Abstract
Acute myeloid leukemia (AML) is the most common myeloid leukemia. It is highly malignant, thus, most patients with AML will relapse and die after traditional treatment. Stat3, a member of the signal transducer and activator of transcription (Stat) family, is involved in the development and progression of many tumors. The purpose of the study was to investigate whether the down-regulation of Stat3 expression by RNA interference is effective against human leukemia HL-60 cells. The results indicated that constitutively expressed Stat3 is present in human leukemia HL-60 cells, and the down-regulation of Stat3 expression caused significant induction of apoptosis as well as inhibition of proliferation in HL-60 cells. These data further demonstrated that Stat3 plays a critical role in human leukemia HL-60 cell apoptosis and proliferation. Thus, targeting Stat3 may be a useful adjunctive treatment strategy in AML.
Introduction
Acute myeloid leukemia (AML), also known as acute myelogenous leukemia, is a malignant disorder of the blood that is characterized by maturation arrest of a malignant clone of myeloid cells.Citation1 It is reported that AML accounts for 70% of newly diagnosed acute leukemias and its incidence increases with age.Citation2–Citation4 Despite considerable advances in antileukemic chemotherapy, the relapse and the low survival rate of AML remain significant problems.Citation5–Citation7 Hence, better approaches for the treatment of AML are needed.
In mammals, the Stat family consists of seven members: Stat1, Stat2, Stat3, Stat4, Stat5a, Stat5b, and Stat6.Citation8 Stat3, a member of Stat family, participates in regulating cell apoptosis, proliferation, and differentiation, and is involved in the development and progression of many tumors.Citation9–Citation13 Constitutive Stat3 activity has been observed in ∼50% of AML cases and patients whose leukemia cells exhibit constitutive Stat3 activity perform shorter disease-free survival than those without such constitutive activity.Citation14Citation14,15
In this report, we further testified whether constitutively expressed Stat3 is present in human leukemia HL-60 cells and examined whether the down-regulation of Stat3 expression by RNA interference (RNAi) could be able to affect the apoptosis and proliferation of human leukemia HL-60 cells.
Materials and Methods
Cell culture
Human leukemia HL-60 cell line was obtained from Academy of Sciences (Shanghai, China). Cells were grown as suspension culture in RPMI-1640 medium supplemented with 10% fetal bovine serum (Gibco; Invitrogen; Carlsbad, CA, USA) in a humidified 37°C incubator and aired with 5% CO2.
Construction of the short hairpin RNA (shRNA) expression plasmid
A double-stranded shRNA oligonucleotide was designed against Stat3 (5′-ACACCGCAGCTGCACCAGCTGTACTTGCTTGAAGTACAGCTGGTGCAGCTGCT-3′); (5′-AAAAAGCAGCTGCACCAGCTGTACTTCAAGCAAGTACAGCTGGTGCAGCTGCG-3′). This oligonucleotide was designed according to the protocol of SilenCircle RNAi transcription kit (Allele Biotechnology, San Diego, CA, USA). After being annealed at 95°C, the products were ligated into the pSilenCircle linearized vector. Then, the Escherichia coli transformation and plasmid DNA preparation were performed according to the standard protocols,Citation16 and the shRNA expression vector sequence was identified.
Cell transfection
Before transfection, about 5×105 cells were seeded into each well of a 24-well plate. FuGENE® HD (Roche Molecular Biochemicals, Indianapolis, IN, USA) was used as the transfection reagent according to the manufacturer’s instructions. In this study, pSilenCircle empty vector was used as a control.
Immunofluorescence staining
HL-60 cells were incubated on the poly-L-lysine pretreated coverslips overnight. After that, cells were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 20 minutes, washed with PBS, permeabilized, and blocked in PBS/Triton containing 5% goat serum albumin at room temperature for 1 hour. Then, 1∶400 dilution of anti-Stat3 antibody (Cell Signaling Technology, Beverly, MA, USA) was added and the cells were incubated overnight at 4°C. In the following day, the cells were washed with PBS and 1∶200 dilution of fluorescein isothiocyanate (FITC)-conjugated secondary antibody was added. After incubation for 1 hour, the cells were washed with PBS, and then mounted with 50% glycerol in PBS. Fluorescence was observed by a Fluoview FV300 confocal laser scanning biological microscope (Olympus, Essex, UK).
Reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was extracted from cultured HL-60 cells with Trizol reagent. cDNA was synthesized from total RNA by using reverse transcription assay according to the previous protocol.Citation17 Primers used to detect Stat3 expression were 5′-GGGTCTGGCTAGACAATATCATCG-3′ and 5′-CACTACCTGGGTCGGCTTCG-3′, and primers against beta-actin as a positive control were 5′-CCAAGGCCAACCGCGAGAAGATGAC-3′ and 5′-AGGGTACATGGTGGTGCCGCCAGAC-3′. After amplification, polymerase chain reaction products were electrophoresed on a 1% agarose gel and visualized by ethidium bromide staining.
Western blot
Total protein was extracted from cultured HL-60 cells with protein lysis buffer. The lysates were centrifuged at 12 000 rpm at 4°C for 10 minutes. Protein samples were electrophoresed on a 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis gel and transferred to polyvinylidene fluoride membranes. The membranes were then incubated with anti-Stat3 antibody (Cell Signaling Technology) or anti-beta-actin antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Immunoreactive bands were detected by enhanced chemiluminescence detection system.
Cell apoptosis assay
HL-60 cells were cultured in 24-well plates. We used an Annexin V-FITC apoptosis detection kit (Bipec Biopharma Corporation, Cambridge, MA, USA) to quantify. In brief, we harvested 106 cells, washed, and resuspended cells in 400 μl binding buffer containing 5 μl Annexin V-FITC, and subsequently incubated cells for 15 minutes at 4°C in the dark. After that, we observed Annexin V-FITC-positive cells under a fluorescence microscope. The percentage of apoptotic cells was determined for each of the treatments by counting five visual fields and at least 100 cells. The experiment was repeated three times.
Cell proliferation assay
HL-60 cells were cultured in 96-well plates. Cell proliferation ability was determined using MTS assay following the manufacturer’s directions (CellTiter 96 AQueous Non-radioactive proliferation kit; Promega, Son Luis, CA, USA). Absorption value was measured at 490 nm with a 96-well plate reader. The cell proliferation of samples was calculated by dividing the absorption value of samples with that of control. The experiment was repeated three times.
Statistical analysis
Data are expressed as mean±SEM. Statistical analysis of the data was performed by one-way analysis of variance. P<0·05 was considered to be significant.
Results
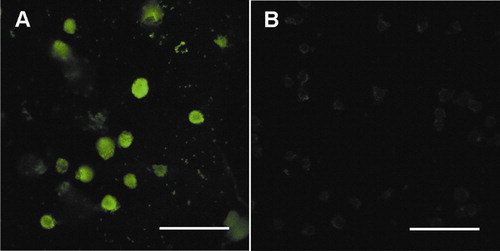
Constitutively expressed Stat3 proteins are present in HL-60 cells
Previous studies have shown that constitutively activated Stat3 is present in many human cancer cells and tumor tissues.Citation18–Citation20 In the current study, the immunofluorescence staining method was used to further testify whether Stat3 proteins are constitutively or transiently expressed in HL-60 cells. The result shows no obvious change in the expression level of Stat3 protein with the passage of time ().
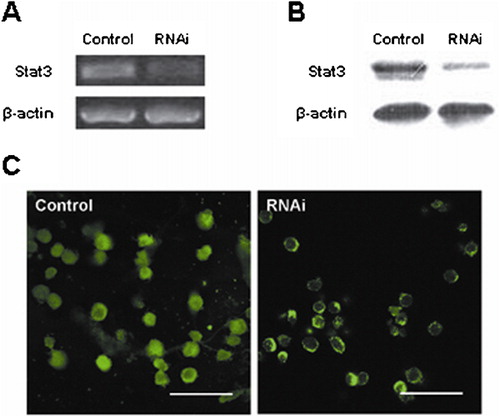
RNAi specifically reduces the expression of Stat3 in HL-60 cells
The effect of RNAi was analyzed by RT-PCR, western blot, and immunofluorescence staining. The results of RT-PCR and western blot showed that Stat3 RNAi effectively down-regulated mRNA and protein expression of Stat3 in HL-60 cells. In contrast, the expression of beta-actin remained unaffected after transfection with Stat3 shRNA (). Immunofluorescence staining further confirmed that Stat3 protein expression was markedly reduced in HL-60 cells after RNAi ().
