Abstract
Objective: Recent reports show the adverse impact of pre-transplantation iron overload on the outcome of haematopoietic stem cell transplantation (HSCT). We studied the pre-transplantation serum iron (SI) parameters including prohepcidin levels — a regulatory peptide of systemic iron homeostasis — and their role in early post-transplantation toxicities in allogeneic HSCT recipients.
Patients and methods: One hundred consecutive patients [36 women and 64 men; median age 27·5 years (range 16–63 years)] who underwent allogeneic HSCT between September 2003 and October 2007 at Gazi University were included in the study.
Results: Pre-transplantation serum prohepcidin levels did not show correlation with SI parameters and interleukin-6 levels (P>0·05). Prohepcidin levels were inversely correlated with the National Cancer Institute grade of mucositis (P = 0·060), neutropenic fever (P<0·001), and the number of days with febrile neutropenia (P = 0·003). SI levels were correlated with the severity of hepatotoxicity (P = 0·015) while pre-transplantation transferrin saturation levels were positively correlated with the severity of hepatotoxicity (P = 0·055), pulmonary toxicity (P = 0·032), and sinusoidal obstruction syndrome (P = 0·049). Pre-transplantation serum ferritin levels were positively correlated with the development of sinusoidal obstruction syndrome (P = 0·010) and inversely correlated with the day of neutrophil engraftment (P = 0·012). Overall survival was 41·26% with a median follow-up time of 13 months (range 0·0–60 months). Pre-transplantation serum prohepcidin levels and iron overload were not associated with survival in Cox regression analysis.
Conclusion: Our results suggest that pre-transplantation iron parameters and prohepcidin levels might predict some of the early post-transplantation toxicities, however, without an impact on overall survival.
Introduction
Early post-transplantation toxic and infectious complications cause significant morbidity and mortality after haematopoietic stem cell transplantation (HSCT). Clinically significant iron overload (IO) is a frequent condition in HSCT candidates due to blood transfusions, increased intestinal iron absorption, ineffective haematopoiesis, and iron released by chemo/radiotherapy-induced tissue injury. The rate and amount of tissue iron accumulation are the two most important factors that determine the risk of iron-related organ toxicity.Citation1,Citation2 Recent studies have suggested that IO is associated with post-transplantation toxicities including sinusoidal obstruction syndrome (SOS), increased susceptibility to infection, disease free survival (DFS), early transplant-related mortality (TRM), and overall survival (OS) in patients with haematological disorders.Citation1,Citation3–Citation8 Serum ferritin has a dual action, not only as a surrogate marker of iron stores, but also as an acute phase marker which might reflect infection, inflammation, or the presence of a malignant disease. Hepcidin, a peptide hormone predominantly expressed in the liver is the homeostatic regulator of iron metabolism and a mediator of host defence and inflammation.Citation9 Pre-prohepcidin, the 84 aminoacid precursor protein, undergoes enzymatic cleavage in the liver, resulting in the export of a 64 aminoacid prohepcidin into the endoplasmic reticulin lumen. Proprotein convertase enzyme leads to the formation of mature bioactive 25 aminoacid form of hepcidin.Citation10 Hepcidin induces the internalization and degradation of transmembrane iron exporter ferroportin which is present on macrophages, hepatocytes, and basolateral site of enterocytes. Body iron levels, inflammation, and erythropoietic activity appear to be the main regulators of hepcidin.Citation11 Although hepcidin mRNA levels were shown to be increased in inflammatory conditions, anaemia is another regulator of hepcidin synthesis by lowering serum levels. The exact mechanism by which body iron stores influence hepcidin metabolism is unknown. In conditions of increased serum transferrin saturation (TS) with iron, free HFE protein at the cell surface triggers a signalling cascade leading to increased synthesis of hepcidin.Citation12
The aim of our study was to determine the relation of serum iron (SI) parameters with prohepcidin as a regulator of iron homeostasis and their impact on early post-transplantation organ toxicities, infectious complications, and TRM in HSCT recipients. We also analysed the markers of acute phase response [C-reactive protein (CRP), erythrocyte sedimentation rate (ESR, mm/hour), and interleukin-6 (IL-6)] and their interaction with iron parameters and prohepcidin.
