination were 64·7, 35·7, 70·0, and 50·0% in patients with eGFRs of ⩾90, 60–89, 30–59, and <30 ml/minute, respectively (P = 0·3). The significance level of difference was >0·10 for all other demographic parameters between groups except age for which it was 0·02 only between groups ⩾90 and 30–59 ml/minute ().
Table 1. Patients’ characteristics
Response rates
Response data were evaluable in a total of 51 patients. Twenty-one (41·2%) and 11 (21·5%) patients had eGFRs of <60 and <30 ml/minute, respectively. Overall response rates (ORRs) were similar between groups with eGFRs of <60 versus ⩾60 ml/minute and ⩾90 versus 60–89 versus 30–59 versus <30 ml/minute (76·2% versus 76·7%, P = 0·7 and 68·8% versus 85·7% versus 80% versus 72·7%, P = 1·0, respectively). In patients with an eGFR of <60 ml/minute, complete response (CR) plus very good partial response (VGPR) rate was 66·4% compared with 60% in patients with an eGFR of ⩾60 ml/minute (P = 0·2). CR plus VGPR rates were 43·8, 78·6, 80, and 63·6% in patients with eGFRs of ⩾90, 60–89, 30–59, and <30 ml/minute, respectively (P = 0·2). CR rates were higher in patients with an eGFR of <60 ml/minute compared with that of ⩾60 ml/minute (52·1% versus 26·7%). But this difference failed to reach the statistical significance (P = 0·06) ().
Table 2. Efficacy data
Overall survivals
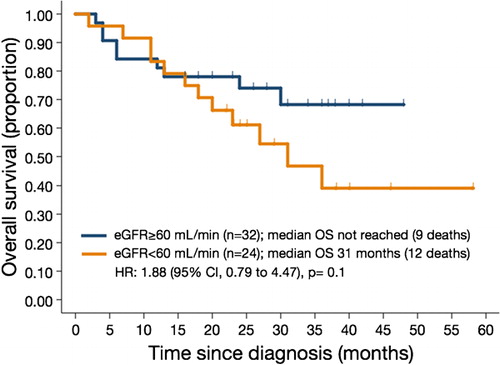
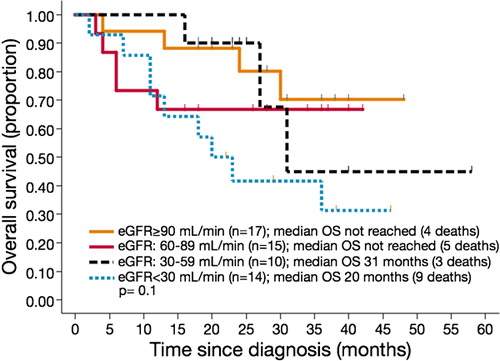
Survival data were estimated in all 56 patients. The median OSs for patients with eGFRs of <60 and ⩾60 ml/minute and the entire population were 31 months, not reached, and 25 months, respectively (P = 0·1) (). Although there is a tendency for shorter OS along lower eGFR, this difference did not reach a statistical significance. The median OS durations for patients with eGFRs of ⩾90 and 60–89, 30–59, and <30 ml/minute were not reached, 31 months, and 20 months, respectively (P = 0·1) (, ).
Adverse events
Adverse event data were evaluated in 56 patients. These patients experienced a total of 51 adverse events. The most common adverse events were neuropathy (19/51, 37%) and thrombocytopenia (13/51, 25%). The adverse event rate was higher in patients with an eGFR of <60 ml/minute than with ⩾60 ml/minute. But this difference did not reach a statistical significance (1·17 versus 0·72 adverse events per patient, P = 0·06). Patients with an eGFR of <30 ml/minute had a higher adverse event rate than those with ⩾90 ml/minute, but not than the others (1·43 versus 0·53 adverse event per patient, P = 0·006). The overall incidences of thrombocytopenia were 41·7% versus 9·4% in patients with eGFRs of <60 versus ⩾60 ml/minute, respectively (P = 0·01). Diarrhea was more prevalent in patients with eGFRs of <60 ml/minute versus ⩾60 ml/minute and <30 versus ⩾90, 60–89, and 30–59 ml/minute (25% versus 3·1%, P = 0·04, 42·9% versus 0% versus 6·7% versus 0%, P = 0·04) ().
