Abstract
Although it has been widely demonstrated that mesenchymal stem cells (MSCs) exert potent immunosuppressive effect, there is little information as to whether adipogenic-differentiated MSCs (adi-MSCs) share the same property. Here, adi-MSCs enhanced alloantigen or mitogen-stimulated lymphocyte proliferation, whereas undifferentiated MSCs (ud-MSCs) inhibited the proliferation. Transwell experiment showed that the stimulatory effect of adi-MSCs was cell–cell contact-independent, and required soluble factors. Furthermore, the supernatant of cultured adi-MSCs could effectively costimulate T and B-lymphocyte proliferation and activation in the presence of anti-CD3 and anti-mu chain treatment, respectively. Production of cytokines interferon-gamma and tumor necrosis factor-alpha by T cells, and Ig secretion by B cells also were increased by the supernatant of cultured adi-MSCs. Mechanism conducted showed that the mRNA and protein expression of costimulatory molecule B-cell activating factor (BAFF) was upregulated, and soluble BAFF was secreted in MSCs after adipogenic differentiation. By blocking the BAFF molecule with specific monoclonal antibody in the culture, T and B-lymphocyte proliferation and activation was stimulated by adi-MSCs or the supernatants were greatly reduced. In conclusion, adipogenic differentiation may alter the immunoregulatory property of MSCs, leading to stimulation of lymphocytes response. The BAFF molecule secreted by the adi-MSCs was responsible for this event.
Introduction
Bone marrow-derived mesenchymal stem cells (MSCs) are non-hematopoietic stem cells that reside in the bone marrow and can differentiate into various tissue cells, including bone, adipose tissues/cells, stromal tissues, muscle, and liver cells, as well as astrocytes under proper conditions.Citation1 Interestingly, MSCs are also recognized to possess immunoregulatory potential without major histocompatibility complex (MHC) restriction.Citation2 An emerging body of data indicated that MSCs could inhibit T-lymphocyte activation and proliferation induced by mitogens, recall antigens, and alloantigens in vitro, while MSCs themselves escaped immune recognition by allogeneic lymphocytes.Citation2–Citation5 Similarly, MSCs also can modulate B lymphocytes,Citation6 natural killer cells,Citation7,Citation8 and dendritic cell maturation or function.Citation5,Citation9,Citation10 The mechanisms underlying these effects are largely unknown but are likely to be mediated by direct cell-to-cell interactions and soluble factors.Citation2,Citation11–Citation15 Based on these, MSCs have been administrated in vivo to improve the outcome of allogeneic transplantation by promoting hematopoietic engraftment, and to hamper graft-versus-host disease and skin-graft rejection.Citation16–Citation18 More recently, systemic administration of MSCs to mice affected by experimental autoimmune encephalomyelitis, a prototypical disease mediated by self-reactive T cells, results in striking disease amelioration mediated by the induction of peripheral tolerance.Citation19 Thus, MSCs may represent a novel therapeutic strategy for immune-mediated disorders.
Studies conducted in human and animal models have demonstrated that infused MSCs are capable of long-term engraftment and in vivo differentiation, and have already produced encouraging clinical results.Citation20–Citation23 However, the xenotransplantation studies using human MSCs in a cardiac infarction model showed conflicting outcomes with either leukocyte infiltration or functionally active chimeras reported.Citation24,Citation25 Currently, little information is available on the capacity of differentiated MSCs. Recently, mature adipocytes are increasingly being shown to have important roles as mediators linking the adipose tissue and inflammation by production of adipokines, e.g. leptin, adiponectin, and resistin.Citation26–Citation28 Thus, the present study aimed to investigate that after adipogenic differentiation, MSCs derived from murine bone marrow showed positive or negative effect on lymphocyte response, similar to undifferentiated MSCs (ud-MSCs) or mature adipocytes.
Materials and Methods
Animals
C57BL/6 and BALB/c mice at 6–8 weeks of age were purchased from the Slack Animal Center of Science Academy of China (Shanghai, China). All animals were bred and maintained under specific pathogen-free conditions. All animal handling and experiment procedures were approved by the Animal Care and Use Committee of Chinese Academy of Medical Sciences.
MSCs preparation and differentiation
Bone marrow MSCs were isolated from 6- to 8-week-old BALB/C mice. In brief, the marrow cells were flushed out of the tibia and femur of the mice by phosphate-buffered saline (PBS). The isolated mononuclear cells were suspended in plating medium (Dulbecco’s modified Eagle’s medium) containing 1 g/l of glucose (DMEM-LG; Hyclone) supplemented with 10% fetal bovine serum (FBS, Hyclone, Beijing, China), 100 U/ml penicillin, and 100 mg/ml streptomycin (Sigma, Beijing, China), and were plated in T25 flasks at a density of 106 cells/cm2. The cells were incubated at 37°C in a humidified atmosphere with 5% CO2. Two days after seeding, floating cells were removed and the medium was replaced by fresh medium. Thereafter, adhered cells (MSCs) were fed every other day by the same medium. Passages were performed when cells were approaching more than 80% confluence. Adherent cells were collected with trypsin/ethylenediaminetetraacetic acid (EDTA; Gibco, Shanghai, China), resuspended, and transferred to new flasks at a density of 5×104 cells/cm2.
After three passages, phenotype and differentiation assays were performed and the cells were used for further experiments. The remaining cells were stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD14, CD31, CD34, phycoerythrin-conjugated anti-CD44, CD105, and peridinin chlorophyll protein-conjugated anti-CD45, and MHC-II antibodies (all from eBioscience, San Diego, CA, USA).
