Abstract
It has been proposed that Vitamin D has a significant influence on disease progression in malignancy. This study aims to investigate whether serum levels of 25-hydroxyvitamin D [25(OH)D] are associated with prognosis in patients with hematological malignancies. This study is based on 105 patients with hematological disease (acute and chronic leukemias, myelodysplastic syndromes, monoclonal gammapathies, and chronic lymphoid disorders), seen over a 6-months period. 25(OH)D deficiency (<20 ng/ml) appeared very common and an inverse relationship was observed between 25(OH)D levels and the response to therapy: lower levels being related to poorer response. In acute leukemias, a significant difference was noted between patients with long-term disease-free survival in those tested at diagnosis (P = 0·001) or in those tested at the time of relapse (P = 0·05). Similarly in patients with Philadelphia-positive leukemias, there was a correlation between molecular response and levels of 25(OH)D (P = 0·01). Previously identified factors, such as age, season, gender, or nutritional index, were not related to circulating 25(OH)D levels. Lower levels of circulating 25(OH)D appeared related to a progressive stage of the disease and poor response to therapy, and, therefore, to the aggressiveness of the disease. It is a potential marker of prognosis in patients with leukemia.
Introduction
Vitamin D, a steroid hormone produced in skin, acts through a nuclear transcription factor to regulate many aspects of cellular growth and differentiation,Citation1,Citation2 and exerts its action via specific intracellular receptors, which are found in normal as well as cancer cells.Citation3 The biologically most active form of vitamin D, calcitriol (1,25-dihydroxyvitamin D [1,25(OH)2D]), is formed from calcidiol (25-hydroxyvitamin D [25(OH)D]) in the kidney, and acts by binding to nuclear vitamin D receptors, and regulates gene transcription. The major circulating metabolite of vitamin D is serum 25(OH)D, which has a half life of between 10 and 19 days, and represents the best indicator of vitamin D status reflecting levels from dietary intake and synthesis in the skin.Citation4 Observational studies have indicated that inadequate 25(OH)D levels are a risk factor for certain types of cancer.Citation5 In several pathologies, a level of 25(OH)D higher than 20 ng/ml at the time of diagnosis and during cancer treatment may improve the prognosis.Citation6,Citation7 Both prospective and retrospective studies have indicated that levels of 25(OH)D below 20 ng/ml are associated with an increased risk of solid tumors, along with higher mortality from these cancers.Citation5,8–Citation11 Recently, results have shown an association of 25(OH)D level less than 50 nmol/l with increased breast cancer risk and provided the first direct evidence that vitamin D may be an important host factor influencing breast cancer prognosis.Citation12 Results from another study have suggested that high levels of 25(OH)D were associated with reduced development of leukemia.Citation8
Based on these first intriguing results, in this study we investigated the relationship between vitamin D levels and disease status and prognosis in patients with hematological malignancies using individual serum levels of 25(OH)D.
Patients and Methods
Study population
25(OH)D levels were examined in a cohort of 105 patients with hematological malignancies at different stages of their disease, hospitalized in the Leukemia Unit (acute leukemias) or followed in consultation (chronic hematological disorders or acute leukemias with long-term follow-up) at the Edouard Herriot Hospital (Lyon, France) between June and November 2008. The patients were split into different groups according to diagnosis and status of their disease. Fifty-four patients had acute myeloid leukemia (AML), of whom one with secondary AML following acute transformation of essential thrombocythemia; 9 had acute lymphoblastic leukemia (ALL), of whom one had transformation of chronic myeloid leukemia (CML); 20 had a myeloproliferative syndrome (MPS), of whom 19 had CML and one essential thrombocythemia; 8 had myelodysplastic syndrome (MDS), 10 had a monoclonal gammapathy, of whom 6 had multiple myeloma (MM) and 4 monoclonal gammapathy of unknown significance (MGUS); and 4 presented with another chronic lymphoproliferative disorder (2 with chronic lymphocytic leukemia and 2 with low grade non-Hodgkin’s lymphoma). Eighty patients had an active disease (37 were at diagnosis, 30 in a chronic phase, and 13 at relapse), while 25 were in complete response. Among patients, 27 presented with Philadelphia chromosome-positive leukemia (19 CML, 7 ALL or ALL-transformed CML, and one AML).