Patients and Methods
We studied the pre-transplantation serum samples and reviewed the records of 100 consecutive patients [36 women and 64 men; median age 27·5 years (range 16–63 years)] who underwent allogeneic stem cell transplantation between September 2003 and October 2007 at our institution. Patient characteristics are shown in . A variety of conditioning regimens were used, while the most common ones were the combination of busulphan (Bu)–cyclophfosphamide (Cy), fludarabine–Bu–Cy, and total body irradiation–Cy. Graft-versus-host disease prophylaxis consisted of the combination of cyclosporine A and long-term methotrexate. The Local Ethics Committee of our university approved this study and written informed consent was obtained from the patients.
Table 1. Clinical characteristics of patients
Data collection
Pre-transplantation patient records were used for serum levels of SI, serum iron binding capacity, TS, ferritin, CRP, and ESR. Baseline serum samples were collected prior to transplantation and stored until use, at −80°C for the analysis of serum hepcidin and IL-6 levels. Serum levels of IL-6 (BioSource Immunoassay Kit; Biosource International Inc., Camarillo, CA, USA) and hepcidin (DRG Hepcidin Prohormone ELISA Kit; DRG International Inc., Marburg, Germany) were assayed using enzyme-linked immunosorbent assay kits according to the manufacturers protocols. Data of toxic and infectious events (organ toxicities, development of SOS, days with febrile neutropenia, presence of fungal infection, transfusion requirement, and engraftment days) in the first 3 months after HSCT were obtained from the toxicity forms within the patient charts. Toxicity was graded from 0 to 4 as defined in the Common Toxicity Criteria from the National Cancer Institute (NCI) CTC version 2.0, 30 April 1999 (http://ctep.cancer.gov/forms/ctcaev3.pdf)
Statistical analysis
Results were analysed with the software programme SPSS for Windows (version 11.; SPSS, Chicago, IL). Means and standard deviations were calculated for all variables. Differences between categorical variables were measured by the chi-square test, and differences between means were calculated with the Student’s t-test. Relationships between iron parameters, hepcidin, acute phase reactants, and toxicities were explored using Pearson’s correlation. Logistic regression was used to evaluate variables related to the appearance of toxicities. Outcomes including OS, DFS, and TRM were estimated using the Kaplan–Meier method. Log-rank univariate comparisons were used to evaluate OS and TRM in univariate survival analysis. Cox proportional hazards analysis was used to identify univariable and multivariable risk factors for OS, TRM, and DFS. All tests were two-tailed and results were assessed as statistically significant when P values were <0·05.
Results
Data of 100 allogeneic HSCT recipients were available for the analysis. Pre-transplantation mean serum prohepcidin levels [137·07±54·69 ng/ml (range 35–320 ng/ml)] showed a near significant inverse correlation with mean serum ferritin [1222·25±1304·37 μg/dl (range 11·4–9066·1 μg/dl)] (r = −0·201; P = 0·051), and IL-6 levels [36·99±56·23 pg/ml (range 2·93–273·48 pg/ml)] (r = −0·190; P = 0·059). Pre-transplantation serum ferritin levels demonstrated a significant positive correlation with serum CRP, ESR, and IL-6 levels [(r = 0·324; P = 0·032), (r = 0·368; P = 0·015), and (r = 0·567; P = 0·000) respectively]. Pre-transplantation serum IL-6 levels showed a significant positive correlation with acute phase reactants [CRP (r = 0·706; P = 0·000), ESR (r = 0·610; P = 0·000), and fibrinogen (r = 0·456; P = 0·002)] and serum ferritin levels (r = 0·567; P = 0·002). Correlation analysis of NCI grade of toxicities, iron parameters, and serum prohepcidin levels is given in for allogeneic HSCT recipients.