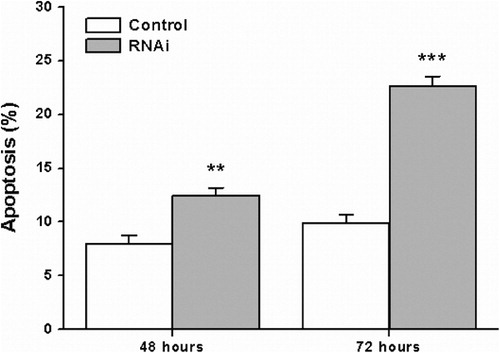
Down-regulation of Stat3 by RNAi induces apoptosis of HL-60 cells
To examine whether down-regulation of Stat3 affects HL-60 cell apoptosis, Annexin V assay was performed. As depicted in , the percentage of apoptotic cells in the Stat3 RNAi treatment group was significantly higher than that in the control group. These results illustrated that the down-regulation of Stat3 resulted in induction of HL-60 cell apoptosis.
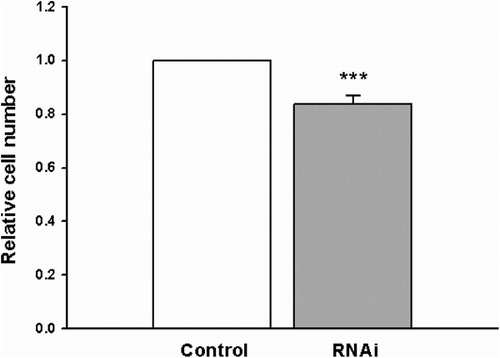
Down-regulation of Stat3 by RNAi inhibits proliferation of HL-60 cells
Seventy-two hours after transfection, the MTS assay was used to examine the cell proliferation. As shown in , the proliferation of HL-60 cells was significantly suppressed by the treatment with Stat3 shRNA, compared with control group cells. These results illustrated that the down-regulation of Stat3 resulted in the inhibition of HL-60 cell proliferation.
Discussion
Since Stat3 acts as a master regulator in many areas of tumor progression, including cell proliferation, apoptosis, and cell cycle regulation, it has been recognized as an oncoprotein.Citation21 In previous researches, scientists have conducted numerous experiments targeting Stat3 to investigate tumor treatment. These studies showed that the inhibition of Stat3 signaling-induced growth inhibition and apoptosis in prostate, ovarian, and breast cancer cells.Citation22–Citation24 Our previous studies also demonstrated that the down-regulation of Stat3 induced apoptosis and decreased the invasion activity in glioma cells.Citation25Citation25,26 All of these findings suggest that targeting Stat3 might pave a new way into cancer therapy.
It has been reported that Stat3 activity is most prevalent in AML.Citation27 Our results also testified that constitutively expressed Stat3 is present in human leukemia HL-60 cells. Subsequently, to examine the role of Stat3 in human leukemia cells, we analyzed the cell apoptosis and proliferation events that occurred in HL-60 cells transfected transiently with Stat3 shRNA. In this study, RNAi-mediated gene silencing was utilized to down-regulate the expression of Stat3. An obvious reduction in both the mRNA level and the protein level of Stat3 in the RNAi treatment group can be detected by RT-PCR, western blot, and immunofluorescence staining analyses. Further analysis revealed that the down-regulation of Stat3 expression by RNAi induces apoptosis and inhibits proliferation in human leukemia HL-60 cells. In addition, induction of apoptosis and inhibition of proliferation only occur in some of HL-60 cells in the RNAi treatment group, suggesting that the apoptosis and proliferation changes in HL-60 cells might be partially due to the inhibition of Stat3 and there might exist other signaling molecules involved in this regulatory process. Our future work will focus on the functional analysis of these molecules and further investigating the molecular mechanism of Stat3 in the pathogenesis of AML, which might foster the development of targeting therapies for AML.
In conclusion, constitutively expressed Stat3 is present in human leukemia HL-60 cells. After the down-regulation of Stat3, human leukemia HL-60 cells underwent induction of apoptosis and inhibition of proliferation. The results of this study imply that Stat3 plays an important role in the apoptosis and proliferation of human leukemia HL-60 cells and may be a potential therapeutic target in AML treatment.
This work was supported by the Natural Science Foundation of Shanghai (10ZR1410800), Leading Academic Discipline Project of Shanghai Municipal Education Commission ‘Molecular Physiology’, National Basic Research Program of China (2010CB529806), and Key Discipline Project of Renji Hospital, Shanghai JiaoTong University School of Medicine (RJ 4101307).
References
- Schiffer CA. Hematopoietic growth factors and the future of therapeutic research on acute myeloid leukemia. N Engl J Med 2003;349:727–9.
- Laubach J, Rao AV. Current and emerging strategies for the management of acute myeloid leukemia in the elderly. Oncologist 2008;13:1097–108.