Table 3. Adverse events
A total of 18 grade III–IV adverse events occurred in 56 patients. The most common grade III–IV event was diarrhea (7/18, 39%), followed by thrombocytopenia (6/18, 33%). Toxicity grade in all seven patients with diarrhea was grade III–IV. On the other hand, 6 out of 13 patients with thrombocytopenia and 4 out of 19 patients with neuropathy had grade III–IV event. Patients with an eGFR of <60 ml/minute had higher rate of grade III–IV adverse events compared with ⩾60 ml/minute (0·50 versus 0·19 adverse event per patient, P = 0·03). Incidences of grade III–IV adverse event rates were 0·06 versus 0·71 events per patient for patients with eGFRs of ⩾90 and <30 ml/minute, respectively (P = 0·002). Grade III–IV diarrhea was experienced by 25% of patients with an eGFR of <60 ml/minute versus 3·1% of those with ⩾60 ml/minute (P = 0·04). Patients with an eGFR of <30 ml/minute had a higher rate of diarrhea than those with ⩾90, 60–89, and 30–59 ml/minute (42·9% versus 0% versus 6·7% versus 0%, P = 0·001, respectively) ().
Adverse events leading to dose modification were experienced more frequently in patients with eGFRs of <60 versus ⩾60 ml/minute (0·38 versus 0·03 adverse event per patient, P = 0·006). The most common adverse event that led to dose modification was thrombocytopenia (5/10). Thrombocytopenia was more prevalent in group with <60 versus ⩾60 ml/minute (20·8% versus 0%, P = 0·03). Patients with moderate to severe renal impairment had more adverse events leading to drug discontinuation without a difference in a particular adverse event (0·21 versus 0 adverse events per patient, P = 0·006) ().
Time-to-adverse event data could only be obtained for diarrhea. Diarrhea was thought to be related with bortezomib use only after the exclusion of other possible causes of diarrhea by blood and stool analyses. Only one patient underwent colonoscopy which revealed non-specific findings for sustained diarrhea. But biopsy was not obtained in this patient. None of the patients were neutropenic during the diarrhea periods. Diarrhea seemed to be experienced at the early courses of therapy. Six out of seven patients with diarrhea had an eGFR of <30 ml/minute and ISS stage III disease as expected. Of patients with diarrhea, two had used bortezomib in combination with dexamethasone. These patients did not experience any adverse event other than diarrhea. Diarrhea affected neither response quality nor OS in those patients ().
Table 4. Patients with diarrhea
Discussion
This study reports the efficacy and safety profiles of bortezomib in patients with moderate to severe renal impairment. Earlier studies had shown that patients with renal impairment had similar efficacy compared with those with normal and mild renal impairments.Citation18–Citation20,22 Various response rates were reported within those studies. ORR in our study population in patients with eGFRs of <60 and ⩾60 ml/minute was 76%. These are comparable with those of Chanan-Khan et al., Roussou et al., GIMEMA group, and Dimopoulos et al. However, bortezomib combinations were heterogeneous in those studies.Citation17–Citation19,21 Only 33% of patients were treated with bortezomib alone or combined with dexamethasone by Chanan-Khan et al.Citation21Dimopoulos et al. had achieved 68% of ORR and 30% of CR in patients with renal impairment treated with bortezomib, melphalan, and prednisone (VMP) combination.Citation18Almost a half of the patients reported by GIMEMA group had received other agents along with bortezemib or bortezomib with dexamethasone.Citation19 Roussou et al. had observed a 65% of ORR and 5% of CR rate in 20 patients with severe renal failure, 13 of which had been treated with bortezomib, dexamethasone, and other agents.Citation17 However, ORR observed in our study population seems to be higher than those achieved in renal impairment subgroup analyses of SUMMIT and CREST (ORR, 25%) and APEX trials (ORR, 40% and CR, 7%). The latter studies had evaluated the efficacy and safety profiles of bortezomib combined with or without dexamethasone in patients with renal impairment as in our study.Citation20Citation20,22 The quality of response in our study population with a CR+VGPR rate of 64% was better than those of previous studies.Citation17–Citation22 Considering the wide range of ORR, the quality of response, and similar characteristics across the studies, there might be additional explanatory factors for heterogeneity of bortezomib efficacy. Greater than 50% plasma cell bone marrow infiltration and older age (⩾65 years) have been shown to be associated with lower response rates.Citation28 A cytogenetic analysis together with beta2-microglobulin has been proposed to be a good predictor of OS.Citation29 We have information about neither cytogenetic profile nor bone marrow biopsy/aspiration to verify the quality of response in all patients. But relatively younger age of our study population compared with the previous studies may explain it in part.
APEX trial which included larger study population has reported similar OS rates in these groups despite a trend for longer OS in patients with normal renal functions.Citation22 Dimopoulos et al. have shown somewhat shorter OS in patients with renal impairment on VMP arm.Citation18 Median OS was similar in patients with normal and impaired renal function in our study. Our results confirmed the efficacy of bortezomib in patients with renal impairment. Although each group included a few patients, the efficacy of bortezomib did not differ significantly between patients with severe, moderate, and mild renal failure and normal renal functions.
