Abstract
**Adult patients with primary immune thrombocytopenia requiring first-line treatment typically receive corticosteroids, which are associated with low response rates and many potential side effects. In a retrospective analysis of two 6-month, placebo-controlled, phase III trials, corticosteroid use decreased from 30 to 26% among patients treated with the novel thrombopoietin-mimetic romiplostim (n = 83) and remained above 30% for placebo-treated patients (n = 42). Moreover, compared to placebo, patients were spared 7 weeks of corticosteroid treatment for every 100 weeks of romiplostim treatment. Thereafter, corticosteroid use continued to decrease significantly, from 35 to 20%, in patients treated with romiplostim for up to 3 years in an open-label extension study (n = 101), and patients were spared a further 8 weeks of corticosteroid treatment for each additional 100 weeks of romiplostim treatment. Such reductions in corticosteroids may improve health-related quality of life in patients with primary immune thrombocytopenia.
Introduction
Primary immune thrombocytopenia (ITP) is an autoimmune disorder characterized by isolated thrombocytopenia, with platelet counts below 100×109/l.Citation1 In adults, ITP typically has an insidious onset and a chronic course.
ITP was historically thought to be a disease of increased antibody-mediated platelet destruction. Hence, traditional treatments have mainly targeted the immune system, with oral corticosteroids given as first-line treatment.Citation2
While initial response rates with corticosteroids range from 60 to 80%, durable response rates are only 10 to 25%.Citation3,Citation4 Moreover, long-term corticosteroid use can be associated with many side effects, including cushingoid syndrome, mood and behavioural alterations, infections due to immune suppression, diabetes, hypertension and musculoskeletal problems,Citation5which can negatively impact health-related quality of life (HRQOL).Citation6 Hence alternative, corticosteroid-sparing, therapies are needed.
In recent years, evidence that impaired platelet production also plays a role in ITP has emerged, and new treatment strategies that stimulate platelet production via the thrombopoietin (TPO)-receptor have been developed.Citation7,Citation8Romiplostim was the first TPO-mimetic to be widely approved for the treatment of chronic ITP in adults, and is recommended for second- and third-line treatment of these patients.Citation2 To date, continuous, long-term romiplostim treatment has been shown to maintain platelet counts for up to 5 years with minimal side effects.Citation9,Citation10
Previous data have shown patients who received romiplostim in two parallel, identically designed, 6-month, placebo-controlled, phase III studies were able to reduce or discontinue concurrent ITP therapies, including baseline corticosteroids.Citation11,Citation12 The analysis presented here further describes the reduced corticosteroid use observed during these studies, and demonstrates that corticosteroid use remains low among patients treated with romiplostim for up to 3 years during an open-label extension study.
Methods
The studies included in this analysis have been reported elsewhere.Citation9,Citation11The study protocols were approved by the relevant institutional review boards. Patients could enter the studies while receiving concurrent ITP therapies at a constant dose and schedule. Dose reductions were permitted during the first 12 weeks of the phase III studies, if platelet counts were above 100×109/l. In the extension study, concurrent therapies could be reduced or discontinued after a platelet count ⩾50×109/l was achieved. Dose increases and the use of rescue therapies were permitted at any time during the phase III studies and when platelet counts fell below 10×109/l in the extension study. Target platelet count was 50–200×109/l in the phase III studies and 50–250×109/l in the extension study.
As reported elsewhere, the demographics and baseline characteristics of patients enrolled in the phase III studies were similar;Citation11 hence data from these studies were pooled for the analyses described here. Incidence of corticosteroid use in each 4-week study period was plotted by treatment group, and mean daily dose plotted for patients continuing to receive prednisone-type corticosteroids. For each treatment group, average duration of exposure to corticosteroids per 100 subject-weeks of exposure to study drug was also calculated.
The analyses described for the extension study include interim data from patients previously enrolled in the phase III studies, collected as of July 2008. Efficacy and safety results from this interim data have been reported elsewhere.Citation13 Incidence of corticosteroid use was plotted for each 24-week period in which at least five patients remained on study. The reduction in corticosteroid use over time was tested for statistical significance using a logistic regression model, using corticosteroid use as a binary response variable, time as a main effect and a random intercept. Mean daily dose was plotted for patients continuing to receive prednisone-type corticosteroids. Average duration of exposure to corticosteroids per 100 subject-weeks of exposure to romiplostim was also calculated.
Results
In total, 125 subjects (83 romiplostim, 42 placebo) were randomized into the phase III studies; 102 of whom subsequently entered the extension study (). At the time of this analysis, patients from the phase III studies had been treated in the extension study for up to 144 weeks (median exposure to romiplostim 102 weeks).
Figure 1. Subject disposition (July 2008). *Reasons for discontinuation from the phase III studies have been reported elsewhereCitation11; †1 subject withdrew consent prior to receiving romiplostim.