Table 2. Correlation between serum iron, ferritin, prohepcidin, IL-6, CRP levels, and early post-transplant complications in allogeneic stem cell transplantation
Mucositis: Serum prohepcidin levels showed a near significant inverse correlation with the grade of mucositis (r = −0·190; P = 0·060).
Cardiac toxicity: Pre-transplantation SI parameters, acute phase reactants, and prohepcidin levels showed no correlation with early cardiac toxicity in allogeneic HSCT recipients (P>0·05).
Pulmonary toxicity: Only pre-transplantation TS and serum IL-6 levels showed a positive correlation with the grade of pulmonary toxicities [(r = 0·221; P = 0·032) and (r = 0·262; P = 0·009) respectively].
Hepatic toxicity: Pre-transplantation SI and TS levels showed a positive correlation with the grade of hepatic toxicity [(r = 0·249; P = 0·015) and (r = 0·198; P = 0·055) respectively]. Similarly serum IL-6 levels and CRP showed a significant correlation with the grade of hepatotoxicity [(r = 0·211; P = 0·036) and (r = 0·207; P = 0·042) respectively].
SOS: Diagnosis of SOS was made according to Seattle criteria.Citation13 TS and serum ferritin levels showed a significant positive correlation with the development of SOS [(r = 0·203; P = 0·049) and (r = 0·263; P = 0·010) respectively)] in allogeneic HSCT recipients. Pre-transplantation TS, ferritin, and CRP levels were significantly higher in patients with SOS compared to HSCT recipients without SOS respectively [(69·35±72·39% versus 46·87±37·17%; P = 0·049), (2670·68±5148·88 μg/dl versus 982·89±810·70 μg/dl; P = 0·010) and (40·68±51·87 mg/dl versus 18·06±42·93 mg/dl; P = 0·027)].
Febrile neutropenia: Pre-transplantation serum prohepcidin levels showed a significant negative correlation (r = −0·298; P = 0·003) while serum IL-6 levels showed a significant positive correlation (r = 0·230; P = 0·023) with the number of febrile days during early post-transplant period. Pre-transplantation serum prohepcidin levels also showed a significant negative correlation with the grade of neutropenic fever (r = −0·387; P = 0·000). We compared the cumulative incidences of bacterial, fungal, and cytomegalovirus infections at day 100 according to pre-transplantation IO and serum prohepcidin levels. There was no correlation of pre-transplantation serum prohepcidin levels and iron parameters with the presence of bacterial infection in allogeneic HSCT recipients (P>0·05). There was a near significant negative correlation between pre-transplantation serum prohepcidin levels and fungal infections. Serum prohepcidin levels were 127·44±46·32 ng/ml in patients with fungal infections while it was 148·29±60·85 ng/ml in patients without fungal infection (r = −0·192; P = 0·059).
Engraftment: SI levels and TS showed a significant positive correlation with the day of platelet engraftment [(r = 0·294; P = 0·007) and (r = 0·361; P = 0·001) respectively].
Transfusion requirement: SI levels and TS showed a significant positive correlation with the requirement of erythrocyte transfusion [(r = 0·366; P = 0·000) and (r = 0·562; P = 0·000) respectively]. Pre-transplantation levels of SI, TS, ferritin, and IL-6 showed a significant positive correlation with platelet transfusion requirement in allogeneic HSCT recipients [(r = 0·262; P = 0·011), (r = 0·656; P = 0·000), (r = 0·287; P = 0·005), and (r = 0·241; P = 0·018) respectively].
Others: Pre-transplantation SI parameters, acute phase reactants, and prohepcidin levels did not show any correlation with the grade of early post-transplant renal toxicity, cytomegalovirus reactivation, and the development of acute graft versus host disease (P>0·05).