- Redaelli A, Lee JM, Stephens JM, Pashos CL. Epidemiology and clinical burden of acute myeloid leukemia. Expert Rev Anticancer Ther 2003;3:695–710.
- Sandler DP, Ross JA. Epidemiology of acute leukemia in children and adults. Semin Oncol 1997;24:3–16.
- Guzman ML, Neering SJ, Upchurch D, Grimes B, Howard DS, Rizzieri DA, et al.. Nuclear factor-kappaB is constitutively activated in primitive human acute myelogenous leukemia cells. Blood 2001;98:2301–7.
- Costello RT, Mallet F, Gaugler B, Sainty D, Arnoulet C, Gastaut JA, et al.. Human acute myeloid leukemia CD34+/CD38− progenitor cells have decreased sensitivity to chemotherapy and Fas-induced apoptosis, reduced immunogenicity, and impaired dendritic cell transformation capacities. Cancer Res 2000;60:4403–11.
- Stone RM, O’Donnell MR, Sekeres MA. Acute myeloid leukemia. Hematology Am Soc Hematol Educ Program 2004:98–117.
- Darnell JE. STATs and gene regulation. Science 1997;277:1630–5.
- Ihle JN. The Stat family in cytokine signaling. Curr Opin Cell Biol 2001;13:211–7.
- Levy DE, Lee CK. What does Stat3 do? J Clin Invest 2002;109:1143–8.
- Takeda K, Akira S. STAT family of transcription factors in cytokine-mediated biological responses. Cytokine Growth Factor Rev 2000;11:199–207.
- Haura EB, Turkson J, Jove R. Mechanisms of disease: insights into the emerging role of signal transducers and activators of transcription in cancer. Nat Clin Pract Oncol 2005;2:315–24.
- Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene 2000;19:2474–88.
- Xia Z, Baer MR, Block AW, Baumann H, Wetzler M. Expression of signal transducers and activators of transcription proteins in acute myeloid leukemia blasts. Cancer Res 1998;58:3173–80.
- Benekli M, Xia Z, Donohue KA, Ford LA, Pixley LA, Baer MR, et al.. Constitutive activity of signal transducer and activator of transcription 3 protein in acute myeloid leukemia blasts is associated with short disease-free survival. Blood 2002;99:252–7.
- Sambrook J, Russell DW. Molecular cloning: a laboratory manual. New York: Cold Spring Harbor Laboratory Press; 2001.
- Chen F, Guo Q, Yang Y, Song H, Wen T. Inhibition of AF116909 gene expression enhances the differentiation of neural stem cells. Neurol Res 2005;27:557–61.
- Bromberg J. Stat proteins and oncogenesis. J Clin Invest 2002;109:1139–42.
- Al Zaid Siddiquee K, Turkson J. STAT3 as a target for inducing apoptosis in solid and hematological tumors. Cell Res 2008;18:254–67.
- Germain D, Frank DA. Targeting the cytoplasmic and nuclear functions of signal transducers and activators of transcription 3 for cancer therapy. Clin Cancer Res 2007;13:5665–9.
- Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, et al.. Stat3 as an oncogene. Cell 1999;98:295–303.
- Mora LB, Buettner R, Seigne J, Diaz J, Ahmad N, Garcia R, et al.. Constitutive activation of Stat3 in human prostate tumors and cell lines: direct inhibition of Stat3 signaling induces apoptosis of prostate cancer cells. Cancer Res 2002;62:6659–66.
- Burke WM, Jin X, Lin HJ, Huang M, Liu R, Reynolds RK, et al.. Inhibition of constitutively active Stat3 suppresses growth of human ovarian and breast cancer cells. Oncogene 2001;20:7925–34.
- Garcia R, Bowman TL, Niu G, Yu H, Minton S, Muro-Cacho CA, et al.. Constitutive activation of Stat3 by the Src and JAK tyrosine kinases participates in growth regulation of human breast carcinoma cells. Oncogene 2001;20:2499–513.
- Ren W, Duan Y, Yang Y, Ji Y, Chen F. Down-regulation of Stat3 induces apoptosis of human glioma cell: a potential method to treat brain cancer. Neurol Res 2008;30:297–301.
- Chen F, Xu Y, Luo Y, Zheng D, Song Y, Yu K, et al.. Down-regulation of Stat3 decreases invasion activity and induces apoptosis of human glioma cells. J Mol Neurosci 2010;40:353–9.
- Benekli M, Baumann H, Wetzler M. Targeting signal transducer and activator of transcription signaling pathway in leukemias. J Clin Oncol 2009;27:4422–32.