Serum 25(OH)D assessment
25(OH)D is the major circulating vitamin D metabolite and the best indicator of an individual’s vitamin D status.Citation13 For each patients the 25(OH)D was measured once; either at the time of diagnosis or during the evolution of the disease. Twenty-five milliliters of peripheral venous blood, collected in tubes with heparin, were drawn from each patient’s sample for the 25(OH)D analyses. Serum/plasma samples were separated and circulating 25(OH)D levels were measured using a radioimmunoassay kit (DIASORIN, Stillwater, MN, USA), coefficient of variation less than 15%.Citation14 All samples were assayed at the same laboratory. Vitamin D deficiency was defined as a 25(OH)D level of less than 20 ng/ml. A level of 21 to 29 ng/ml was considered to indicate a relative insufficiency of vitamin D, and a level of 30 ng/ml or greater was considered to indicate sufficient vitamin D.Citation2
Statistical analysis
From the existing literature, a number of factors are known to influence circulating 25(OH)D concentrations; among these, sunlight exposure and vitamin D intake are the most important.Citation15 Other factors related to these main factors included region as a surrogate of residential ultraviolet radiation exposure,Citation16 physical activity level, skin pigmentation, nutritional indexes such as body mass index (BMI),Citation8 season,Citation17 cigarette smoking, gender and age. Clinical information and functional status at time of analysis was retrieved from the medical records. Patient information from the medical records was collected without the knowledge of the individual 25(OH)D level. Performance status, defined by Eastern Cooperative Oncology GroupCitation18 was <2 for all patients. Data on sunlight exposure behaviors or outdoor activities, vitamin D intake from diet and supplements and cigarette smoking were not available. Nutritional index was assessed in patients with acute leukemia tested at the time of diagnosis. Body mass index was defined by a person’s weight in kilograms divided by height in meters squared. The relationship between 25(OH)D levels and molecular response in leukemias with Philadelphia chromosome was assessed by comparison of 25(OH)D levels in patients with no major molecular response (%Bcr-Abl/Abl ⩾0·1%), and patients having a major molecular response (%Bcr-Abl/Abl <0·1%). In the statistical analysis, 25(OH)D levels were considered as continuous variables. The relationship between 25(OH)D levels and quantitative characteristics were studied by the Spearman rank correlation test. The relationships between 25(OH)D levels and qualitative parameters were studied by the Mann-Whitney or Kruskal-Wallis tests. All reported P values were from two-sided tests. Computations were performed using BMDP PC90 statistical program (BMDP Statistical Software, Los Angeles, CA, USA).
Results
Characteristics of patients
A cohort of 105 patients (median age, 57 years; range: 21–85 years) was studied. Mean serum 25(OH)D level±standard deviation (SD) was 22·9±10·5 ng/ml. The range of 25(OH)D concentrations was 6 to 50 ng/ml. Forty-nine patients (46%) had 25(OH)D levels less than 20 ng/ml and were defined as vitamin D deficiency, while 27 (26%) ranged between 20 and 30 ng/ml, and 29 (28%) were at 30 ng/ml or more. Patient characteristics (age, sex, diagnosis, and disease status) and 25(OH)D are presented in .
Table 1. Serum 25(OH)D levels according to patients characteristics
Assessment of factors potentially influencing circulating 25(OH)D
In the entire cohort, 25(OH)D levels were not associated with gender (23·3±10·5 ng/ml in males versus 24·3±13·7 ng/ml in females). Increasing age was also not associated with a lower level of 25(OH)D (20·0±7·6 ng/ml in patients <30 years old, 23·8±11·4 ng/ml between 30 and 60 years, and 22·0±10·0 ng/ml over 60 years). This was still the case when considering only patients tested at the time of diagnosis (17·0±6·5 ng/ml in patients <30 years old, 18·6±10·7 ng/ml between 30 and 60 years, and 20·1±7·1 ng/ml over 60 years). No difference was noted when 25(OH)D was drawn in summer (June–August) versus autumn (September–November) months, as well as in the entire cohort (21·6±10·3 ng/ml versus 25·6±10·3 ng/ml) than in the group of patients tested at the time of diagnosis (16·5±9·1 ng/ml versus 16·0±6·7 ng/ml). In acute leukemia patients studied at the time of diagnosis for this factor (24 patients), the BMI appeared not related to circulating 25(OH)D level (r = 0·025). However, the two patients presenting with under weight at diagnosis (BMI<18·5 kg/m2) showed low 25(OH)D levels (15 and 16 ng/ml). Over weight (BMI>25 kg/m2) was observed in 14 patients (58%) and showed circulating 25(OH)D levels similar to those from patients with BMI in the normal range (16·7±6·7 ng/ml versus 20·6±8·3 ng/ml, respectively).
