Abstract
This study evaluates the prognostic value of MAGE-A3 expression in 28 diffuse large B-cell lymphoma (DLBCL) patients. A significant association was observed between MAGE-A3 expressions, assessed by quantitative real-time RT-polymerase chain reaction (PCR), with advanced stages of disease (P<0·05). Elevated serum lactate dehydrogenase (LDH) levels and International Prognostic Index (IPI) score were significantly higher in MAGE-A3-positive patients (P = 0·025 and P = 0·004, respectively). Expression of MAGE-A3 was associated with poor response to treatment and a significantly shorter overall survival (P<0·001). Our data address new information in the association of MAGE-A3 expression and poor prognosis in DLBCL patients.
Keywords:
Introduction
Despite modern chemotherapeutic agents and monoclonal antibody therapy, the prognosis for many patients with hematological malignancies, particularly those with aggressive diseases such as diffuse large B-cell lymphoma (DLBCL), mantle cell lymphoma, and multiple myeloma, remains poor.Citation1–Citation3DLBCL is the most common type of non-Hodgkin lymphoma and accounts for 30%–40% of new diagnoses.Citation4 Neither the International Prognostic Index (IPI) nor the revised IPI identifies a risk group with less than a 50% chance of survival. Therefore, other predictors like molecular prognostic markers must be elucidated to identify those patients most in need of alternate therapies.Citation5
The MAGE-A3 gene belongs to a group of genes called cancer–testis antigen genes whose abnormal expression has been observed in many tumor types, including DLBCL.Citation6Citation6,7 MAGE-A transcripts predict an unfavorable outcome in patients with lung cancer,Citation8 multiple myeloma,Citation9 cervical cancer,Citation10and carcinoma of the urinary bladder.Citation11However, in DLBCL patients, it has been reported that MAGE-A3 expression showed no prognostic value.Citation6 We have now investigated the potential of MAGE-A3 expression as a prognosis marker in this group of patients. The MAGE-A3 gene showed prognostic value in DLBCL patients.
Methods
Patients and controls
Twenty eight de novo DLBCL patients who visited the Hematology Service at the General Hospital of Mexico were included in the study. The median age was 45·5 years (range: 18–69 years), and six patients (21·4%) were over 60 years old. There were 13 (46%) males and 15 (54%) females. The patients were treated with cyclophosphamide, doxorubicine, vincristine, and prednisone (CHOP). They were to receive 6–8 cycles of CHOP and if there was a residual mass after chemotherapy, the patients were treated with involved field radiation therapy. None of these patients received monoclonal antibodies and none underwent stem cell transplantation. Patients whose lymphoma recurred despite first line therapy were treated with dexamethasone, etoposide, and cisplatin as second-line chemotherapy at the discretion of the treating physician.
Peripheral blood lymphocytes (PBLs)
Normal testicular tissue from a 61-year-old patient with prostatic carcinoma was used as positive control. Informed consent was obtained from patients and healthy donors. The study was approved by Ethics Committee of the Hospital and complied with the Helsinki Declaration.
RT-polymerase chain reaction (PCR), quantitative real-time RT-PCR, and sequencing assays
RNA was extracted from the specimens using TRIzol regent (Life Technologies, Paisley, UK) and 1 μg of RNA was used to synthesize cDNA by M-MLV reverse transcriptase (Life Technologies).Citation12PCR amplification was performed with the previously described primers.Citation12 Each cDNA was also tested by PCR using specific primers for beta2-microglobulin to exclude samples with strongly degraded RNA. The PCR reaction and sequencing were carried out as described previously.Citation12
For quantitative real-time RT-PCR, 2 μl of cDNA was used. Amplification was performed in a i-Cycler iQ Detection System (Bio-Rad, Hercules, CA, USA) as described.Citation12 All reactions were run in triplicate. Results were expressed as the relative expression level between the target gene and the 18S rRNA.
Western blotting
Whole cell protein extracts were electrophoresed in SDS–polyacrylamide gels in the conditions previously described.Citation13The gels were transferred to nitrocellulose filters,Citation14 and the blots treated with the MAGE-A (FL-309) rabbit polyclonal IgG and a beta-actin mouse monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA). After incubation with secondary horseradish peroxidase-conjugated goat anti-rabbit or goat anti-mouse antibodies (Santa Cruz Biotechnology), the blot was treated for protein bands detection according to instructions by Immun-Blot Assay Kit (Bio-Rad). Densitometric semiquantification of the protein bands was performed using the Quantity One software (Bio-Rad).