Univariate regression analysis: Variables significant in correlation analysis were evaluated in univariate regression analysis. A near significant association was present between lower serum hepcidin levels and higher NCI grade of mucositis in allogeneic HSCT recipients (P = 0·060). The number of febrile days and higher NCI grade of neutropenic fever was associated with low serum prohepcidin levels (P = 0·003 and P = 0·000 respectively). Pre-transplantation TS and serum IL-6 levels were associated with the higher NCI grade of pulmonary toxicities (P = 0·032 and P = 0·009 respectively). Higher serum TS was significantly associated with the higher NCI grade of hepatotoxicity, development of SOS, late platelet engraftment, increased erythrocyte, and platelet transfusion requirement (P = 0·055, P = 0·049, P = 0·001, P = 0·000, and P = 0·000 respectively). Higher pre-transplantation SI levels were significantly associated with the higher NCI grade of hepatotoxicity, delayed platelet engraftment, increased erythrocyte, and platelet transfusion requirement (P = 0·015, P = 0·007, P = 0·000, and P = 0·011 respectively). Higher pre-transplantation serum ferritin levels were associated with the increased platelet transfusion requirement (P = 0·005).
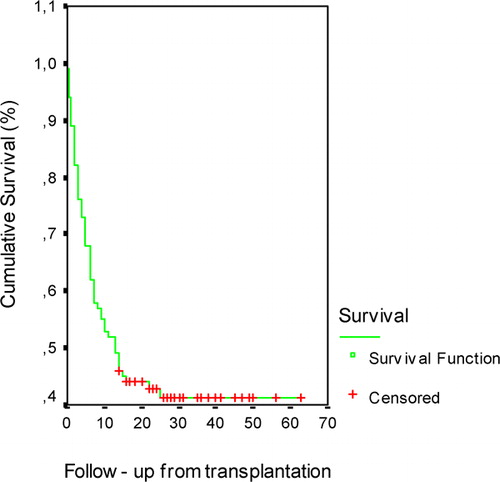
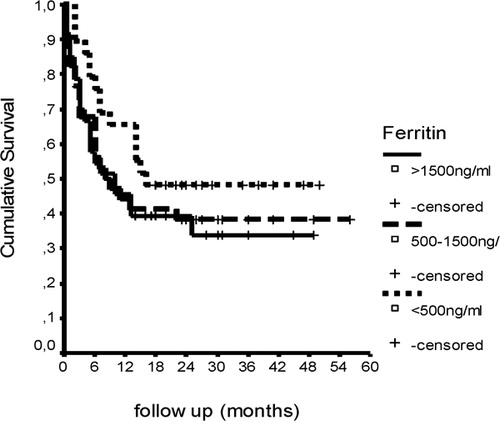
OS: Outcomes of patients were estimated using the Kaplan–Meier method. First 100-day survival was 76% and OS was 41·26% (95% CI: 7·13–18·87) with a median survival time of 13 months (range 0·0–63 months) () in allogeneic HSCT recipients consisting the study cohort. No correlation was found between pre-transplantation serum ferritin levels and OS (). In Cox regression analysis, pre-transplantation serum prohepcidin levels and iron parameters did not show any correlation with OS in allogeneic HSCT recipients (P>0·05).