Most of the earlier studies including randomized controlled trials have reported similar toxicity profiles for bortezomib in patients with normal and impaired renal functions. However, a recent cohort analysis of VISTA study has shown that AEs of grade IV–V and serious AEs were somewhat higher among patients with an eGFR of ⩽50 ml/minute compared with those with an eGFR of >50 ml/minute. But dose reduction and treatment discontinuation rates have appeared similar between groups.Citation18 Total and severe adverse events, dose modification, and treatment discontinuation rates were higher in patients with renal impairment in our study. Our data provide additional information about the safety profile of bortezomib in patients with renal impairment.
We observed thrombocytopenia and diarrhea to be more prevalent in patients with renal impairment. Platelet budding from megakaryocyte progenitors is thought to be dependent on NF-kappaB. Bortezomib may temporarily suppress this process and may lead to thrombocytopenia.Citation30 Thrombocytopenia was reported by previous studies in rates between 26 and 34% as observed similarly in patients with renal impairment in our study.Citation21Citation21,22 But only 9% of patients with normal renal functions in our study had thrombocytopenia which is less than that observed by other studies. Nevertheless thrombocytopenia experienced by patients with renal impairment was mild to moderate and manageable by only dose modification.
Diarrhea was mostly severe, but transient and disappeared despite the use of bortezomib at same doses in most of our patients. It was observed during the earlier courses of therapy. Although it did not affect the quality of response, it led to serious adverse events in two patients who experienced acute deterioration of chronic renal failure. Patients who used bortezomib alone experienced it more frequently and these patients had extra adverse events other than diarrhea. Diarrhea had persisted more than 7 days in patients using bortezomib alone. Considering that a half of patients with an eGFR of <30 ml/minute had used bortezomib alone and five out of these six patients had diarrhea, bortezomib seems to render patients with renal impairment a series of inflammatory events that lead to diarrhea and the use of dexamethasone combined with bortezomib prevents its occurrence or at least decreases its severity. This observation was supported by other studies as well. Frequency of diarrhea was reported in a wide range in several studies. There is no information regarding the rate of diarrhea or gastrointestinal complications in a retrospective study by Chanan-Khan et al. in which only two out of 20 patients received bortezomib alone.Citation21 Diarrhea was experienced in rates of 57, 59, and 71% by patients with eGFRs of >50, ⩽50, and <30 ml/minute, respectively, in bortezomib alone arm of subgroup analysis of the phase 3 APEX study which has included 17 patients with an eGFR of <30 ml/minute. However, diarrhea prevalences in the dexamethasone subgroup of the same study were 21, 19, and 9% in patients with eGFRs of >50, ⩽50, and <30 ml/minute, respectively.Citation22 Recently GIMEMA group reported that only a total of five out of 117 patients had gastrointestinal complications in a retrospective study. All of these patients had an eGFR of ⩽50 ml/minute that corresponds to an 8% diarrhea rate within this group. None of the patients was treated with bortezomib alone in the latter study.Citation19
Bortezomib has been associated with inflammatory and vasculitic processes. Gerecitano et al. reviewed 140 patients enrolled in various bortezomib clinical trials. Nineteen percent of these patients had a vasculitic rash.Citation31 Bortezomib-induced inflammatory pulmonary complications and pauci-immune pulmonary capillaritis have been reported.Citation32Citation32,33 Direct vascular toxicity, delayed hypersensitivity, cellular immune response, and drug-associated cytokine release are the potential mechanisms claimed to cause these inflammatory reactions.Citation31 All these complications had resolved with the use of corticosteroids.Citation31–Citation33 Considering lower diarrhea rates by bortezomib use combined with dexamethasone, diarrhea might be a result of immune, inflammatory, or vasculitic processes. Inhibition of NF-kappaB signaling during the resolution of inflammation may protract the inflammatory response.Citation34 In vivo, this inhibition leads to spontaneous development of intestinal inflammation in mice.Citation35 Pharmacokinetics of bortezomib is independent of renal function.Citation16 But NF-kappaB levels are increased in renal diseases and NF-kappaB affects cytochrome p450 by direct and indirect mechanisms.Citation36Citation36,37 NF-kappaB lowering affects of bortezomib may be exaggerated in patients with renal failure. This may be an explanation for the possible high prevalence of thrombocytopenia and diarrhea in those patients.