Statistical analysis
Correlations between MAGE-A3 gene expression and clinical variables were examined with the chi-square test and Fisher’s exact test. Patient’s survival data were analyzed with the Kaplan–Meier methodCitation15 and were compared by means of the log-rank test.Citation16 Data were analyzed with SSPS software (version 15·0 for Windows; SPSS Inc., Chicago, IL, USA).
Results
Association of MAGE-A3 expression with advanced-stage disease
MAGE-A3 mRNA expression was detected in 39·3% (11/28) of DLBCL patients. Representative results of the gels are shown in , where testicular tissue or PBLs from a healthy donor were used as a positive control or a negative control, respectively. Expression of the gene was detected in none (0/12) of the controls. The characteristics of patients and summarized results are described in . The median age at diagnosis was 45·5 years (range: 18–69 years). Sequence analyses were performed to validate the RT-PCR specificity. Nucleotide sequences obtained were compared with the reported sequence for the MAGE-A3 gene, and as expected, the amplified bands corresponded to the MAGE-A3 gene (not shown). Expression of MAGE-A3 mRNA was associated with advanced-stage disease: quantitative analyses using real-time RT-PCR showed a higher expression of MAGE-A3 in stages III and IV DLBCL patients (P<0·05) (). The expression of MAGE-A3 in stage III and stage IV samples was approximately seven- and 13-fold higher than that in stage II samples (average: 0·93 and 1·60 versus 0·12, respectively). These results indicate that tumors from advanced stages of disease in DLBCL patients are, on average, high expressers of MAGE-A3 gene.
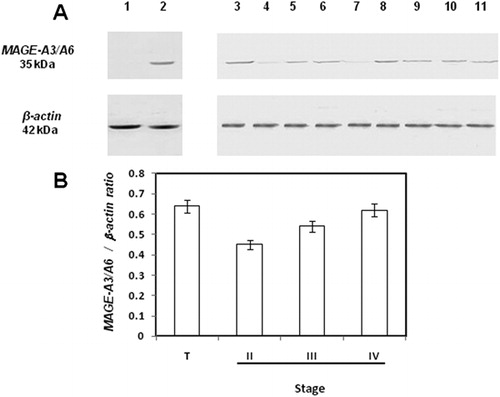
Figure 1. mRNA expression levels of MAGE-A3 in DLBCL stages. (A) Expression of MAGE-A3 in DLBCL. Lane 1, 100-bp ladder; lane 2, testicular tissue; lane 3, PBLs from a healthy donor; lanes 4–11, DLBCL representative samples. (B) Real-time RT-PCR. The expression results were obtained from eight samples from stage II DLBCL patients; nine from stage III and eight from stage IV. Significant differences between results (P<0·05) were obtained by means of parametric test. T, testicular tissue.
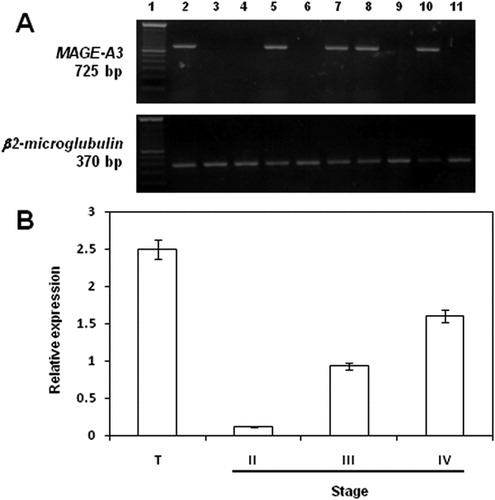
Table 1. Association between clinical characteristics and MAGE-A3 expression in DLBCL patients
We investigated the protein expression of MAGE-A3 gene in DLBCL patients. As MAGE-A3 and MAGE-A6 show 95% of protein sequence homology, binding of anti-MAGE-A3/A6 antibody to MAGE-A6 seems very likely; therefore, the protein recognized in the immunoassays was designated MAGE-A3/A6. Western blot analyses revealed that MAGE-A3/A6 was expressed in the same patients expressing MAGE-A3 at the mRNA level ().Densitometric semiquantification of the protein bands verified the higher expression of MAGE-A3 in stages III and IV DLBCL patients ().
