Abstract
Endocan is a soluble proteoglycan expressed only by vascular endothelium and is also found circulating in the bloodstream. Inflammatory cytokines as well as proangiogenic growth factors increase its expression, and increased serum levels are found in immunocompetent patients with sepsis. We investigated serum endocan levels in patients with untreated acute myeloid leukemia (AML) and AML patients during chemotherapy-induced bone marrow failure. We observed increased levels in 40 AML patients compared with healthy controls, which was also confirmed in a second cohort. The serum levels decreased after intensive chemotherapy and subsequent severe chemotherapy-induced cytopenia, and increased levels were thereafter observed during bone marrow regeneration. However, even for these severely immunocompromized patients, serum endocan levels increased during complicating bacterial infections before a decrease was seen during antibiotic therapy. To conclude, serum endocan is a disease marker in AML, but serum levels are also affected by complicating infections and bone marrow regeneration.
Introduction
Acute myeloid leukemia (AML) is a hematologic malignancy characterized by an accumulation of immature myeloblasts in the bone marrow.Citation1 Standard treatment of AML is induction chemotherapy to achieve disease control followed by consolidation therapy to maintain the control and prevent relapse.Citation1 Long-time survival after this intensive and potentially curative chemotherapy is only 40–50%, but for selected subsets of younger patients the prognosis can be improved by allogeneic stem cell transplantation.Citation1However, patients above 60–70 years of age cannot tolerate the intensive therapeutic strategies due to the bone marrow toxicity; for this reason, they receive either less intensive but still potentially curative treatment or they receive only disease-stabilizing therapy.Citation1–Citation3
The leukemia-induced increase in bone marrow microvascularity is important both for leukemogenesis and chemosensitivity in human AML,Citation4 and antiangiogenic therapy is now considered as a potential combination with both intensive chemotherapy and low-toxicity disease-stabilizing treatment.Citation4 Identification of biomarkers for leukemia-associated angiogenesis may be important for disease prognosis. Furthermore, monitoring for minimal residual disease is now considered important for early detection of post-treatment AML relapse.Citation5AML is a heterogeneous disease, and the most common strategy is therefore to use flow cytometry or polymerase chain reaction analyses to examine combinations of markers that can be expressed by the AML cells.Citation5Citation5,6 However, because angiogenesis is important in leukemogenesis, it may be useful not only to include markers expressed by the leukemic cells but also markers that reflect other essential contributors in leukemogenesis, e.g. endothelial cells. Endocan is an endothelial cell-specific molecule,Citation7 and in the present study, we therefore investigated whether serum endocan can be used as a biomarker in AML.
Patients and Methods
Acute leukemia patients
All studies were approved by the regional Ethics Committee (Health Region III, Bergen, Norway) and conducted in accordance with the Declaration of Helsinki. Samples were collected after informed consent and biobanks approved by the Royal Ministry of Health and the Directorate for Health and Social Affairs.
Group 1 acute leukemia patients: Serum samples collected three times weekly from start of intensive chemotherapy and until hematopoietic reconstitution. The sera were collected from 21 consecutive acute leukemia patients during a period of 25 months, and they represent all acute leukemia patients admitted to our department during this period. Sixteen patients were examined during induction cycles. A total of 19 consolidation cycles were also studied, thus two cycles were then examined for three unselected patients whereas for the other patients only one consolidation cycle was investigated. Blood samples were collected three times weekly from start of chemotherapy until hematopoietic reconstitution with neutrophil counts exceeding 0·5×109/l and stable platelet counts without platelet transfusions was achieved. The 21 patients included 7 females and 14 males with a mean age of 46·6 years (range 18–67 years); 17 patients had AML (3 with relapsed disease) and 4 patients had acute lymphoblastic leukemia (ALL) (3 with relapse). All patients received intensive cytarabine-based chemotherapy resulting in a period of 14–22 days with severe treatment-induced cytopenia with neutrophil counts below 0·5×109/l and dependency of regular platelet transfusions to maintain peripheral blood platelet counts above 10–20×109/l. All infections were documented by growth in blood cultures (six incidents) or local signs of bacterial infections, i.e. catheter infections, radiological signs of pneumonia or clinical signs of perianal infections or typhlitis. Invasive fungal infections were not documented in any patient. None of the patients died from infections or had signs of septic shock.