To stimulate adipogenic differentiation, MSCs were cultured in the adipogenic medium [DMEM-LG containing 10% FBS, 10−7 mol/l dexamethasone (Sigma), 50 mg/ml indomethacin (Sigma), 0·5 mmol/l of 3-isobutyl-1-methyl-xanthine (Sigma), and 0·01mg/ml of insulin (Sigma)] for 14 days. The adipocyte differentiation of MSCs was confirmed by phase-constructed microscopic observation and oil red O staining of the accumulated lipid vesicles in the cells.
To stimulate osteogenesis, 10−8 mmol/l dexamethasone, 0·2 mmol/l ascorbic acid-2-phosphate, and 10 mmol/l beta-glycerophosphate were added into basic medium, and the medium changed twice weekly. Alkaline phosphatase (ALP) expression was examined at day 14. ALP staining was carried out using an ALP kit according to the manufacturer’s instructions (Sigma).
To stimulate chondrogenic differentiation, insulin (6·25 μg/ml; Sigma), transferrin (6·25 μg/ml; Sigma), BSA (1·25 mg/ml; Sigma), dexamethasone (0·1 μmol/ml; Sigma), proline (0·35 mmol/l; Sigma), pyruvate (1 mmol/l; Sigma), and selenous acid (6·25 ng/ml; Sigma) were added into DMEM-HG (Hyclone) containing 2% FBS, and the medium replaced every 3–4 days for 21 days. The cells were then fixed in formalin, examined morphologically, and stained with toluidine blue O.
T and B-lymphocyte isolation
The spleen of C57BL/6 mice were removed and snipped into pieces, and mononuclear cells (MNCs) were isolated by Ficoll–Hypaque density gradient (Sigma). Cell viability was 95% by trypan blue exclusion. For T-lymphocyte purification, CD3+ T cells were isolated from MNCs preparations by magnetic separation (MACS; Miltenyi Biotec, Sydney, Australia). The cells were routinely 95% CD3+. For B-lymphocyte purification, MNCs suspensions were treated with magnetic activated cell sorting (MACS) CD19-conjugated microbeads, according to the instructions of the manufacturer (Miltenyi Biotech, Auburn, CA, USA). Positively selected cells contained on average 95% B cells, as assessed by flow cytometric analysis with anti-CD20 monoclonal antibody. All cell cultures were performed in RPMI 1640 medium supplemented with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin (Sigma).
Mixed lymphocytes cultures (MLCs) and mitogenic stimulation
To study the effect of MSCs on lymphocyte reactivity, irradiated MSCs in various concentrations were added at the beginning of the one-way MLCs. MSCs were allogeneic or syngenic to responder lymphocytes. Lymphocytes of 1×105 were stimulated with irradiated allogeneic lymphocytes (1×105) in V-bottomed 96-well culture plates. After 3 days, uptake of [3H]-thymidine was measured on an LKB Betaplate counter (Wallac Oy, Finland).
For mitogenic stimulation, concanavalin A (ConA) (5 μg/ml) and pokeweed mitogen (PWM) (10 μg/ml) were used. Triplicates of 2×105 lymphocytes (0·2 ml/well) were cocultured with MSCs in the presence of mitogen. After 2 days, uptake of [3H]-thymidine was measured on an LKB Betaplate counter (Wallac Oy).
Transwell cultures
Adipogenic-differentiated MSCs (adi-MSCs) (2×105) were seeded onto the transwell membrane of the inner chamber 1–2 hours before the beginning of the culture. Adi-MSCs were added directly to ConA (5 μg/ml) or PWM (10 μg/ml)-treated lymphocyte culture, as control cultures. After 3 days, uptake of [3H]-thymidine was measured on an LKB Betaplate counter (Wallac Oy).
In selected experiments, adi-MSCs were replaced with the supernatant from adi-MSCs cell cultures. A day before being split, cell culture supernatant was harvested, centrifuged, and filtered through a 0·2-μm Millipore filter.
T-cell stimulation with anti-CD3
To examine the costimulatory effect of adi-MSCs on T lymphocytes, ud- or adi-MSCs were added into the culture containing anti-CD3-treated T cells. In briefly, 2×105 T cells (responders) were added into 96 wells microplates coated with anti-CD3 antibody (2 μg/ml). After 3 days, uptake of [3H]-thymidine was measured on an LKB Betaplate counter (Wallac Oy). Meanwhile, the lymphocytes were harvested and ready for activation detection by flow cytometry analysis. The cells were stained with FITC-conjugated anti-CD25 antibody (eBioscience). The supernatant was harvested and determined for the level of cytokines interferon-gamma (IFN-gamma) and tumor necrosis factor-alpha (TNF-alpha) by enzyme-linked immunosorbent assay (ELISA). ELISA kits were purchased from R&D, Beijing, China. In some experiments, the supernatant of cultured adi-MSCs was harvested and used to be stimulation agent in the assay. To confirm the role of BAFF in the assay, anti-BAFF mAb (R&D Systems) was added into the cultures.