The non-systematic, non-randomized, and retrospective properties of this analysis limit its validity. Time-to-event data are important to report and we could only determine it for diarrhea data. Physicians need to know when an adverse event can occur during the treatment. Cumulative risk for any adverse event may be similar between two or more arms, but instantaneous risk might be different. Reporting adverse events by periods of time is another important issue. Safety profiles may be similar between the study arms at the end of the study period. But certain adverse events can be seen during a particular period of time in different study groups. If adverse events were compared by yearly or quarterly periods, there could be differences which might be attributed to that particular arm during the specified period.Citation38 Our safety analysis is incomplete for these aspects. But previous studies had the same limitations as well. Although we did not make a power analysis due to the retrospective nature of the study, a type II error for both efficacy and safety analyses is possible.
In conclusion, our data support the activity of bortezomib in patients with renal impairment. Overall and severe adverse event, dose modification, and treatment discontinuation rates are higher in those patients. Patients with renal failure experience more thrombocytopenia and diarrhea. Particularly diarrhea may be a problem by leading to serious adverse events in those patients with MM using bortezomib as monotherapy.
References
- Bladé J, Fernández-Llama P, Bosch F, Montolíu J, Lens XM, Montoto S, et al.. Renal failure in multiple myeloma: presenting features and predictors of outcome in 94 patients from a single institution. Arch Intern Med 1998;158:1889–93.
- Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, et al.. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc 2003;78:21–33.
- Knudsen LM, Hippe E, Hjorth M, Holmberg E, Westin J. Renal function in newly diagnosed multiple myeloma — a demographic study of 1353 patients. The Nordic Myeloma Study Group. Eur J Haematol 1994;53:207–12.
- Eleutherakis-Papaiakovou V, Bamias A, Gika D, Simeonidis A, Pouli A, Anagnostopoulos A, et al.. Renal failure in multiple myeloma: incidence, correlations, and prognostic significance. Leuk Lymphoma 2007;48:337–41.
- Dimopoulos MA, Kastritis E, Rosinol L, Bladé J, Ludwig H. Pathogenesis and treatment of renal failure in multiple myeloma. Leukemia 2008;22:1485–93.
- Munshi NC. Plasma cell disorders: an historical perspective. Hematology Am Soc Hematol Educ Program 2008:297.
- Abbott KC, Agodoa LY. Multiple myeloma and light chain associated nephropathy at end-stage renal disease in the United States: patient characteristics and survival. Clin Nephrol 2001;56:207–10.
- Augustson BM, Begum G, Dunn JA, Barth NJ, Davies F, Morgan G, et al.. Early mortality after diagnosis of multiple myeloma: analysis of patients entered onto the United Kingdom Medical Research Council trials between 1980 and 2002 — Medical Research Council Adult Leukaemia Working Party. J Clin Oncol 2005;23:9219–26.
- Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, et al.. Improved survival in multiple myeloma and the impact of novel therapies. Blood 2008;111:2516–20.
- Brenner H, Gondos A, Pulte D. Recent major improvement in long-term survival of younger patients with multiple myeloma. Blood 2008;111:2521–6.
- Hideshima T, Anderson KC. Preclinical studies of novel targeted therapies. Hematol Oncol Clin North Am 2007;21:1071–91viii–xi.
- Richardson PG, Sonneveld P, Schuster M, Irwin D, Stadtmauer E, Facon T, et al.. Extended follow-up of a phase 3 trial in relapsed multiple myeloma: final time-to-event results of the APEX trial. Blood 2007;110:3557–60.
- Harousseau JL, Attal M, Leleu X, Troncy J, Pegourie B, Stoppa AM, et al.. Bortezomib plus dexamethasone as induction treatment prior to autologous stem cell transplantation in patients with newly diagnosed multiple myeloma: results of an IFM phase II study. Haematologica 2006;91:1498–505.
- Raab MS, Podar K, Breitkreutz I, Richardson PG, Anderson KC. Multiple myeloma. Lancet 2009;374:324–39.
- Pekol T, Daniels JS, Labutti J, Parsons I, Nix D, Baronas E, et al.. Human metabolism of the proteasome inhibitor bortezomib: identification of circulating metabolites. Drug Metab Dispos 2005;33:771–7.
- Mulkerin D, Remick S, Takimoto C, Ivy P, Karol M. Safety, tolerability, and pharmacology of bortezomib in cancer patients with renal failure requiring dialysis: results from a prospective phase 1 study. Blood (ASH Annual Meeting Abstracts) 2007;110:Abstract 3477.
- Roussou M, Kastritis E, Migkou M, Psimenou E, Grapsa I, Matsouka C, et al.. Treatment of patients with multiple myeloma complicated by renal failure with bortezomib-based regimens. Leuk Lymphoma 2008;49:890–5.