Association between 25(OH)D levels and malignant cell burden
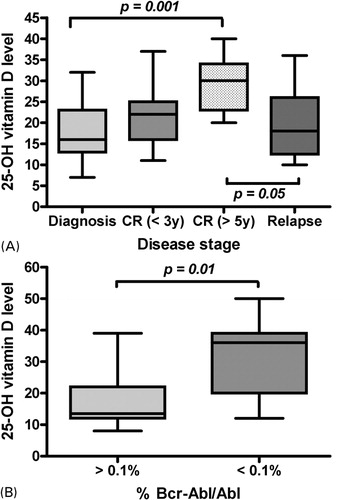
Although there was no correlation between 25(OH)D level and the percentage of leukemic cells infiltrating bone marrow (BM) in patients with acute leukemia at diagnosis, as well as when studied as a continuous variable (r = −0·23) or when divided into two groups by the median value (19·8±6·8 ng/ml for BM blasts <60% versus 18·8±7·6 ng/ml for BM blasts ⩾60%), lower 25(OH)D levels were associated with higher malignant cell burden as indicated by the correlation observed between the stage of the disease in acute leukemias and levels of 25(OH)D. The difference was significant between patients with long-term disease-free survival and those tested at diagnosis (29·2±6·1 ng/ml versus 17·7±6·7 ng/ml; P = 0·001) or those tested at the time of relapse (29·2±6·1 ng/ml versus 19·9±9·1 ng/ml; P = 0·05) (). Similarly in patients with Philadelphia-positive leukemias, the study of molecular residual disease showed a correlation between molecular response and levels of 25(OH)D (31·2±12·0 ng/ml in patients with %Bcr-Abl/Abl<0·1% versus 17·4±8·9 ng/ml in patients with %Bcr-Abl/Abl⩾0·1%; P = 0·01) (). In those patients, serum levels of parathyroid hormone (PTH) and calcium remained in the normal range: 15 to 65 ng/l (median: 45 ng/l) and 2·12 to 2·52 mmol/l (median: 2·22 mmol/l), respectively. Although the cohort of patients with monoclonal gammapathy was small, there was a trend toward higher circulating levels of 25(OH)D for patients with an active disease as compared to those in MGUS (18·0±6·1 ng/ml versus 28·5±23·1 ng/ml, respectively). In patients with active disease, 25(OH)D levels were not significantly different among myeloid and lymphoid malignancies (21·5 ±10·2 ng/ml versus 23·2±14·1 ng/ml, respectively). In relapsing patients with acute leukemia, we assessed whether the value of circulating 25(OH)D was modified by the time interval between relapse and last complete response to therapy, defined into two groups by the median value (32 months). No difference was noted between the two groups (15·6 ±6·0 ng/ml for patients with shorter remission duration versus 15·5 ±3·8 ng/ml for those with longer remission duration). No correlation was found when considering the duration of last remission as a continuous variable (r = 0·17), but the analysis involved only 9 patients.
Figure 1. Correlations between outcome and 25(OH)D levels are illustrated by (A) the relationship between 25(OH)D levels and the stage of the disease in acute leukemias: patients at the time of diagnosis (n = 27), patients with CR of less than 3 years (n = 11), patients with CR of more than 5 years (n = 13), and patients in relapse (n = 12); and (B) the relationship between 25(OH)D levels and molecular response in leukemias with Philadelphia chromosome: patients with no major response (n = 12), and patients with major response (n = 13).