Discussion
HSCT recipients are at increased risk of developing IO. Prolonged dyserythropoiesis, transfusion burden, increased growth differentiation factor 15 (GDF-15), increased intestinal iron absorption due to mucositis, release of iron from injured tissues, and the tumour cells after chemotherapy and radiotherapy are the main underlying causes of iron-overloaded state in HSCT recipients.Citation14,Citation15 Several recent reports have demonstrated an association with pre-transplant IO and early post-transplant toxicities in HSCT recipients.Citation6–Citation8
In the present study, we analysed the role of pre-transplantation IO and serum levels of iron regulatory protein prohepcidin on early transplant outcome. We found that pre-transplantation serum prohepcidin levels showed a near significant negative correlation with serum ferritin and IL-6 levels in allogeneic HSCT recipients. The inverse correlation with prohepcidin and ferritin is surprising as conditions associated with IO result in overexpression of hepcidin mRNA in hepatocytes which is a regulatory response defending against adverse effects of IO.Citation16 Increased hepcidin released by the liver negatively regulates the expression of divalent metal transporter 1 in the enterocytes and inhibits intestinal iron absorption by the enterocyte.Citation17 On the other hand, similar to our results hepcidin levels have been demonstrated to be significantly low in thalassaemia major patients who are heavily iron overloaded and this contradiction has been explained with increased GDF-15 levels.Citation18 Decreased prohepcidin levels might be the cause rather than being the consequence of IO. Similarly the inverse correlation with IL-6 and prohepcidin is rather confusing. It is well known that inflammatory cytokines, IL-6 in particular activates hepcidin transcription which in turn decreases iron absorption contributes to the anaemia associated with many chronic disease and anaemia of cancer and infection.Citation19 In this respect, a positive rather than an inverse correlation is expected with IL-6 and prohepcidin. However, IL- 6 and inflammation also might be the consequence of IO rather than being the cause of it, and suggests a possible variability of iron hemostasis and regulation in various haematological disorders requiring HSCT. Matched degree of IO is known to present with different patterns of tissue distribution of iron and iron regulatory proteins. Extrahepatic manifestations of IO and the levels of GDF-15 for example are different in sickle cell anaemia and thalassaemia major.Citation20 Hepcidin on the other hand may not be the sole iron regulatory protein contributing to the pathogenesis of IO in HSCT recipients.
Pre-transplantation serum ferritin levels demonstrated a significant positive correlation with serum CRP, ESR, and IL-6 levels which might predict the presence of inflammatory condition prior to transplantation. Another possibility is that IO might have triggered inflammation and inflammatory markers through oxidative stress-induced tissue injury.Citation21,Citation22 A near significant association was present between lower serum prohepcidin levels and higher NCI grade of mucositis in allogeneic HSCT recipients (P = 0·060). Whereas the number of febrile days and higher NCI grade of neutropenic fever was significantly associated with low serum prohepcidin levels (P = 0·003 and P = 0·000 respectively). In the light of the near significant inverse correlation with prohepcidin and ferritin and IL-6, the lower prohepcidin levels seem to be contributing to the IO which in turn causes decreased defence against infections. IO is known to cause impaired cellular immunity, chemotaxis, and phagocytosis which increase the susceptibility to infections.Citation23–Citation28 Recently Kanda et al. reported the association between pre-transplantation high serum hepcidin levels and the increased incidence of documented bacterial infections early after allogeneic HSCT.Citation15 We have not found a similar association with documented infections, whereas both the grade and the number of days with febrile neutropenia were higher in patients with lower prohepcidin levels suggesting an association with IO and infection.
Although iron is a critical element for cell function, it is potentially toxic to the host via free radicals. The increase in serum non-transferrin-bound iron has been observed early post-transplantation due to conditioning regimen-related cell damage and release of iron from the bone marrow and liver. This free form of iron can mediate the production of reactive oxygen species and may cause organ toxicity in the early post-transplant period.Citation29–Citation31 Increased iron in the gastrointestinal mucosa as a result of increased iron absorption due to low prohepcidin levels in the present study might be a factor contributing to the degree of mucositis by causing oxidative stress and decreased antioxidant capacity.Citation32,Citation33
Serum TS and IL-6 levels showed a significant positive correlation with the NCI grade of pulmonary toxicities. Our group demonstrated a similar association with invasive pulmonary fungal infections and IO.Citation24 Whereas SI and TS showed a significant positive correlation with the grade of hepatic toxicity and TS and ferritin levels showed a significant positive correlation with the development of SOS. Similarly a recent report has documented elevated levels of ferritin as a risk factor for SOS and a value of 1000 ng/ml was claimed to show the best sensitivity and specificity with the development of SOS.Citation34
SOS is one of the most common life-threatening complications of HSCT. Morado et al. were the first to demonstrate the association of elevated levels of ferritin and the development of SOS in patients undergoing autologous HSCT.Citation8 We confirm the negative impact of pre-transplantation IO on the development of SOS in the present study. A significant positive correlation between serum ferritin levels and histological grade of iron in the hepatocytes has been reported both in patients with elevated liver enzymes late after HSCT and early in an autopsy series of HSCT patients dying at 50–100 days after transplantation.Citation35–Citation37 On the other hand, IO is one of the most common aetiological factors causing liver dysfunction after HSCT.Citation38
SI and TS levels showed a positive correlation with the day of platelet engraftment and the requirement of erythrocyte transfusion. Pre-transplantation levels of SI, TS, ferritin, and IL-6 have also shown a significant positive correlation with the requirement of platelet transfusion. Patients with high pre-transplantation IL-6 and iron parameters might indicate and predict the patients with a more aggressive disease behaviour or complicated course with infection and/or inflammation thus causing a more complicated transplant course. It should also be noted that patients with SOS also have increased platelet transfusion requirement.