B-cell stimulation with anti-mu
To examine the effect of adi-MSCs on B cells, ud- or adi-MSCs were added into the culture containing anti-mu-treated B cells. In briefly, 2×105 B lymphocytes (responders) were added into 96 wells microplates coated with anti-mu antibody (2 μg/ml). After 3 days, uptake of [Citation3H]-thymidine was measured on an LKB Betaplate counter (Wallac Oy). Meanwhile, the lymphocytes were harvested and ready for activation detection by flow cytometry analysis. The cells were stained with FITC-conjugated anti-CD25 antibody (eBioscience). The supernatant was harvested and determined for the Ig level by ELISA. In some experiments, the supernatant of cultured adi-MSCs was harvested and used to be stimulation agent in the assay. To confirm the role of BAFF in the assay, anti-BAFF mAb (R&D Systems) was added into the cultures.
Determination of BAFF mRNA level by conventional reverse transcription polymerase chain reaction
Total RNA was extracted from MSCs using Trizol reagent (Invitrogen), and reverse transcription was performed using the ImProm-II Reverse Transcription System (Promega, Beijing, China) according to the manufacturer’s recommendations. Oligonucleotide primers were as follows: BAFF, 5′-TTGGAAACACAGTGAGAGGCT-3′ and 5′-GGCAGGGAAGAGAAGGACTTA-3′; and beta-actin, 5′-TGTCCACCTTCCAGCAGATGT-3′ and 5′-AGCTCAGTAACAGTCCGCCTAGA-3′. The polymerase chain reaction products were electrophoresed on 1% agarose gels, visualized by ethidium bromide staining, and documented using an FX-20M electrophoresis and documentation system.
Determination of BAFF protein expression level by western blot analysis
After experimental treatment, MSCs were washed twice with ice-cold PBS and lysed with extraction buffer containing 50 mM Tris-HCl (pH 7·5), 150mM NaCl, 1% NP-40, 1 mM EDTA, 10 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 100mM NaF, 1 mM PMSF, and protease inhibitor cocktail. The total concentration of extracted proteins was determined using the Bradford method. The proteins were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes. After blocking for 1 hour at room temperature with TBS-T (10 mM Tris, 150 mM NaCl, and 0·05% Tween-20, pH 7·6) containing 5% non-fat milk, the membranes were incubated with the appropriate primary antibodies. To detect the antigen-bound antibodies, the blots were treated with secondary antibodies conjugated to horseradish peroxidase. Immunoreactivity was detected using the ECL western blotting detection system. Antibody against BAFF was purchased from R&D Systems (Minneapolis, MN, USA). Antibody against GAPDH was purchased from Cell Signaling Technology (Danvers, MA, USA).
Quantification of BAFF secretion in supernatant by ELISA
Measurement of BAFF secretion in supernatant is performed with mouse BAFF/BLyS/TNFSF13B immunoassay (Quantikine; R&D Systems) according to the manufacturer’s instruction.
Statistics analysis
All results are expressed as mean±standard deviation (SD) of three replicate determinations, and statistical comparisons are based on one-way analysis of variance or Student’s t-test. A probability value of P<0·05 was considered significant.
Results
Characteristics of murine bone marrow-derived MSCs (BM-MSCs)
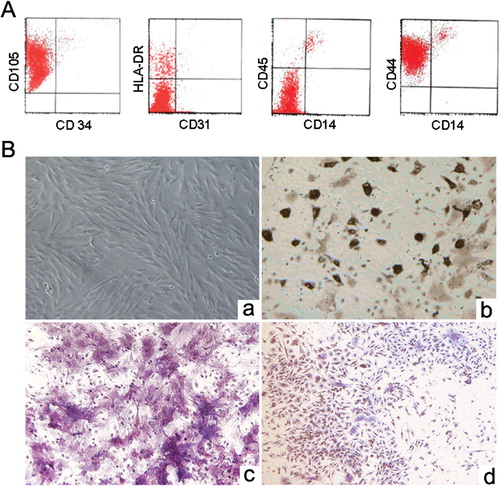
Cultured BM-MSCs were isolated from second to third passage. According to previous reports, we identified the characteristics of isolated murine BM-MSCs, including immunophenotype, and differentiation potentials. Analysis of flow cytometry showed that cultured BM-derived cells shared classical immunophenotype of MSC, including positivity for CD44 and CD105, but negativity for CD31, CD34, CD45, and CD14, and low expression for HLA-DR (). As shown in , the cells formed a monolayer of homogenous bipolar spindle-like cells with a whirlpool-like array within 2 weeks. We further tested the abilities of primary cultures on passage 2–3 to undergo osteogenic, adipogenic, and chondrogenic differentiation after applying the corresponding conditioned media. The tested cultures demonstrated good propagation and three lineages differentiation abilities (). Collectively, these data showed that we have successfully obtained abundant MSCs from murine bone marrow.
Figure 1. Characteristics of murine bone marrow-derived mesenchymal stem cells (MSCs). (A) Immunophenotype of murine bone marrow-derived MSCs. (B) MSCs morphology and capacity for differentiation. (a) Culture-expanded murine bone marrow-derived MSCs show a spindle-shaped fibroblastic morphology (magnification, ×100). (b) For adipogenic induction, the accumulated lipid vacuoles were stained with oil red O at day 14 (magnification, ×100). (c) For osteogenic induction, the cells were positive for alkaline phosphatase staining at day 14 (magnification, ×200). (d) For chondrogenic induction, the cells were positive for toluidine blue O staining at day 21 (magnification, ×100).