Group 2 acute leukemia patients: Serum samples collected from patients with chemotherapy-induced neutropenia at four predefined time points. Sixteen patients (12 males and 4 females, mean age 47·5 years with range 32–69 years) were included during a 12-month period; 12 AML patients, 3 ALL patients, and 1 patient with myeloid sarcoma were studied during 36 chemotherapy cycles (18 induction cycles and 18 consolidation cycles). All patients also received intensive cytarabine-based chemotherapy and developed severe treatment-induced pancytopenia. Serum samples were collected at four time points: (1) pre-therapy samples collected immediately before start of chemotherapy; (2) during the first part of chemotherapy-induced cytopenia, after 4–8 days of cytopenia if patients still showed no clinical or laboratory signs of complicating infections; (3) during complicating infections within 24 hours after development of fever >38·5°C; and (4) from cytopenic patients showing clinical and laboratory improvement during antibiotic therapy [no fever, decreasing serum levels of C-reactive protein (CRP)]. Thus, the times for sampling of the two last samples varied between patients and were determined by the time of febrile neutropenia and treatment response. All infections were documented by growth in blood cultures or local signs of bacterial infections as described above. Invasive fungal infection was not documented in any patient. None of the patients died from complicating infections or had signs of septic shock during the infections.
Healthy controls
Two different control groups were analyzed. Samples from the untreated AML patients and the first treatment group were collected according to the same guidelines and were compared with a group of 21 healthy individuals (mean age 55·2 years, 10 males and 11 females). The controls for the second patient group consisted of 20 healthy individuals (mean age 40 years, 11 females and 9 males).
Analysis of serum endocan levels
Venous blood samples were collected into sterile glass (group 1 and corresponding controls) or plastic tubes (all other samples) and allowed to coagulate for 90 minutes at ambient temperature before centrifugation (300 g for 10 minutes) and serum collection. All samples were immediately frozen at −70°C until analyzed. Repeated freezing and thawing were avoided. A sandwich-based enzyme-linked immunosorbent assay using two monoclonal anti-human endocan antibodies was used to measure serum endocan levels.Citation8
Statistical analysis
Differences between groups were analyzed using the Mann–Whitney U-test and Wilcoxon’s rank sum test for comparing paired samples. Endocan serum levels were either used as a continuous variable or as a binary variable with the median endocan level as the cut-off value. For correlation analysis, the Spearman’s rho test was used. Analyses were performed using PASW Statistics 17 (SPSS Inc., Chicago, IL, USA) and the P values are two-sided and considered statistically significant when P<0·05.
Results
Serum endocan levels are increased for patients with untreated AML and ALL
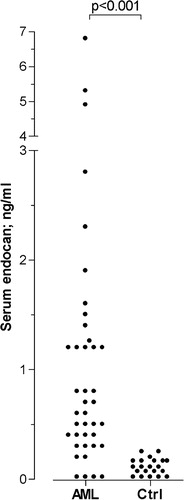
We investigated serum endocan levels for a consecutive group of 40 patients with untreated AML (median age 61 years with range 19–21 years, 21 men and 19 women) and six patients with ALL, and these levels were compared with a group of 21 healthy controls that were sampled during the same time period as the patients. The AML patients (median level 0·7 ng/ml, range not detectable to 6·8 ng/ml) and the ALL patients (median level 0·9 ng/ml, range not detectable to 1·3 ng/ml) showed significantly increased endocan levels compared with healthy individuals (range not detectable to 0·2 ng/ml) (). Finally, patients from the second treatment group who received induction therapy represent an additional group of untreated patients, and their serum endocan levels were significantly increased compared with the corresponding healthy controls (median patient level 2·0 ng/ml, range 1·0–2·8 ng/ml, P = 0·03).
Figure 1. Serum endocan levels for untreated acute myeloid leukemia (AML) patients and healthy controls. The figure presents endocan levels (ng/ml) for a consecutive group of 40 patients with untreated AML (AML) and a group of 21 healthy controls (Ctrl). The corresponding P value is indicated at the top of the figure.
The serum endocan levels were analyzed more in detail for the AML patients with regard to clinical and biological characteristics. No statistically significant association was found between endocan levels and age, morphology (i.e. French–American–British classification), expression of stem cell markers (CD34 and CD117), cytogenetic abnormalities (low-risk abnormalities 2·5% of patients, high-risk 12%, intermediate risk 30·5%, normal karyotype 45%, and no data available 10%), genetic Flt3-abnormalities (31% of patients with internal tandem duplication and 5% of patients with point mutation) or NPM-1 mutations (12% of patients with mutation) (data not shown). Furthermore, 27 patients received induction chemotherapy with conventional cytarabine combined with an anthracycline, but pre-therapy endocan levels did not differ between patients with or without complete remission after one induction cycle (data not shown).