Pre-transplantation SI parameters, acute phase reactants, and prohepcidin levels did not show any correlation with the grade of early post-transplant renal toxicity, cytomegalovirus reactivation, and the development of acute graft versus host disease in our allogeneic HSCT cohort.
Cox regression analysis of pre-transplantation serum prohepcidin levels and iron parameters did not show any correlation with OS in allogeneic HSCT recipients in the current study.
There are conflicting reports regarding the impact of pre-transplantation iron status on overall survival. While Armand et al. have found an impact of elevated ferritin on OS and DFS after allogeneic HSCT, this impact was limited to patients with acute myeloid leukaemia and myelodysplastic syndrome.Citation5 On the other hand, Mahindra et al. attributed the decreased OS to relapse mortality.Citation39 We have not demonstrated an impact of pre-transplantation prohepcidin levels and/or iron parameters on OS; however, it should be mentioned that a subgroup analysis according to underlying disease and the cause of death was not made in the current study.
The role of serum ferritin as an indicator of IO is questioned due to its dual role as a marker of body iron content and inflammatory marker. As a matter of fact, we have demonstrated a positive correlation with pre-transplantation serum ferritin levels and acute phase reactants suggesting that infection and/or inflammation might be compounding the picture. Iron particularly in its free form might as well trigger inflammatory reaction and acute phase response as previously mentioned.Citation40,Citation41 We have not found any significant correlation with serum prohepcidin levels and iron parameters and acute phase reactants. This may be due to the different control mechanisms of prohepcidin synthesis.Citation9–Citation11,Citation42
In summary, IO and serum prohepcidin are associated with the severity of various early complications and have a negative impact on the clinical outcome of allogeneic HSCT. The intricate interaction between the iron parameters and the iron regulatory protein hepcidin in the complex milieu of HSCT remains to be validated in further prospective studies. Pre-transplantation increased body iron stores and iron regulatory proteins seemed to be important in the clinical risk assessment of patients undergoing HSCT.
This work was supported by a research grant of Turkish Society of Hematology. We are also grateful to all the members of our transplant team for their dedicated patient care.
References
- Majhail NS, Lazarus HM, Burns LJ. Iron overload in hematopoietic cell transplantation. Bone Marrow Transplant 2008;41:997–1003.
- Knovich MA, Storey JA, Coffman LG, Torti SV, Torti FM. Ferritin for the clinician. Blood Rev 2009;23:95–104.
- Lucarelli G, Gaziev J. Advances in the allogeneic transplantation for thalassemia. Blood Rev 2008;22:53–63.
- Gaziev J, Sodani P, Polchi P, Andreani M, Lucarelli G. Bone marrow transplantation in adults with thalassemia: treatment and long-term follow-up. Ann NY Acad Sci 2005;1054:196–205.