Discussion
There are increasing molecular and clinical evidence in favor of an anticarcinogenic effect of vitamin D.Citation19,Citation20 Epidemiologic studies have shown that low levels of 25(OH)D may be associated with increased cancer incidence and mortality.Citation4,Citation8,Citation21 This hypothesis has received experimental support and the demonstration that activation of the vitamin D receptor by 1,25(OH)2D induces differentiation and apoptosis, and inhibits proliferation, invasiveness and angiogenesis.Citation22 The vitamin D-sensitive cancers that have been found associated with predicted 25(OH)D levels were those of rapidly proliferating tissue, such as the bone marrow.Citation8 These results are of interest for leukemias given that 1,25(OH)2D induces differentiation of mouse myeloid leukemia cellsCitation23 and improves survival in mice inoculated with murine myeloid leukemia cells.Citation24 However, there is still controversy, a recent study showing that a high circulating 25(OH)D level may be associated with an increased risk of aggressive disease.Citation25
In the present study, we observed a significant association between circulating 25(OH)D and malignant cell burden. To our knowledge, this is the first study examining and demonstrating such an association. Mechanisms that may explain this association are highly speculative because there is a lack of understanding of the molecular mechanism by which vitamin D regulate the expression of genes involved in carcinogenesis. Several limitations of our study deserve comments. Our analysis used a single measurement of plasma 25(OH)D. Potentially 25(OH)D level could represent a confounder such as exercise, outdoor sunlight exposure, or correlated dietary factor. Although the series is large, sample sizes drop in the subgroup analyses by diagnosis or different stages. In addition, there is the problem of missing or non-sufficient data regarding certain subgroups. The strength of our study includes its prospective design with 25(OH)D status being assessed only on few months, thereby reducing the influence of reverse causality. Although the magnitude of the difference was relatively small in our series, patients who were diagnosed with cancer of the breast, colon, prostate, lung or lymphoma during summer or autumn were found to have better prognosis than patients diagnosed during the winter months.Citation6,Citation26–Citation28 We also did not observe significant interactions by gender, age, or nutritional status that have been previously described as factors influencing circulating 25(OH)D levels.Citation8 Higher BMI or obesity has been found to be associated with substantially lower circulating concentrations of 25(OH)D probably as a result of decreased bioavailability of 25(OH)D because of its deposition in body fat compartments.Citation29 Although we did not confirm those results, we cannot exclude an impact of those factors, our study having limited power to observe interactions involving these factors because of our small cohort or because few subjects were out of the normal range regarding nutritional index (BMI). Other influencing factors previously described, but not tested in our study, could also be suspected for influencing our results. Residual confounding by cigarette smoking is possible,Citation8 however not likely because few subjects were current smokers. A single measurement of 25(OH)D may also not reflect long-term vitamin D status. In a steady-state context, it represents the past several weeks to several months of exposureCitation30 and associations between one measure of 25(OH)D and cancer have already been reported.Citation4,Citation31 Although most of the patients included had inadequate levels of 25(OH)D, we do not know whether there is an optimal level with respect to leukemias. There is no general consensus on the optimal level of 25(OH)D for maintaining health. Vitamin D intake has been shown to predict a relatively small proportion of the variance in 25(OH)D.Citation8 It has been hypothesized that vitamin D can amplify the effect of cancer treatment; a synergistic effect that has been observed in both experimental and clinical studies.Citation32,Citation33 In a case report, adequate vitamin D intake (with apparently no other treatment) was associated with clinical remission of CLL for at least 16 years.Citation34
The disease may, directly or indirectly, have an impact on the 25(OH)D level if patients with advanced disease are less able to attend outdoor activities or have unsatisfactory dietary habits with respect of 25(OH)D. This could be the situation for the group of patients with acute leukemia, for whom we found a significant difference between those with progressive disease as compared to those with long-term CR. However, this hypothesis is not consistent with our results regarding minimal residual disease detected by molecular biology in Philadelphia-positive leukemias. In those patients treated by imatinib mesylate, previous studies have strongly suggested that imatinib treatment results in decreased serum phosphate levels and an increase in serum levels of PTH, secondary to decreased calcium levels, and increased serum 1,25(OH)2D.Citation35–Citation38 However, none of our patients with Philadelphia-positive leukemia exhibited PTH levels or calcium levels outside the normal range. Imatinib has shown direct effects on bone-resorbing osteoclasts and bone-forming osteoblasts through inhibition of c-fms, c-kit, carbonic anhydrase II, and the platelet-derived growth factor receptor.Citation39 The strength of the association of 25(OH)D and minimal residual disease suggests that leukemia patients can benefit from increasing the level of serum 25(OH)D. A randomized study, giving or not vitamin D in addition to the treatment with tyrosine kinase inhibitor, should find here an application. In the present study, correlations observed between lower 25(OH)D levels and active diseases are also consistent with the inverse relationship between serum 25(OH)D levels and early mortality that have been previously reported for solid tumors.Citation7,Citation40 In those studies, high 25(OH)D levels were also associated with high vitamin D intake. However, we cannot exclude the possibility that a correlate to serum 25(OH)D status that is unknown and not controlled could also explain the associations we observed.