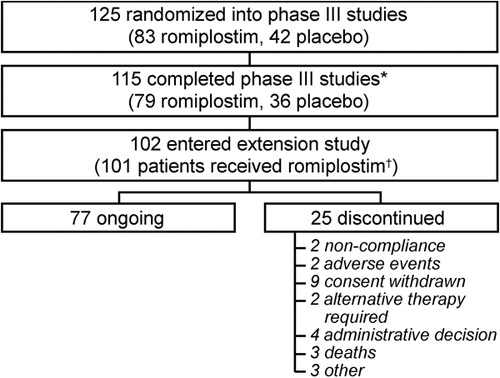
Patients receiving romiplostim and placebo in the phase III studies had similar demographics and baseline characteristics.Citation11Overall, median age was 52 years (range 21–88 years), mean platelet count was 16×109/l (range 2–31×109/l), 65% (81/125) patients were female, 50% (63/125) were splenectomized and 94% (118/125) had previously received corticosteroids for ITP. At baseline, use of concurrent ITP medications was lower in the romiplostim group (23/83 [28%] vs 16/42 [38%]).
During the phase III studies, cumulative exposure to study drug was 1891 and 774 weeks for romiplostim- and placebo-treated patients, respectively. Cumulative exposure to corticosteroids was 443 and 236 weeks, respectively. Hence, for every 100 weeks of exposure to romiplostim and placebo, patients received corticosteroids for an average of 23·4 and 30·5 weeks.
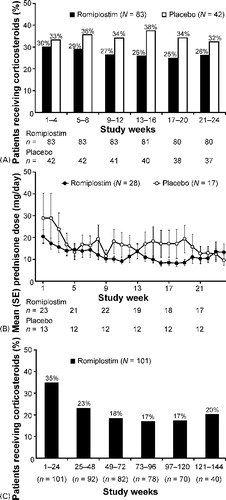
In the phase III studies, the rate of corticosteroid use among romiplostim-treated patients decreased from 30% during weeks 1–4 to 26% during weeks 21–24. Corticosteroid use among placebo-treated patients remained above 30% (). In both treatment groups, the majority of patients requiring corticosteroids received prednisone-type steroids (romiplostim: 28/31 [90%], placebo: 17/19 [89%]). Among romiplostim-treated patients, mean daily prednisone dose decreased from 20 to 7 mg by week 19, before increasing slightly to 13 mg by week 24. Mean daily dose also decreased in placebo-treated patients ().
Figure 2. (A) Corticosteroid use decreased in romiplostim-treated patients during two, 6-month, phase III studies. Percentages calculated from n = number of subjects remaining on study at the start of the relevant time period. (B) Prednisone dose decreased in patients continuing to receive corticosteroids during the phase III studies. N = Number of subjects receiving prednisone-type corticosteroids during the phase III studies; n = number of subjects receiving prednisone-type corticosteroids at specified time point. (C) Corticosteroid use decreased significantly over time in patients treated with romiplostim for up to 3 years in an open-label extension study. n = number of subjects remaining on study.
During the extension study, cumulative exposure to romiplostim and corticosteroids was 9437 and 1417 weeks, respectively. Hence, for every 100 weeks of exposure to romiplostim, patients received corticosteroids for an average of 15·0 weeks.
Corticosteroid use continued to decrease during the extension study (). During weeks 1–24, 35% of patients received corticosteroids, compared with 20% during weeks 121–144. A logistic regression model showed this decrease to be statistically significant (P = 0·0003), and estimated that the odds of receiving corticosteroids decreased by 13% for every 24 weeks of romiplostim treatment received. The majority of patients requiring corticosteroids received prednisone-type steroids (31/39 [79%]). Mean daily prednisone dose decreased from 15 to 5 mg after 1 year of romiplostim treatment.
Discussion
Corticosteroids, the conventional first-line treatment in adult patients with ITP, have limited, short-term efficacy, and are associated with many side effects which can have a detrimental effect on HRQOL. Hence alternative, corticosteroid-sparing, therapies are needed.
Romiplostim is a novel, TPO-mimetic which is approved for the treatment of chronic ITP in adults. To date, continuous long-term treatment has been shown to maintain platelet counts for up to 5 years with minimal side effects.Citation9,Citation10In the retrospective analysis presented here, concomitant corticosteroid use among patients treated with romiplostim for up to 3 years was analyzed.
In two placebo-controlled, phase III studies, the rate of corticosteroid decreased from 30 to 26% among romiplostim-treated patients, and remained above 30% in placebo-treated patients. While the analyses presented had insufficient power to demonstrate statistical significance, the differences observed are deemed clinically relevant. Indeed, patients were spared 7 weeks of corticosteroid treatment for every 100 weeks of romiplostim treatment. In an open-label extension study, corticosteroid use continued to decrease. After 3 years of continuous romiplostim treatment, the rate of corticosteroid use had decreased from 35 to 20%, a reduction that was statistically significant. Moreover, patients were spared an additional 8 weeks of corticosteroid treatment for each additional 100 weeks of romiplostim treatment.