- Armand P, Kim HT, Cutler CS, Ho VT, Koreth J, Alyea EP, et al.. Prognostic impact of elevated pretransplantation serum ferritin in patients undergoing myeloablative stem cell transplantation. Blood 2007;109:4586–8.
- Altes A, Remacha AF, Sureda A, Martino R, Briones J, Canals C, et al.. Iron overload might increase transplant-related mortality in hematopoietic stem cell transplantation. Bone Marrow Transplant 2002;29:987–9.
- Altes A, Remacha AF, Sarda P, Baiget M, Sureda A, Martino R, et al.. Early clinical impact of iron overload in stem cell transplantation. A prospective study. Ann Hematol 2007;86:443–7.
- Morado M, Ojeda E, Garcia-Bustos J, Aguado MJ, Arrieta R, Quevedo E, et al.. Serum ferritin as a risk factor for veno-occlusive disease of the liver. Prospective cohort study. Hematology 2000;6:505–12.
- Ganz T. Hepcidin — a regulator of intestinal iron absorbtion and iron recycling by macrophages. Best Pract Res Clin Haematol 2005;18:171–82.
- Kemna EHJM, Tjalsma H, Willems HL, Swinkels DW. Hepcidin: from discovery to differential diagnosis. Haematologica 2008;93:90–7.
- Ganz T. Hepcidin in iron metabolism. Curr Opin Hematol 2004;11:251–4.
- Vyoral D, Petrak J. Hepcidin: a direct link between iron metabolism and immunity. Int J Biochem Cell Biol 2005;37:1768–73.
- McDonald GB, Hinds MS, Fisher LD, Schoch HG, Wolford JL, Banaji M, et al.. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med 1993;118:255–67.
- Koreth J, Antin JH. Iron overload in hematologic malignancies and outcome of allogeneic hematopoietic stem cell transplantation. Haematologica 2010;95:364–6.
- Kanda J, Mizumoto C, Kawabata H, Ichinohe T, Tsuchida H, Tomosugi N, et al.. Clinical significance of serum hepcidin levels on early infectious complications in allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2009;15:956–62.
- Means RT. Hepcidin and anemia. Blood Rev 2004;18:219–25.
- Mena NP, Esparza AL, Nunez MC. Regulation of transepithelial transport of iron by hepcidin. Biol Res 2006;39:191–3.
- Tanno T, Bhanu NV, Oneal PA, Goh SH, Staker P, Lee YT, et al.. High levels of GDF15 in thalassemia suppress expression of the iron regulatory protein hepcidin. Nat Med 2007;13:1096–101.
- Nemeth E, Ganz T. Regulation of iron metabolism by hepcidin. Annu Rev Nutr 2006;26:323–42.
- Porter JB. Pathophysiology of transfusional iron overload: contrasting patterns in thalassemia major and sickle cell disease. Hemoglobin 2009;33(Suppl 1):S37–45.
- Evens AM, Mehta J, Gordon LI. Rust and corrosion in hematopoietic stem cell transplantation: the problem of iron and oxidative stress. Bone Marrow Transplant 2004;34:561–71.
- McCord JM. Iron: free radicals, and oxidative injury. Semin Hematol 1998;35:5–12.
- Ganz T. Iron in innate immunity: starve the invaders. Curr Opin Immunol 2009;21:63–7.
- Özyılmaz E, Aydoğdu M, Sucak G, Akı SZ, Özkurt ZN, Yegin ZA, et al.. Risk factors for fungal pulmonary infections in hematopoietic stem cell transplantation recipients: the role of iron overload. Bone Marrow Transplant 2010;45:1528–33.
- Tunccan O, Yegin ZA, Ozkurt ZN, Erbas G, Akı SZ, Senol E, et al.. High ferritin levels are associated with hepatosplenic candidiasis in hematopoietic stem cell transplant candidates. Int J Infect Dis 2010;14(Suppl 3):e104–7.
- Marx JJM. Iron and infection: competition between host and microbes for a precious element. Best Pract Res Clin Haematol 2002;15:411–26.