Effect of adi-MSCs on lymphocyte response in primary mixed culture
MSCs were classified as being low immunogenic, and did not trigger a significant proliferation of allogeneic lymphocytes.Citation2–Citation6 Thus, to determine whether adi-MSCs could induce proliferation response by allogeneic lymphocytes, spleen lymphocytes were cultured with irradiated allogeneic adi-MSCs in various doses. As shown in , no response of lymphocytes was observed when allogeneic adi-MSCs were added to lymphocytes. The finding was independent of the number of adi-MSCs added to the culture. As control group, ud-MSCs also could not induce the response by allogeneic lymphocytes.
Figure 2. The effects of adi-MSCs on mixed lymphocyte cultures (MLCs). (A) Effect of adi-MSCs on lymphocyte response in primary mixed culture. Irradiated allogeneic adi- or ud-MSCs in various cell doses were added to the lymphocyte culture. Three days after coculture, cells proliferation was determined by [3H]-thymidine incorporation. (B and C) Effect of adi-MSCs on lymphocyte response in secondary mixed culture. Irradiated ‘third party’ allogeneic (B) or syngenic (C) MSCs were added to MLCs on day 0 in the designated doses per well. The number of stimulating and responding lymphocytes were kept constant in all experiments. Three days after coculture, cells proliferation was determined by [Citation3H]-thymidine incorporation. The data are expressed as mean±SD of triplicates of three separate experiments. *P<0·01, compared to the control group.
![Figure 2. The effects of adi-MSCs on mixed lymphocyte cultures (MLCs). (A) Effect of adi-MSCs on lymphocyte response in primary mixed culture. Irradiated allogeneic adi- or ud-MSCs in various cell doses were added to the lymphocyte culture. Three days after coculture, cells proliferation was determined by [3H]-thymidine incorporation. (B and C) Effect of adi-MSCs on lymphocyte response in secondary mixed culture. Irradiated ‘third party’ allogeneic (B) or syngenic (C) MSCs were added to MLCs on day 0 in the designated doses per well. The number of stimulating and responding lymphocytes were kept constant in all experiments. Three days after coculture, cells proliferation was determined by [Citation3H]-thymidine incorporation. The data are expressed as mean±SD of triplicates of three separate experiments. *P<0·01, compared to the control group.](/cms/asset/2f01a9f6-4c7e-43b7-ba58-b959c4b51447/yhem_a_11646142_f0002_b.jpg)
Effect of adi-MSCs in MLCs
To further determine whether allogeneic adi-MSCs affect the proliferation of lymphocytes in response to alloantigens, spleen lymphocytes were stimulated with irradiated allogeneic lymphocytes in the absence or presence of various concentrations of adi-MSCs, which were allogeneic to the responding lymphocytes. As shown in , the enhancement of lymphocyte proliferation in one-way MLCs occurred in a dose-dependent fashion when various allogeneic adi-MSCs were added in the two experiments. In contrast, as the control group, allogeneic ud-MSCs significantly suppressed the MLCs.
The effect of syngenic adi-MSCs in MLCs was also investigated. The same trend with enhancement of MLCs was observed when adi-MSCs were syngenic to the responding lymphocytes (). Again, we also observed the inhibitory effect of syngenic ud-MSCs on the MLCs.
Effect of adi-MSCs on mitogenic stimulation
We further investigate the effect of adi-MSCs on mitogenic stimulation. As shown in , both allogeneic and syngenic adi-MSCs could enhance mitogenic responses. In the presence of allogeneic adi-MSCs, ConA response was largely higher than that of the controls not cultured with adi-MSCs. Lymphocyte response to PWM was also enhanced with increasing cell dose of allogeneic adi-MSCs. Moreover, in the two experiments using syngenic adi-MSCs, the enhanced response to ConA or PWM was also observed, similar to that of allogeneic adi-MSCs treatment (). However, contrastly, both allogeneic and syngenic ud-MSCs showed inhibitory effect on lymphocyte response to ConA or PWM.
Figure 3. The effect of adi-MSCs on mitogen activation of lymphocytes. Lymphocytes were stimulated with ConA (5 μg/ml) or PWM (10 μg/ml) in the presence of allogeneic (A) or syngenic (B) MSCs in the indicated cell doses. After cocultured for 2 days, cells proliferation was determined by [3H]-thymidine incorporation. The data are expressed as mean±SD of triplicates of three separate experiments. *P<0·01, compared to the control group.
![Figure 3. The effect of adi-MSCs on mitogen activation of lymphocytes. Lymphocytes were stimulated with ConA (5 μg/ml) or PWM (10 μg/ml) in the presence of allogeneic (A) or syngenic (B) MSCs in the indicated cell doses. After cocultured for 2 days, cells proliferation was determined by [3H]-thymidine incorporation. The data are expressed as mean±SD of triplicates of three separate experiments. *P<0·01, compared to the control group.](/cms/asset/9ceddf46-0d73-4a34-aacb-2ce99d6b08f0/yhem_a_11646142_f0003_b.jpg)
Soluble factor is required for adi-MSCs-mediated immunostimulatory property
To determine whether allogeneic or syngenic adi-MSCs immunostimulation of lymphocytes requires intercellular contact or soluble factors, we used a transwell chamber system to separate lymphocytes from MSCs. Allogeneic or syngenic adi-MSCs in the transwell chamber system still could enhance ConA or PWM-induced lymphocytes proliferation, similar to the usual coculture system (). Furthermore, the supernatant of adi-MSCs cultures added to the test, also showed stimulatory effect on lymphocyte proliferation in a dose-dependent fashion (). Moreover, both the supernatant from allogeneic and syngenic adi-MSCs have similar enhancement of lymphocyte response by mitogen stimuli (). These data indicated that the interaction between adi-MSCs and target cells was not required for the proliferation of lymphocytes, and that the action of soluble factors was involved in the event.