The serum endocan levels showed an inverse correlation with the platelet count at the time of diagnosis. The median peripheral blood platelet count was 34×109/l (variation range <5–248×109/l). Patients with serum endocan levels exceeding the median level had significantly lower platelet counts than patients with endocan levels below the median (median platelet counts 39 versus 30×109/l; Mann–Whitney test, P<0·0005).
Pre-therapy serum endocan levels are increased also for patients in hematologic remission receiving consolidation therapy
The group 1 patients’ samples were collected before and regularly following intensive chemotherapy (21 patients, 35 chemotherapy cycles). Pre-therapy endocan levels were relatively high and did not differ when comparing the 16 induction cycles (median level 1·2 ng/ml, variation range not detectable to 2·9 ng/ml) versus the 19 consolidation cycles (median level 1·1 ng/ml, range 0·3–2·8 ng/ml). Endocan serum levels in all of the preinduction samples, except one patient, and all of the preconsolidation samples exceeded the highest level found in the corresponding control group (>0·2 ng/ml).
Serum endocan levels decrease following intensive chemotherapy
To investigate the effect of intensive chemotherapy, we determined the endocan levels immediately before start of intensive chemotherapy and early during severe treatment-induced cytopenia with neutrophil counts below 0·5×109/l and dependency on platelet transfusions. The first group of patients was then examined, and at this second time of sampling, none of the patients had either fever or other systemic or local clinical signs of complicating infections. These samples were collected during 23 chemotherapy cycles, and the mean duration from development of neutropenia until sampling was 2·7 days. We observed a significant decrease in serum endocan levels when patients developed severe cytopenia; this was observed when all samples were included in the statistical analysis (; Wilcoxon’s test for paired samples, n = 23, P<0·0004) and when only consolidation cycles were analyzed (n = 15, P = 0·017). At the time of the second sample, the patients had no microscopic signs of AML: (1) all patients receiving consolidation therapy were in remission prior to the chemotherapy as well as after hematopoietic reconstitution, and (2) patients receiving induction therapy showed no residual leukemia blasts in bone marrow smears collected during cytopenia, and later they all reached complete hematologic remission.
Figure 2. Serum endocan levels for acute leukemia patients before treatment (B) and after development (A) of severe chemotherapy-induced pancytopenia with peripheral blood neutrophil counts <0·5×109/l and dependency on regular platelet transfusions. The figure shows the results for those patients in patient group 1 that could be sampled before start of chemotherapy and after the development of treatment-induced cytopenia but before the development of complicating infections. When the second sample was collected, the patients were cytopenic, and had no fever and neither other systemic nor local signs of bacterial infection, and a stable serum CRP level below 50 mg/l. The figure shows the overall results for patients receiving induction (stippled line) or consolidation chemotherapy (solid line) and the corresponding P value is indicated at the top.

Serum endocan levels increase in neutropenic patients with complicating bacterial infections
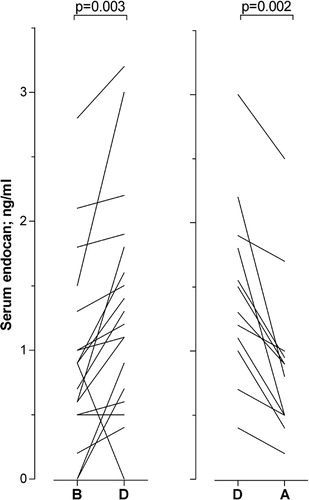
The patients in treatment group 1 were regularly sampled three times weekly from start of chemotherapy until regeneration with increasing peripheral blood cell counts. During the 35 chemotherapy cycles, it was possible to identify 21 cycles with febrile neutropenia due to a bacterial infection when either (1) the infection started during neutropenia and it was possible to analyze an additional preinfection sample that had been collected <5 days before the infection when the patient was cytopenic without signs of infection; and/or (2) the patient showed improvement during antibiotic therapy so that a serum sample could be collected when the patient was still neutropenic but afebrile with normalizing CRP. The mean duration of neutropenia until sampling during infection was 7·9 days, and the mean duration from start of chemotherapy until sampling was 12·1 days. A comparison of serum endocan levels for preinfection versus infection was possible for 18 cycles and infection versus post-infection for 12 cycles (). The neutropenic patients showed increased serum endocan levels during infections compared with the preinfection samples (Wilcoxon’s test for paired samples, n = 18, P = 0·003). Similarly, for those patients that responded to antibiotic therapy and became afebrile while still cytopenic, decreased serum endocan levels were observed during improvement compared with samples collected during the infections (P = 0·002). Thus, serum endocan levels are increased in response to complicating bacterial infections.