Association of MAGE-A3 expression with survival
Expression of MAGE-A3 was not found to be associated with age, sex, performance status, and extranodal involvement ().
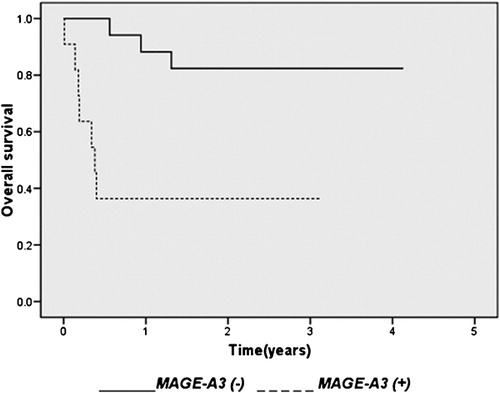
Elevated serum lactate dehydrogenase (LDH) levels were observed mainly in positive samples (8/11, 72·7%). This incidence was significantly higher than that in the MAGE-A3 negative samples (5/17, 29·4%; P = 0·025). IPI score was significantly higher in MAGE-A3-positive samples (P = 0·004, ).The patients were treated with CHOP. A complete response was achieved in 8/17 (47%) of the MAGE-A3-negative patients, while none (0/11) of the positive patients responded to treatment. Tumor expression of MAGE-A3 was associated with a significantly shorter overall survival (P<0·001). Seven positive patients (7/11, 63·6%) died within 3 years after the diagnosis. In contrast, 3/17 (17·6%) negative patients died within the same time. The median observation time of surviving patients was 4·5 and 38·5 months for positive and negative patients, respectively. The 3-year overall survival rates were 36·4% and 82·4% for positive and negative patients, respectively. The survival curve of MAGE-A3 negative patients was significantly better than that of positive patients ().
Discussion
In this report, we have investigated the potential of MAGE-A3 expression as a prognosis marker in DLBCL patients. We found that the expression of this gene is associated with poor prognosis in these patients. Our data differ from previously published results by Han et al.,Citation6 who reported that MAGE-A3 expression was not associated with clinical characteristics or prognosis in DLBCL patients. Possible explanations of this discrepancy are: (1) DLBCL, as identified in the current classification schemes, is not a uniform disease; (2) a different treatment regimen is used. It has been reported that prognostic factors for non-Hodgkin’s lymphoma patients treated with chemotherapy may not predict outcome in patients treated with rituximab.Citation17
In our study, we observed significant correlation between MAGE-A3 mRNA expression and advanced stages of the disease, serum LDH levels, IPI, response to treatment, and overall survival. In previous analyses of relatively small numbers of patients with this disease, a variety of clinical characteristics were consistently associated with stage-disease and outcome: the serum LDH concentration, the number of nodal and extranodal sites of disease, tumor size, and the distinction between localized disease (Ann Arbor stage I or II) and advanced disease (stage III or IV).Citation18The IPI has been currently used to predict prognosis.Citation19
Our results show that stage III and IV DLBCL patients express more MAGE-A3 transcripts than those from stages I and II. This is consistent with previous reports of increased CT antigen expression being associated with more aggressive diseases, or advanced stages of disease.Citation20–Citation22 Recently, it was reported that cell lines derived from more aggressive lymphoma subtypes generally expressed CT transcripts at higher frequency.Citation23
We observed that MAGE-A3 expression was associated with worse overall survival. There are reports of higher levels of CT expression correlating with poorer prognosis in multiple myeloma and operable non-small-cell lung cancer.Citation8Citation8,9Expression of around 30 genes has been reported to predict the survival in DLBCL.Citation24 Hans et al.Citation25 had reported that expression of MUM1 or cyclin D2 was associated with worse overall survival. Using microarray techniques has demonstrated that patients with a germinal center B cell-like signature have a more favorable course than those with an activated B cell-like profile. These differences exist within each risk group identified by the IPI.Citation26 Lossos et al.Citation24 subsequently demonstrated that they could develop a new predictor of survival of DLBCL based on the expression of six genes assayed by quantitative real-time polymerase chain reaction. So, quantitative real-time PCR of MAGE-A3 transcripts could also be used to predict survival in DLBCL.