While some patients continued to receive corticosteroids, the dose administered in these patients decreased. This trend was observed for both romiplostim- and placebo-treated patients. The decrease in the placebo group may be due to the widespread use of rescue medications among these patients, which has been reported elsewhere.Citation11,Citation14 Furthermore, in the phase III studies corticosteroids were reduced when platelet counts were below 100×109/l, a higher threshold than used in clinical practice. Hence, the corticosteroid-sparing effect of romiplostim may be underestimated in this analysis.
Conclusion
The analyses presented demonstrate that concomitant corticosteroid use is reduced among patients treated with romiplostim, and remains low during long-term treatment with this agent. In addition, data in patients treated for up to 5 years show that continuous, long-term, romiplostim treatment safely and effectively maintains platelet counts.Citation9,Citation10 Hence romiplostim is an important corticosteroid-sparing therapy for adult ITP, and may improve HRQOL by alleviating the side effects associated with corticosteroids. This improvement in HRQOL needs to be quantified in controlled, prospective studies.
This work was funded by Amgen Inc., Thousand Oaks, CA, USA. The authors would like to thank Claire Desborough, of Amgen Ltd, UK, who provided medical writing assistance.
Notes
**A Corrigendum was subsequently published for this paper in Vol. 16, No. 6. See: http://dx.doi.org/10.1179/ 102453311X13025568942041.
References
- Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, et al. Standardization of terminology, definitionsand outcome criteria in immune thrombocytopenic purpura of adults and children:report from an international working group. Blood 2009;113:2386–93.
- Provan D, Stasi R, Newland AC, Blanchette VS, Bolton-Maggs P, Bussel JB, et al. International consensus report on theinvestigation and management of primary immune thrombocytopenia. Blood 2010;115:168–86.
- Arnold DM, Kelton JG. Current options for the treatment of idiopathicthrombocytopenic purpura. Semin Hematol 2007;44:S12–S23.
- Stasi R, Evangelista ML, Amadori S. Novel thrombopoietic agents: a review of theiruse in idiopathic thrombocytopenic purpura. Drugs 2008;68:901–12.
- McDonough AK, Curtis JR, Saag KG. The epidemiology of glucocorticoid-associatedadverse events. Curr Opin Rheumatol 2008;20:131–7.
- Mathias SD, Gao SK, Miller KL, Cella D, Snyder C, Turner R, et al. Impact of chronic Immune ThrombocytopenicPurpura (ITP) on health-related quality of life: a conceptual model startingwith the patient perspective. Health Qual Life Outcomes 2008;6:13.
- Nugent D, McMillan R, Nichol JL, Slichter SJ. Pathogenesis of chronic immune thrombocytopenia:increased platelet destruction and/or decreased platelet production. BrJ Hematol 2009;146:585–96.
- Newland A. Thrombopoietin receptor agonists in the treatmentof thrombocytopenia. Curr Opin Hematol 2009;16:357–64.
- Bussel JB, Kuter DJ, Pullarkat V, Lyons RM, Guo M, Nichol JL. Safety and efficacy of long-term treatmentwith romiplostim in thrombocytopenic patients with chronic ITP. Blood 2009;113:2161–71.
- Kuter DJ, Bussel JB, Newland A, Wasser JS, Lyons RM, George JN, et al. Long-term efficacy and safety of romiplostimtreatment of adult patients with chronic immune thrombocytopenia (ITP): finalreport from an open-label extension study. ASH AnnualMeeting Abstracts 2010;116: Abstract68.
- Kuter DJ, Bussel JB, Lyons RM, Pullarkat V, Gernsheimer TB, Senecal FM, et al. Efficacy of romiplostim in patientswith chronic immune thrombocytopenic purpura: a double-blind randomised controlledtrial. Lancet 2008;37:395–403.
- Michel M, Desborough C. Reduced corticosteroid treatment in adultswith ITP receiving romiplostim. EHA Annual MeetingAbstracts 2009; 0226.
- Kuter DJ, Bussel JB, Newland A, de Wolf JTM, Guthrie T, Wasser J, et al. Long-term treatment with romiplostimin patients with chronic immune thrombocytopenic purpura (ITP): 3-year updatefrom an open-label extension study. ASH Annual MeetingAbstracts 2008;112:402.
- Pullarkat VA, Gernsheimer TB, Wasser JS, Newland A, Guthrie TH, de Wolf JT, et al. Quantifying the reduction in immunoglobulinuse over time in patients with chronic immune thrombocytopenic purpura receivingromiplostim (AMG 531). Am J Hematol 2009;84:538–40.