Figure 4. Soluble factors were responsible for the stimulatory effect of adi-MSCs. Adi-MSCs (2×105) were seeded onto the transwell membrane of the inner chamber, and various numbers of lymphocytes from C57BL/6 mice were cultured in the lower chamber of transwell plate in the presence of ConA (5 μg/ml) or PWM (10 μg/ml). (A) Allogeneic adi-MSCs treatment group. (B) Syngenic adi-MSCs treatment group. (C) In a parallel experiment, supernatant from MSC cultures was added into the lymphocytes cultures. After 2 days, uptake of [3H]-thymidine was measured. The data are expressed as mean±SD of triplicates of three separate experiments. MSC/R indicates the MSC-to-responder cells ratio.
![Figure 4. Soluble factors were responsible for the stimulatory effect of adi-MSCs. Adi-MSCs (2×105) were seeded onto the transwell membrane of the inner chamber, and various numbers of lymphocytes from C57BL/6 mice were cultured in the lower chamber of transwell plate in the presence of ConA (5 μg/ml) or PWM (10 μg/ml). (A) Allogeneic adi-MSCs treatment group. (B) Syngenic adi-MSCs treatment group. (C) In a parallel experiment, supernatant from MSC cultures was added into the lymphocytes cultures. After 2 days, uptake of [3H]-thymidine was measured. The data are expressed as mean±SD of triplicates of three separate experiments. MSC/R indicates the MSC-to-responder cells ratio.](/cms/asset/41c404a4-dd28-4d3f-8117-ee37bba21d00/yhem_a_11646142_f0004_b.jpg)
Effect of the supernatant of adi-MSCs cultures on T-lymphocyte proliferation and activation
We further investigated the effect of supernatant of adi-MSC cultures on lymphocytes response by anti-CD3 stimulation. In the assay of anti-CD3-stimulated T-cell proliferation, we observed that adi-MSCs effectively enhanced cell proliferation of suboptimally anti-CD3-treated T cells, to a level more than that observed with anti-CD28 stimulation (). Furthermore, addition of the supernatant from cultured allogeneic or syngenic adi-MSCs into the test, T-cell proliferation was also increased in the presence of anti-CD3 (). The activation marker CD25 in the surface of T cells, was remarkably upregulated in adi-MSCs and their supernatant treated groups (). However, ud-MSCs showed inhibitory effects (). These data suggested that adipogenic differentiation might promote the secretion of costimulatory molecules of MSCs, which provided costimulatory signaling for T-cell proliferation and activation.
Figure 5. The effect of the supernatant of adi-MSCs cultures on T-lymphocyte proliferation and activation. (A) T cells were cultured with ud- or adi-MSCs at a ratio of 1∶1, in the absence or presence of anti-CD3 Ab (2 μg/ml). (B) Various titrations of the supernatant of adi-MSCs were added into T-cell culture in the presence of anti-CD3 Ab. After culture for 3 days, T-cell proliferation was determined by [3H]-thymidine incorporation. (C) Activation of T cells was determined. Allogeneic T cells were incubated for 3 days with ud- and adi-MSCs, and the supernatant of adi-MSCs in the presence of anti-CD3 Ab, and stained for the surface expression of the activation maker CD25. Dashed lines indicate isotype-matched IgG antibody control staining. (D) The level of pro-inflammatory cytokines secreted by T cells. T cells were incubated for 3 days with various titrations of the supernatant of adi-MSCs in the presence of anti-CD3 Ab, and pro-inflammatory cytokines IFN-gamma and TNF-alpha in the culture were determined by ELISA. *P<0·01, compared to the control group.
![Figure 5. The effect of the supernatant of adi-MSCs cultures on T-lymphocyte proliferation and activation. (A) T cells were cultured with ud- or adi-MSCs at a ratio of 1∶1, in the absence or presence of anti-CD3 Ab (2 μg/ml). (B) Various titrations of the supernatant of adi-MSCs were added into T-cell culture in the presence of anti-CD3 Ab. After culture for 3 days, T-cell proliferation was determined by [3H]-thymidine incorporation. (C) Activation of T cells was determined. Allogeneic T cells were incubated for 3 days with ud- and adi-MSCs, and the supernatant of adi-MSCs in the presence of anti-CD3 Ab, and stained for the surface expression of the activation maker CD25. Dashed lines indicate isotype-matched IgG antibody control staining. (D) The level of pro-inflammatory cytokines secreted by T cells. T cells were incubated for 3 days with various titrations of the supernatant of adi-MSCs in the presence of anti-CD3 Ab, and pro-inflammatory cytokines IFN-gamma and TNF-alpha in the culture were determined by ELISA. *P<0·01, compared to the control group.](/cms/asset/75b95f2d-8415-4a81-a168-e8aa0614d904/yhem_a_11646142_f0005_b.jpg)
Figure 6. The effect of the supernatant of adi-MSCs cultures on B-lymphocyte proliferation, activation, and IgG secretion. (A) B cells were cultured with ud- or adi-MSCs at a ratio of 1∶1, in the absence or presence of anti-mu Ab (2 μg/ml). (B) Various titrations of the supernatant of adi-MSCs were added into B-cell culture in the presence of anti-mu Ab. After culture for 3 days, B-cell proliferation was determined by [3H]-thymidine incorporation. (C) Activation of B cells was determined. Allogeneic T cells were incubated for 3 days with ud- and adi-MSCs, and the supernatant of adi-MSCs in the presence of anti-mu Ab, and stained for the surface expression of the activation maker CD25. Dashed lines indicate isotype-matched IgG antibody control staining. (D) The level of IgG secreted by B cells. B cells were incubated for 3 days with various titrations of the supernatant of adi-MSCs in the presence of anti-mu Ab, and IgG secretion in the culture was determined by ELISA. *P<0·01, compared to the control group.