In this report, we provide evidence for a role of MAGE-A3 in mediating resistance of DLBCL cells to CHOP. A number of studies have documented roles for genes in chemoresistance of DLBCL cells.Citation27–Citation29 Of particular interest is the microarray study by Kawano et al.Citation28showing that DLBCL cases with resistance to anthracycline-containing chemotherapy regimens showed high expression of PRAME (a CT antigen), and that PRAME-positive patients had significantly worse progression-free survival than PRAME-negative patients. It will be interesting to assess the relation of MAGE-A3 expression and treatment response or prognosis in patients treated with rituximab and CHOP.
Finally, with regard to MAGE-A3 expression in DLBCL patients, our results are consistent with published results by Han et al.,Citation6 but differ from a previous studyCitation30 in which none of 28 DLBCL patients expressed this gene. The reasons for this discrepancy are unclear. However, Han et al.’s data and our results were confirmed by real-time PCR. In the present report, we additionally observed MAGE-A3 protein expression in DLBCL patients, a prerequisite for design of a therapeutic vaccine. Protein expression is more relevant than mRNA expression in terms of selecting targets for lymphoma immunotherapy. A cytolytic T-cell response to a cancer-testis antigen (PASD1) in DLBCL has been reported.Citation31 We conclude that expression of MAGE-A3 is associated with poor prognosis in DLBCL patients.
IO is a student of ‘Posgrado en Ciencias Biológicas-UNAM’ and was supported by CONACyT Fellowship (168939). We thank Melissa T. Castro Epperson for critical reading of the manuscript. This work was supported by CONACyT 48015-M and 80085; and by ‘Dirección de Investigación-HGM’.
References
- Ng AK. Diffuse large B-cell lymphoma. SeminRadiat Oncol 2007;17:169–75.
- Weigert O, Unterhalt M, Hiddemann W, Dreyling M. Current management of mantle cell lymphoma. Drugs 2007;67:1689–1702.
- Richardson PG, Mitsiades C, Schlossman R, Munshi N, Anderson K. New drugs for myeloma. Oncologist 2007;12:664–89.
- Coiffier B. Diffuse large cell lymphoma. CurrOpin Oncol 2001;13:325–34.
- Lossos IS, Morgensztern D. Prognostic biomarkers in diffuse large B-celllymphoma. J Clin Oncol 2006;24:995–1007.
- Han MH, Eom HS, Park WS, Yun T, Park S, Kim HJ, et al.. Detection of circulating lymphoma cellsin patients with non-Hodgkin lymphoma using MAGE-A3 geneexpression in peripheral blood. Leuk Res 2010;34:1127–31.
- Miranda EI. MAGE, biological functions and potential clinicalapplications. Leuk Res 2010;34:1121–2.
- Sienel W, Mecklenburg I, Dango S, Ehrhardt P, Kirschbaum A, Passlick B, et al.. Detection of MAGE-A transcripts inbone marrow is an independent prognostic factor in operable non-small-celllung cancer. Clin Cancer Res 2007;13:3840–7.
- Condomines M, Hose D, Raynaud P, Hundemer M, de Vos J, Baudard M, et al.. Cancer/testis genes in multiple myeloma:expression patterns and prognosis value determined by microarray analysis. JImmunol 2007;178:3307–15.
- Napoletano C, Bellati F, Tarquini E, Tomao F, Taurino F, Spagnoli G, et al.. MAGE-A and NY-ESO-1 expression in cervicalcancer: prognostic factors and effects of chemotherapy. AmJ Obstet Gynecol 2008;198:99.e1–7.
- Kocher T, Zheng M, Bolli M, Simon R, Forster T, Schultz-Thater E, et al.. Prognostic relevance of MAGE-A4 tumorantigen expression in transitional cell carcinoma of the urinary bladder:a tissue microarray study. Int J Cancer 2002;100:702–5.
- Martinez A, Olarte I, Mergold MA, Gutierrez M, Rozen E, Collazo J, et al.. mRNA expression of MAGE-A3gene in leukemia cells. Leuk Res 2007;31:33–7.
- Miranda EI, Garrido-Guerrero E, García-Carrancá A, Gariglio P. Immunoprecipitation of SV40 replicating minichromosomescomplexed with bacteriophage T4 gene 32 protein. NuclAcids Res 1992;20:903–7.