Figure 3. Serum endocan levels for acute leukemia patients with severe chemotherapy-induced pancytopenia before, during, and after the development of complicating bacterial infections (patient group 1). All patients had peripheral blood neutrophil counts <0·5×109/l and were dependent on regular transfusions to maintain platelet counts above 10–20×109/l at the time of sampling. The figure compares (left part) the serum endocan levels (ng/ml) for those patients that could be sampled before (B) and during the bacterial infection (D); and (right part) serum levels for patients that were cytopenic both during the infection (D) and also after clinical improvement due to antibiotic therapy (A). Clinical improvement means that they were afebrile with normalization of the serum CRP level. Corresponding P values are indicated at the top of the figure.
In this first group, a total of 73 samples were available from 21 patients with severe treatment-induced cytopenia. For all these samples, we compared the serum levels of endocan and the acute phase marker CRP. The CRP levels were significantly correlated with endocan, but despite the significant P value, the corresponding r value was relatively low (Spearman’s rho, P = 0·003, r = 0·350).
We then compared serum endocan levels for patients in the second treatment group. These patients were examined either before, during, or after infections, but the number of samples in these three different groups differed because: (1) some patients develop infections early or before severe neutropenia; (2) other patients recovered from infections after hematopoietic reconstitution; (3) if residual leukemic disease was detected 2 weeks after start of induction chemotherapy, the patients received a new induction cycle while still being neutropenic; and (4) for a minority of cycles, the patients did not develop febrile neutropenia. For these reasons, we compared these results as categorized data. Only samples collected during infections differed significantly from the healthy controls (Mann–Whitney U-test, P = 0·004), whereas samples collected from stable patients or during clinical improvement due to antibiotic therapy did not. Furthermore, the levels during infections (median level 1·80 ng/ml, range 0·80–20 ng/ml) were significantly higher than for samples collected during stable cytopenia (median level 1·45 ng/ml, variation range 0·45–2·8 ng/ml, P = 0·02) and during clinical improvement (median 1·1 ng/ml, range 0·7–3·1 ng/ml, P = 0·05).
Taken together, our overall results show that the serum endocan levels in two independent patient groups show a similar variation with relatively high pre-chemotherapy endocan levels, decreased levels during stable chemotherapy-induced cytopenia, and increased levels during complicating infections.
Discussion
Most molecular AML biomarkers considered for detection of minimal residual disease, early diagnosis of relapse, or evaluation of treatment responses after achievement of complete remission are markers expressed by the leukemic cells.Citation5Citation6Citation5,6,9 However, leukemogenesis not only consists of transformation and proliferation of the leukemic cells; the disease development also involves interactions with non-malignant neighboring bone marrow cells, including increased bone marrow angiogenesis.Citation4Collection of biopsies is not suitable for frequent follow-up of AML patients, and an alternative is therefore to use endothelial cell markers for follow-up evaluation of the angiogenic component of leukemogenesis.Citation7 Endocan is a proteoglycan produced only by endothelial cells and can also be found in serum.Citation7 In the present study, we describe increased endocan serum levels in patients with untreated AML, these levels decrease when the leukemia cell burden is reduced by induction chemotherapy but they increase during complication infections and bone marrow regeneration.
We investigated serum endocan levels following chemotherapy in two independent patient groups. For both groups we compared: (1) pre-therapy levels with levels during uncomplicated chemotherapy-induced pancytopenia; and (2) serum levels in cytopenic patients immediately before, early after development of, and during antibiotic treatment for complicating infections. The larger number of patients/cycles and the regular sampling with 2–3 days intervals made it possible to analyze the results from the first patient group as paired samples, whereas for the second group, the samples were classified as pre-therapy/stable cytopenia/infection/recovery and the statistical analysis was based on categorized data.
Patients with untreated AML had increased serum endocan levels; this is probably not caused by aberrant expression of endocan in the malignant cells because we recently showed that in vitro cultured primary AML cells did not release detectable endocan.Citation10Furthermore, a decrease in serum levels was seen during stable chemotherapy-induced neutropenia, and this decrease probably reflects a reduced leukemia cell burden. Previous studies in a small group of patients also demonstrated that serum endocan levels decrease during palliative AML therapy even though these patients still had a large burden of AML blasts in their bone marrow.Citation11 Furthermore, the serum endocan levels increased during complicating infections, suggesting that endothelial cells are affected by the acute phase reaction in these patients.Citation12Finally, increased endocan levels persisted for patients in complete hematologic remission examined immediately before the consolidation treatment that was started within 10 days after the initial signs of hematopoietic reconstitution when peripheral blood cell counts were still increasing. The most likely explanation for increased levels before consolidation therapy is therefore ongoing hematopoietic regeneration after the previous chemotherapy.