![Figure 6. The effect of the supernatant of adi-MSCs cultures on B-lymphocyte proliferation, activation, and IgG secretion. (A) B cells were cultured with ud- or adi-MSCs at a ratio of 1∶1, in the absence or presence of anti-mu Ab (2 μg/ml). (B) Various titrations of the supernatant of adi-MSCs were added into B-cell culture in the presence of anti-mu Ab. After culture for 3 days, B-cell proliferation was determined by [3H]-thymidine incorporation. (C) Activation of B cells was determined. Allogeneic T cells were incubated for 3 days with ud- and adi-MSCs, and the supernatant of adi-MSCs in the presence of anti-mu Ab, and stained for the surface expression of the activation maker CD25. Dashed lines indicate isotype-matched IgG antibody control staining. (D) The level of IgG secreted by B cells. B cells were incubated for 3 days with various titrations of the supernatant of adi-MSCs in the presence of anti-mu Ab, and IgG secretion in the culture was determined by ELISA. *P<0·01, compared to the control group.](/cms/asset/6b626fd0-f66a-4818-b637-85aa90c2a101/yhem_a_11646142_f0006_b.jpg)
The effect of the supernatant of cultured adi-MSCs on the production of pro-inflammatory cytokines by T cells was also investigated. After anti-CD3 stimulation alone, IFN-gamma and TNF-alpha produced by T cells were moderately increased. However, compared to the control group, the supernatant of adi-MSCs significantly promoted both cytokines production in a dose-dependent fashion ().
Effect of the supernatant of adi-MSCs culture on B-lymphocyte proliferation and activation
To analyze the stimulatory effect of the supernatant of adi-MSCs on B lymphocyte, B cells were cocultured with adi-MSCs or their supernatant in the presence of anti-mu treatment. We observed the costimulatory effect of adi-MSCs on B-cell response. Cell proliferation of anti-mu-treated B cells could be significantly enhanced by the adi-MSCs (). We also observed the similar effect of the supernatant from both allogeneic and syngenic adi-MSCs on B-cell proliferation in a dose-dependent manner (). In addition, flow cytometry data revealed that CD25 expression was also upregulated in B cells by adi-MSCs and the supernatants induction, compared to that of the group by anti-mu treatment alone (). In contrast, ud-MSCs also showed inhibitory effects on B-cell response ().
To clarify whether the supernatant of adi-MSCs also affect the function of B cells, an ELISA was performed to measure the level of IgG secretion by B cells. Similarly, IgG secretion was significantly released with increasing concentration of the supernatant compared to that of the group by anti-mu treatment alone (). This result suggested that the secreted factor by adi-MSCs might also contribute to proliferation and activation of B cells.
Involvement of the secreted BAFF molecule in adi-MSCs-mediated immunostimulation
The aforementioned data indicated that soluble factor by adi-MSCs contributed to T and B-cell responses. It is well known that BAFF plays important roles in T or B-cell proliferation, activation, and B-cell maturation and survival. Moreover, human Simpson–Golabi–Behmel syndrome and 3T3-L1 adipocytes have been shown to express TNFSF13B (another term for BAFF)Citation29,Citation30 Thus, we examined mRNA and protein expression levels of BAFF in adi-MSCs using conventional reverse transcription polymerase chain reaction and western blot analysis, respectively. BAFF mRNA expression levels were increased as differentiation progressed (). Similarly, protein expression levels were increased in accordance with differentiation (). Ud-MSCs showed no expression of the molecule (). However, using flow cytometry analysis, membrane-bound form of BAFF was not detected in adi-MSCs (data not shown). In contrast, we found that increasing amount of secreted BAFF in the culture supernatant was detected by ELISA (), suggesting that the secreted BAFF molecule by adi-MSCs might be involved in the these events.
Figure 7. Secreted BAFF molecule mediated the immunostimulation of adi-MSCs. (A) mRNA level of BAFF increased during adipocyte differentiation of MSCs. Data are expressed relative to the mRNA levels of beta-actin and are the mean±SD of six independent experiments. *P<0·01 versus day 0. (B) Protein level of BAFF on MSCs during adipocyte differentiation was determined by western blotting. Data are expressed relative to the protein level of GAPDH and are the mean±SD of six independent experiments. *P<0·01, versus day 0. (C) Soluble BAFF level in the supernatant of adi-MSCs. The supernatant of MSCs during adipocyte differentiation was harvested and determined for BAFF concentration by ELISA. The data are expressed as mean±SD of triplicates of three separate experiments. *P<0·01 versus day 0. (D–G) Costimulation effect of adi-MSCs or the supernatant of adi-MSCs on lymphocytes was suppressed by anti-BAFF treatment. Anti-CD3-treated T or anti-mu Ab-treated B cells were cultured with adi-MSCs at 1∶1 ratio or the supernatant of adi-MSCs in the presence of anti-BAFF mAb (10 μg/ml) for 3 days. Lymphocytes proliferation was determined by [3H]-thymidine incorporation. (F and G) The expression of activation marker CD25 in lymphocytes was reduced with anti-BAFF treatment. The data are expressed as mean±SD of triplicates of three separate experiments. *P<0·01, compared to the control cultures.