In conclusion, 25(OH)D deficiency appeared very common in hematological malignancies since only 28% of the patients studied were 25(OH)D sufficient. The lowest blood levels appeared related to active stages of the disease, poor response to therapy, and therefore aggressiveness of the disease. Whether lower 25(OH)D levels have other explanations remains a challenge, like the exact role of vitamin D in malignant cells and its potential need for a therapeutic or preventive approach. Additional efforts to understand the mechanisms through which the vitamin D pathway influences hematological malignancies are warranted.
References
- Holick MF. The influence of vitamin D on bone health across the life cycle. J Nutr 2005;135:2726S–7S.
- Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266–81.
- Elias J, Marian B, Edling C, Lachmann B, Noe CR, Rolf SH, et al.. Induction of apoptosis by vitamin D metabolites and analogs in a glioma cell line. Recent Results Cancer Res 2003;164:319–32.
- Freedman DM, Looker AC, Chang SC, Graubard BI. Prospective study of serum vitamin D and cancer mortality in the United States. J Natl Cancer Inst 2007;99:1594–602.
- Garland CF, Garland FC, Gorham ED, Lipkin M, Newmark H, Mohr SB, et al.. The role of vitamin D in cancer prevention. Am J Public Health 2006;96:252–61.
- Robsahm TE, Tretli S, Dahlback A, Moan J. Vitamin D from sunlight may improve the prognosis of breast-, colon- and prostate cancer (Norway). Cancer Causes Control 2004;2:149–58.
- Zhou W, Heist RS, Liu G, Asomaning K, Neuberg DS, Hollis BW, et al.. Circulating 25-hydroxyvitamin D levels predict survival in early-stage non-small lung cancer patients. J Clin Oncol 2007;25:479–85.
- Giovannucci E, Liu Y, Rimm EB, Hollis BW, Fuchs CS, Stampfer MJ, et al.. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst 2006;98:451–9.
- Gorham ED, Garland CF, Garland FC, Grant WB, Mohr SB, Lipkin M, et al.. Vitamin D and prevention of colorectal cancer. J Steroid Biochem Mol Biol 2005;97:179–94.
- Ahonen MH, Tenkanen L, Teppo L, Hakama M, Tuohimaa P. Prostate cancer risk and prediagnostic serum 25-hydroxyvitamin D levels (Finland). Cancer Causes Control 2000;11:847–52.
- Feskanich D, Ma J, Fuchs CS, Kirkner GJ, Hankinson SE, Hollis BW, et al.. Plasma vitamin D metabolites and risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev 2004;13:1502–8.
- Goodwin PJ, Ennis M, Pritchard KI, Koo J, Hood N. Prognostic effects of 25-hydroxyvitamin D levels in early breast cancer. J Clin Oncol 2009;27:3757–63.
- Dietary supplement fact sheet: vitamin D. Bethesda (MD): Office of Dietary Supplements, National Institutes of Health. 2011. Available from: http://ods.od.nih.gov/factsheets/vitaminD.
- Hollis BW, Kamerud JW, Selvaag SR, Lorenz JD, Napoli JL. Determination of vitamin D status by radioimmunoassay using I[125]-labelled tracer. Clin Chem 1993;39:529–33.
- Skinner HG, Michaud DS, Giovannucci E, Willett WC, Colditz GA, Fuchs CS. Vitamin D intake and the risk for pancreatic cancer in two cohort studies. Cancer Epidemiol Biomarkers Prev 2006;15:1688–95.
- Stolzenberg-Solomon RZ, Hayes RB, Horst RL, Anderson KE, Hollis BW, Silverman DT. Serum vitamin D and risk of pancreatic cancer in the prostate, lung, colorectal, and ovarian screening trial. Cancer Res 2009;69:1439–47.
- Stolzenberg-Solomon RZ, Vieth R, Azad A, Pietinen P, Taylor PR, Virtamo J, et al.. A prospective nested case-control study of vitamin D status and pacreatic cancer risk in male smokers. Cancer Res 2006;66:10213–9.
- Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al.. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649–55.
- Peterlik M, Cross HS. Dysfunction of the vitamin D endocrine system as common causes for multiple malignant and other chronic diseases. Anticancer Res 2006;26:2581–8.
- Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr 2007;85:1586–91.
- Tangpricha V, Colon NA, Kaul H, Wang SL, Decastro S, McFadden ET, et al.. Prevalence of vitamin D deficiency in patients attending an outpatient cancer care clinic in Boston. Endocr Pract 2004;10:292–3.
- Holick MF. Vitamin D; its role in cancer prevention and treatment. Prog Biophys Mol Biol 2006;92:549–59.
- Abe E, Miyaura C, Sakagami H, Takeda M, Konno K, Yamazaki T, et al.. Differentiation of mouse myeloid leukemia cells induced by 1[alpha],25-dihydroxyvitamin D3. Proc Natl Acad Sci USA 1981;78:4990–4.
- Honma Y, Hozumi M, Abe E, Konno K, Fukushima M, Hata S, et al.. 1[alpha],25-dihydroxyvitamin D3 and 1[alpha]-hydroxyvitamin D3 prolong survival time of mice inoculated with myeloid leukemia cells. Proc Natl Acad Sci USA 1983;80:201–4.
- Ahn J, Peters U, Albanes D, Purdue HP, Abnet CC, Chatterjee N, et al.. Serum vitamin D concentration and prostate cancer risk: a nested case-control study. J Natl Cancer Inst 2008;100:796–804.
- Porojnicu AC, Robsahm TE, Ree AH, Moan J. Season of prognosis is a prognostic factor in Hodgkin’s lymphoma: a possible role of sun-induced vitamin D. Br J Cancer 2005;93:571–4.
- Porojnicu AC, Robsahm TE, Dahlback A, Berg JP, Christiani D, Bruland OS, et al.. Seasonal and geographical variations in lung cancer prognosis in Norway. Does vitamin D from the sun play any role? Lung Cancer 2007;55:263–70.
- Lim HS, Roychoudhuri R, Peto J, Schwartz G, Baade P, Møller H. Cancer survival is dependent on season of diagnosis and sunlight exposure. Int J Cancer 2006;119:1530–6.
- Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 2000;72:690–3.
- Holick MF. The use and interpretation of assays for vitamin D and its metabolites. J Nutr 1990;120(Suppl. 11):1464–9.
- Wu K, Feskanich D, Fuchs CS, Willett WC, Willett WC, Hollis BW, Giovannucci EL. A nested case control study of plasma 25-hydroxyvitamin D concentrations and risk of colorectal cancer. J Natl Cancer Inst 2007;99:1120–29.
- Dunlap N, Schwartz GG, Eads D, Cramer SD, Sherk AB, John V, et al.. 1α,25-Dihydroxyvitamin D3 (calcitriol) and its analogue, 19-nor-1α,25(OH)2D2 potentiate the effect of ionising radiation on human prostate cancer cells. Br J Cancer 2003;89:746–53.
- Deeb KK, Trump DL, Johnson CS. Vitamin D: signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer 2007;7:684–700.
- Politzer WM. Long-term clinical remission of chronic lymphocytic leukaemia by dietary means. South African Med J 2005;95:321–2.
- Berman E, Nicolaides M, Maki RG, Fleisher M, Chanel S, Scheu K, et al.. Altered bone and mineral metabolism in patients receiving imatinib mesylate. N Engl J Med 2006;354:2006–13.
- Grey A, O’Sullivan S, Reid IR, Browett P. Imatinib mesylate increased bone formation, and secondary hyperparathyroidism. N Engl J Med 2006;355:2494–5.
- Fitter S, Dewar AL, Kostakis P, To LB, Hughes TP, Roberts MM, et al.. Long-term imatinib therapy promotes bone formation in CML patients. Blood 2008;111:2538–47.
- Osorio S, Noblejas AG, Duran A, Steegmann JL. Imatinib mesylate induces hypophosphatemia in patients with chronic myeloid leukemia in late chronic phase, and this effect is associated with response. Am J Hematol 2007;82:394–5.
- Vandyke K, Fitter S, Dewar AL, Hughes TP, Zannettino ACW. Dysregulation of bone remodeling by imatinib mesylate. Blood 2010;115:766–74.
- Ng K, Meyerhardt JA, Wu K, Feskanich D, Hollis BW, Giovannucci EL, et al.. Circulating 25-hydroxyvitamin D levels and survival in patients with colorectal cancer. J Clin Oncol 2008;36:2984–91.