- Maertens J, Demuynck H, Verbeken EK, Zachée P, Verhoef GE, Vandenberghe P, et al.. Mucormycosis in allogeneic bone marrow transplant recipients: report of five cases and review of the role of iron overload in the pathogenesis. Bone Marrow Transplant 1999;24:307–12.
- Altes A, Remacha AF, Sadra P, Sancho FJ, Sureda A, Martino R, et al.. Frequent severe liver iron overload after stem cell transplantation and its possible association with invasive aspergillosis. Bone Marrow Transplant 2004;34:505–9.
- Dürken M, Nielsen P, Knobel S, Finckh B, Herrnring C, Dresow B, et al.. Nontransferrin bound iron in serum of patients receiving bone marrow transplantation. Free Radical Biol Med 1997;22:1159–63.
- Sahlstedt L, Ebeling F, Bonsdorff L, Parkkinen J, Ruutu T. Non-transferin bound iron during allogeneic stem cell transplantation. Br J Haematol 2001;113:836–8.
- Dürken M, Herrnring C, Finckh B, Nagel S, Nielsen P, Fischer R, et al.. Impaired plasma antioxidative defense and increased nontransferrin-bound iron during high-dose chemotherapy and radiochemotherapy preceding bone marrow transplantation. Free Radical Biol Med 2000;28:887–94.
- Emerit J, Beaumont C, Trivin F. Iron metabolism, free radicals, and oxidative injury. Biomed Pharmacother 2001;55:333–9.
- Gordon LI, Brown SG, Tallman MS, Rademaker AW, Weitzman SA, Lazarus HM, et al.. Sequential changes in serum iron and ferritin in patients undergoing high- dose chemotherapy and radiation with autologous bone marrow transplantation: possible implications for treatment related toxicity. Free Radical Biol Med 1995;18:383–9.
- Maradei SC, Maiolino A, Azevedo AM, Colares M, Bouzas LF, Nucci M. Serum ferritin as a risk factor for sinusoidal obstruction syndrome of the liver in patients undergoing hematopoietic stem cell transplantation. Blood 2009;114:1270–5.
- Sucak GT, Yeğin ZA, Özkurt ZN, Akı ŞZ, Karakan T, Akyol G. The role of liver biopsy in the work- up of liver dysfunction late after SCT: is the role of iron overload underestimated? Bone Marrow Transplant 2008;42:461–7.
- Strasser SI, Kowdley KV, Sale GE, McDonald GB. Iron overload in bone marrow transplant recipients. Bone Marrow Transplant 1998;22:167–73.
- Kamble RT, Selby GB, Mims M, Kharjan-Dabaja MA, Ozer H, George JN. Iron overload manifesting as apparent exacerbation of hepatic graft versus host disease after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2006;12:506–10.
- Ho GT, Parker A, MacKenzie JF, Morris JA, Stanley AJ. Abnormal liver function tests following bone marrow transplantation: aetiology and role of liver biopsy. Eur J Gastroenterol Hepatol 2004;16:157–62.
- Mahindra A, Bolwell B, Sobecks R, Rybicki L, Pohlman B, Dean R, et al.. Elevated ferritin is associated with relapse after autologous hematopoietic stem cell transplantation for lymphoma. Biol Blood Marrow Transplant 2008;14:1239–44.
- Hershko C. Mechanism of iron toxicity. Food Nutr Bull 2007;28(4 Suppl):S500–9.
- Walter PB, Fung EB, Killilea DW, Jiang Q, Hudes M, Madden J, et al.. Oxidative stress and inflammation in iron-overloaded patients with beta-thalassaemia or sickle cell disease. Br J Haematol 2006;135:254–63.
- Kemma EHJM, Kartikasari AER, van Tits LJH, Pickkers P, Tjalsma H, Swinkels DW. Regulation of hepcidin: insights from biochemical analyses on human serum samples. Blood Cells Mol Dis 2008;40:339–46.