![Figure 7. Secreted BAFF molecule mediated the immunostimulation of adi-MSCs. (A) mRNA level of BAFF increased during adipocyte differentiation of MSCs. Data are expressed relative to the mRNA levels of beta-actin and are the mean±SD of six independent experiments. *P<0·01 versus day 0. (B) Protein level of BAFF on MSCs during adipocyte differentiation was determined by western blotting. Data are expressed relative to the protein level of GAPDH and are the mean±SD of six independent experiments. *P<0·01, versus day 0. (C) Soluble BAFF level in the supernatant of adi-MSCs. The supernatant of MSCs during adipocyte differentiation was harvested and determined for BAFF concentration by ELISA. The data are expressed as mean±SD of triplicates of three separate experiments. *P<0·01 versus day 0. (D–G) Costimulation effect of adi-MSCs or the supernatant of adi-MSCs on lymphocytes was suppressed by anti-BAFF treatment. Anti-CD3-treated T or anti-mu Ab-treated B cells were cultured with adi-MSCs at 1∶1 ratio or the supernatant of adi-MSCs in the presence of anti-BAFF mAb (10 μg/ml) for 3 days. Lymphocytes proliferation was determined by [3H]-thymidine incorporation. (F and G) The expression of activation marker CD25 in lymphocytes was reduced with anti-BAFF treatment. The data are expressed as mean±SD of triplicates of three separate experiments. *P<0·01, compared to the control cultures.](/cms/asset/ceef8873-52dd-429e-9708-e95c3523af15/yhem_a_11646142_f0007_b.jpg)
To further examine whether BAFF molecule was responsible for modulating the immunostimulatory property of adi-MSCs, anti-BAFF monoclonal antibody was used for blocking experiments. As shown in , the adi-MSCs or their cultured supernatant significantly enhanced anti-CD3 or anti-mu stimulation of lymphocyte response, whereas addition of anti-BAFF antibody decreased the effect. Moreover, the expression of activation marker CD25 on lymphocytes was also significantly reduced by anti-BAFF treatment, compared to the control group ().
Discussion
MSCs are the focus of regenerative and immunological studies due to their potential usage in tissue engineering and immunotherapeutical approaches. Because of their differentiation properties, easy accessibility, and proliferative capacity, MSCs have emerged as a promising therapeutic modality for tissue regeneration. Although accumulative evidence demonstrated that MSCs modulated several immume cells functions, including T, B, and DC cells,Citation2–Citation10 MSCs might change certain properties to suit specific functions of their terminally differentiated progenies after adopting different lineage pathways. In contrast to numerous and excellent publications describing the role of MSCs in immune regulation,Citation31,Citation32 investigations on the immune regulatory capacity of differentiated MSCs are rare.Citation33
It is well known that MSCs can express human leukocyte antigen MHC class I and negligible levels of both MHC class II, and they do not express B7-1, B7-2, CD40, or CD40L.Citation34,Citation35 In the present study, compared to previous experiments in which MSCs had an inhibitory effect on MNCs (including some T cells and B lymphocytes), the adi-MSCs took a dissimilar effect on immune cells, and facilitated lymphocytes proliferation and activation. Moreover, transwell experiments demonstrated that soluble factor, cell–cell contact-independent, was required for this event. Thus, we postulate that adipogenic differentiation might not upregulate some costimulatory molecules or MHC-related molecules in the surface of adi-MSCs. Furthermore, we found that adi-MSCs not only could stimulate the response of T lymphocytes, but also enhance B-lymphocyte proliferation through souble factors. Accumulative evidence demonstrated that BAFF (also known as BLyS, TALL-1, zTNF-4, THANK, and TNFSF13B), is an important regulator of B-cell and T-cell responses.Citation36–Citation38 Recently, Kim et al. reported the expression of BAFF and BAFF receptors in 3T3-L1 adipocytes.Citation29 Indeed, we observed that adi-MSCs could secrete BAFF costimulatory molecule, which stimulated B and T-cell proliferation and activation. Of course, whether adi-MSCs could affect other immune cells function needs further investigation.
While there have been reports of fat grafting for over a century, the results and success of this approach, even using autologous fat, have been variable.Citation39 In tissue engineering, the limited amount of preadipocyte cells that can be obtained from an individual, the limited life span, and incapacity to withstand freeze/thaw procedures are drawbacks. MSCs have the potential to substitute for adipocyte owing to their capacity for immortalization, self-renewal, multi-differentiation potential, and the fact that they can be frozen and thawed repeatedly and used when needed. Our findings could be of relevance for clinical applications of MSCs. Though MSCs possess a hypoimmunogenic character,Citation12,Citation40 further investigation and observation should be performed when they are used to differentiate into such terminal cells as adipocytes in vivo.
In summary, this study represents what we believe to be the first report to demonstrate that adipogenic differentiation could alter the immunoregulatory property of MSCs, leading to stimulation of T and B-cell response. The soluble BAFF molecule secreted by the adi-MSCs may be responsible for this event.
This study was partially supported by National Natural Science Foundation of China (30771093) and Shanghai Pujiang Program (07pj14020) to T. Chen.
References
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al.. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143–7.
- Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, et al.. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002;99:3838–43.
- Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringdén O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol 2003;57:11–20.