- Salazar AM, Ostrosky-Wegman P, Menéndez D, Miranda EI, García-Carrancá A, Rojas E. Induction of P53 protein expression by sodiumarsenite. Mutat Res 1997;381:259–65.
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. JAm Stat Assoc 1958;53:457–81.
- Mantel N. Evaluation of survival data and two new rankorder statistics arising in its consideration. CancerChemother Rep 1966;50:163–70.
- Czuczman MS, Grillo-López AJ, Alkuzweny B, Weaver R, Larocca A, McLaughlin P. Prognostic factors for non-Hodgkin’slymphoma patients treated with chemotherapy may not predict outcome in patientstreated with rituximab. Leuk Lymphoma 2006;47:1830–40.
- Fisher RI, Miller TP, O’Connor OA. Diffuse aggressive lymphoma. Hematology Am Soc HematolEduc Program 2004;:221–36.
- TheInternational Non-Hodgkin’s Lymphoma Prognostic Factors Project. Apredictive model for aggressive non-Hodgkin’s lymphoma. NEngl J Med 1993;329:987–94.
- Brasseur F, Rimoldi D, Liénard D, Lethé B, Carrel S, Arienti F, et al.. Expression of MAGE genes in primaryand metastatic cutaneous melanoma. Int J Cancer 1995;63:375–80.
- Kurashige T, Noguchi Y, Saika T, Ono T, Nagata Y, Jungbluth A, et al.. NY-ESO-1 expression and immunogenicityassociated with transitional cell carcinoma: correlation with tumor grade. CancerRes 2001;61:4671–4.
- Dhodapkar MV, Osman K, Teruya-Feldastein J, Filippa D, Hedvat CV, Iversen K, et al.. Expression of cancer/testis (CT) antigensMAGE-A1, MAGE-A3, MAGE-A4, CT-7 and NY-ESO-1 in malignant gammopathies isheterogeneous and correlates with site, stage and risk status of disease. CancerImmun 2003;3:9–16.
- Liggins AP, Lim SH, Soilleux EJ, Pulford K, Banham AH. A panel of cancer–testis genes exhibitingbroad-spectrum expression in haematological malignancies. CancerImmun 2010;10:8–19.
- Lossos IS, Czerwinski DK, Alizadeh AA, Wechser MA, Tibshirani R, Botstein D, et al.. Prediction of survival in diffuse largeB-cell lymphoma based on the expression of six genes. NEngl J Med 2004;350:1828–37.
- Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, et al.. Confirmation of the molecular classificationof diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004;103:275–82.
- Rosenwald A, Wright G, Chan WC, Connors JM, Campo E, Fisher RI, et al.. The use of molecular profiling to predictsurvival after chemotherapy for diffuse large B-cell lymphoma. NEngl J Med 2002;346:1937–47.
- Shipp MA, Ross KN, Tamayo P, Weng AP, Kutok JL, Aguiar RC, et al.. Diffuse large B-cell lymphoma outcomeprediction by gene-expression profiling and supervised machine learning. NatMed 2002;8:68–74.
- Kawano R, Karube K, Kikuchi M, Takeshita M, Tamura K, Uike N, et al.. Oncogene associated cDNA microarrayanalyses shows PRAME gene expression is a marker for response to anthracyclinecontaining chemotherapy in patients with diffuse large B-cell lymphoma. JClin Exp Hematopathol 2009;49:1–7.
- Maxwell SA, Li Z, Jaya D, Ballard S, Ferrell J, Fu H. 14-3-3ζ mediates resistance of diffuselarge B-cell lymphoma to an anthracycline-based chemotherapeutic regimen. JBiol Chem 2009;284:22379–89.
- Xie X, Wacker H, Huang S, Regitz E, Preuss K-D, Romeike B, et al.. Differential expression of cancer testisgenes in histological subtypes of non-Hodgkin’s lymphomas. ClinCancer Res 2003;9:167–73.
- Ait-Tahar K, Liggins AP, Collins GP, Campbell A, Barnardo M, Lawrie C, et al.. Cytolytic T-cell response to the PASD1cancer testis antigen in patients with diffuse large B-cell lymphoma. BrJ Haematol 2009;146:396–407.