- Dimopoulos MA, Richardson PG, Schlag R, Khuageva NK, Shpilberg O, Kastritis E, et al.. VMP (bortezomib, melphalan, and prednisone) is active and well tolerated in newly diagnosed patients with multiple myeloma with moderately impaired renal function, and results in reversal of renal impairment: cohort analysis of the phase III VISTA study. J Clin Oncol 2009;27:6086–93.
- Morabito F, Gentile M, Ciolli S, Petrucci MT, Galimberti S, Mele G, et al.. Safety and efficacy of bortezomib-based regimens for multiple myeloma patients with renal impairment: a retrospective study of Italian Myeloma Network GIMEMA. Eur J Haematol 2010;84:223–8.
- Jagannath S, Barlogie B, Berenson JR, Singhal S, Alexanian R, Srkalovic G, et al.. Bortezomib in recurrent and/or refractory multiple myeloma. Initial clinical experience in patients with impared renal function. Cancer 2005;103:1195–200.
- Chanan-Khan AA, Kaufman JL, Mehta J, Richardson PG, Miller KC, Lonial S, et al.. Activity and safety of bortezomib in multiple myeloma patients with advanced renal failure: a multicenter retrospective study. Blood 2007;109:2604–6.
- San-Miguel JF, Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, et al.. Efficacy and safety of bortezomib in patients with renal impairment: results from the APEX phase 3 study. Leukemia 2008;22:842–9.
- Huber JM, Tagwerker A, Heininger D, Mayer G, Rosenkranz AR. The proteasome inhibitor bortezomib aggravates renal ischemia-reperfusion injury. Am J Physiol Renal Physiol 2009;297:F451–60.
- Palumbo A, Ambrosini MT, Benevolo G, Pregno P, Pescosta N, Callea V, et al.. Bortezomib, melphalan, prednisone, and thalidomide for relapsed multiple myeloma. Blood 2007;109:2767–72.
- Durie BG, Harousseau JL, Miguel JS, Bladé J, Barlogie B, Anderson K, et al.. International uniform response criteria for multiple myeloma. Leukemia 2006;20:1467–73.
- Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, et al.. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 2003;139:137–47.
- Bauer C, Melamed ML, Hostetter TH. Staging of chronic kidney disease: time for a course correction. J Am Soc Nephrol 2008;19:844–6.
- Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, et al.. Clinical factors predictive of outcome with bortezomib in patients with relapsed, refractory multiple myeloma. Blood 2005;106:2977–81.
- Avet-Loiseau H, Attal M, Moreau P, Charbonnel C, Garban F, Hulin C, et al.. Genetic abnormalities and survival in multiple myeloma: the experience of the Intergroupe Francophone du Myélome. Blood 2007;109:3489–95.
- Mateos MV, San Miguel JF. Bortezomib in multiple myeloma. Best Pract Res Clin Haematol 2007;20:701–15.
- Gerecitano J, Goy A, Wright J, MacGregor-Cortelli B, Neylon E, Gonen M, et al.. Drug-induced cutaneous vasculitis in patients with non-Hodgkin lymphoma treated with the novel proteasome inhibitor bortezomib: a possible surrogate marker of response? Br J Haematol 2006;134:391–8.
- Miyakoshi S, Kami M, Yuji K, Matsumura T, Takatoku M, Sasaki M, et al.. Severe pulmonary complications in Japanese patients after bortezomib treatment for refractory multiple myeloma. Blood 2006;107:3492–4.
- Pitini V, Arrigo C, Altavilla G, Naro C. Severe pulmonary complications after bortezomib treatment for multiple myeloma: an unrecognized pulmonary vasculitis? Leuk Res 2007;31:1027–8.
- Lawrence T, Gilroy DW, Colville-Nash PR, Willoughby DA. Possible new role for NF-kappaB in the resolution of inflammation. Nat Med 2001;7:1291–7.
- Pasparakis M. IKK/NF-kappaB signaling in intestinal epithelial cells controls immune homeostasis in the gut. Mucosal Immunol 2008;1(Suppl 1):S54–7.
- Guijarro C, Egido J. Transcription factor-kappa B (NF-kappa B) and renal disease. Kidney Int 2001;59:415–24.
- Zordoky BN, El-Kadi AO. Role of NF-kappaB in the regulation of cytochrome P450 enzymes. Curr Drug Metab 2009;10:164–78.
- Yazici Y. Some concerns about adverse event reporting in randomized clinical trials. Bull NYU Hosp Jt Dis 2008;66:143–5.