- Aggarwal S, Pittenger M. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005;105:1815–22.
- Beyth S, Borovsky Z, Mevorach D, Liebergall M, Gazit Z, Aslan H, et al.. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T cell unresponsiveness. Blood 2005;105:2214–9.
- Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, et al.. Human mesenchymal stem cells modulate B-cell functions. Blood 2006;107:367–72.
- Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, Moretta L. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood 2006;107:1484–90.
- Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells 2006;24:74–85.
- Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, et al.. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood 2005;105:4120–6.
- Ramasamy R, Fazekasova H, Lam EW, Soeiro I, Lombardi G, Dazzi F. Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation 2007;83:71–6.
- Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, et al..: Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 2003, 101, 3722–3729.
- Potian JA, Aviv H, Ponzio NM, Harrison JS, Rameshwar P.: Veto-like activity of mesenchymal stem cells: functional discrimination between cellular responses to alloantigens and recall antigens. J Immunol 2003, 171, 3426–3434.
- Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC.: Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation 2003, 75, 389–397.
- Djouad F, Plence P, Bony C, Tropel P, Apparailly F, Sany J, et al.. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood 2003;102:3837–44.
- Meisel R, Zibert A, Laryea M, Gobel U, Daubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase- mediated tryptophan degradation. Blood 2004;103:4619–21.
- Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, et al.. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 2002;30:42–8.
- Lazarus HM, Koc ON, Devine SM, Curtin P, Maziarz RT, Holland HK, et al.. Cotransplantation of HLA-identical sibling culture- expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant 2005;11:389–98.
- Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, et al.. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 2004;363:1439–41.
- Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, et al.. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T cell anergy. Blood 2005;106:1755–61.
- Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, et al.. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc Natl Acad Sci USA 2002;99:8932–7.
- Koc ON, Day J, Nieder M, Gerson SL, Lazarus HM, Krivit W. Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrophy (MLD) and Hurler syndrome (MPS-IH). Bone Marrow Transplant 2002;30:215–22.
- Koc ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI, et al.. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol 2000;18:307–16.
- Liechty KW, MacKenzie TC, Shaaban AF, Radu A, Moseley AB, Deans R, et al.. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med 2000;6:1282–6.
- MacDonald DJ, Luo J, Saito T, Duong M, Bernier PL, Chiu RC, et al.. Persistence of marrow stromal cells implanted into acutely infarcted myocardium: Observations in a xenotransplant model. J Thorac Cardiovasc Surg 2005;130:1114–21.
- Grinnemo KH, Mansson A, Dellgren G, Klingberg D, Wardell E, Drvota V, et al.. Xenoreactivity and engraftment of human mesenchymal stem cells transplanted into infarcted rat myocardium. J Thorac Cardiovasc Surg 2004;127:1293–300.
- Fantuzzi G. Adipose tissue, adipokines and inflammation. J Allergy Clin Immunol 2005;115:911–9.
- Kim YH, Choi BH, Do MS. The interaction of adipose tissue with immune system and related inflammatory molecules. Immune Network 2006;6:169–78.
- Tilg H, Moschen AR. Adipokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 2006;6:772–83.
- Kim YH, Choi BH, Cheon HG, Do MS. B cell activation factor (BAFF) is a novel adipokine that links obesity and inflammation. Exp Mol Med 2009;41:208–16.
- Do MS, Jeong HS, Choi BH, Hunter L, Langley S, Pazmany L, et al.. Inflammatory gene expression patterns revealed by DNA microarray anaysis in TNF-α-treated SGBS human adipocytes. Yonsei Med J 2006;47:729–36.
- Rasmusson I. Immune modulation by mesenchymal stem cells. Exp Cell Res 2006;312:2169–79.
- Uccelli A, Moretta L, Pistoia V. Immunoregulatory function of mesenchymal stem cells. Eur J Immunol 2006;36:2566–73.
- Chen X, McClurg A, Zhou GQ, McCaigue M, Armstrong MA, Li G. Chondrogenic differentiation alters the immunosuppressive property of bone marrow-derived mesenchymal stem cells, and the effect is partially due to the upregulated expression of B7 molecules. Stem Cells 2007;25:364–70.
- Deans RJ, Moseley AB. Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol 2000;28:875–84.
- Tse WT, Beyer W, Pendleton JD, D’Andrea A, Guinan EC. Bone marrow derived mesenchymal stem cells suppress T cell activation without inducing allogeneic anergy. Blood 2000;96:1034a.
- Huard B, Schneider P, Mauri D, Tschopp J, French LE. T cell co-stimulation by the TNF ligand BAFF. J Immunol 2001;167:6225–31.
- Ng LG, Sutherland AP, Newton R, Qian F, Cachero TG, Scott ML, et al.. B cell-activating factor belonging to the TNF family (BAFF)-R is the principal BAFF receptor facilitating BAFF costimulation of circulating T and B cells. J Immunol 2004;173:807–17.
- Schneider P, MacKay F, Steiner V, Hofmann K, Bodmer JL, Holler N, et al.. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med 1999;189:1747–56.
- Tran TT, Kahn CR. Transplantation of adipose tissue and stem cells: role in metabolism and disease. Nat Rev Endocrinol 2010;6:195–213.
- Maitra B, Szekely E, Gjini K, Laughlin MJ, Dennis J, Haynesworth SE, et al.. Human mesenchymal stem cells support unrelated donor hematopoietic stem cells and suppress T-cell activation. Bone Marrow Transplant 2004;33:597–604.