The serum endocan levels in patients with untreated AML showed no association with age, morphology, expression of stem cell markers, or the genetic abnormalities. However, a highly significant inverse correlation was observed between platelet counts and endocan levels. Endocan is regarded as a marker of endothelial cell dysfunction in patients with cancer,Citation7and our present results suggest that a relatively low degree of dysfunction (i.e. lower endocan levels) is associated with better maintenance of remaining normal hematopoiesis reflected in the higher platelet counts.
Our study included unselected AML patients, only the younger patients received intensive chemotherapy, and their consolidation treatment varied (conventional or cytarabine, allogeneic stem cell transplantation). This heterogeneity makes it difficult to analyze whether endocan levels correlate with survival. However, all younger patients received the same induction chemotherapy (conventional cytarabine plus anthracycline), and pretreatment endocan levels then showed no association with the prognostically important response to the first induction cycle, i.e. achievement of remission after the first cycle.
We detected increased endocan levels during bacterial infections in our severely immunocompromized patients and this is similar to observations in immunocompetent patients with sepsis.Citation13Neutropenic patients with complicating infections show increased systemic levels of several soluble mediators, including tumor necrosis factor-alpha and interleukin-1-beta.Citation12 Both these cytokines increase endocan expression by endothelial cells and may therefore contribute to the increased serum levels during infections.Citation7
Collectively, our results demonstrate that serum endocan is a disease biomarker in untreated AML, probably reflecting the importance of angiogenesis and/or endothelial cell activation in leukemogenesis. Analysis of proteomic or gene expression profiles is a possible strategy for detection of minimal residual disease, early leukemia relapse, or treatment responses in human AML,Citation5–Citation7and endocan levels may then be used for additional evaluation of the angiogenic component of leukemogenesis. Serum endocan would then represent an endothelium-specific and easily available marker. Even though the serum levels in addition are influenced by complicating infections and bone marrow regeneration, this should not be a major problem because these patients usually have a stable clinical situation after completion of the intensive chemotherapy.
Authors are grateful to Dr Maryse Delehedde and Ms Geneviève Marchandise for her help and technical support at the Institute Pasteur de Lille and at Lunginnov’s lab. EndoMark H1 kits were also graciously provided by Dr Delehedde (Lunginnov, Lille, France). The study received financial support from the Norwegian Cancer Society, the Helse-Vest Foundation and the Solveig and Ove Lundes Foundation.
References
- Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK, et al.. Diagnosis and management of acute myeloidleukemia in adults: recommendations from an international expert panel, onbehalf of the European LeukemiaNet. Blood 2010;115:453–74.
- Ryningen A, Stapnes C, Paulsen K, Lassalle P, Gjertsen BT, Bruserud Ø. In vivo biologicaleffects of ATRA in the treatment of AML. Exp Opin Investig Drugs 2008;17:1623–33.
- Bruserud Ø, Stapnes C, Tronstad KJ, Ryningen A, Anensen N, Gjertsen B. Protein lysine acetylation in normal and leukaemichaematopoiesis: HDACs as possible therapeutic targets in adult AML. ExpOpin The Targets 2006;10:51–68.
- Hatfield KJ, Olsnes AM, Gjertsen BT, Bruserud Ø. Antiangiogenic therapy in acute myelogenousleukemia: targeting of vascular endothelial growth factor and interleukin8 as possible antileukemic strategies. Curr CancerDrug Targets 2005;5:229–48.
- Al-Mawali A, Gillis D, Lewis I. The role of multiparameter flow cytometryfor detection of minimal residual disease in acute myeloid leukemia. AmJ Clin Pathol 2009;131:16–26.
- Steinbach D, Schramm A, Eggert A, Onda M, Dawczynski K, Rump A, et al.. Identification of a set of seven genesfor the monitoring of minimal residual disease in pediatric acute myeloidleukemia. Clin Cancer Res 2006;12:2434–41.
- Sarrazin S, Adam E, Lyon M, Depontieu F, Motte V, Landolfi C, et al.. Endocan or endothelial cell specificmolecule-1 (ESM-1): a potential novel endothelial cell marker and a new targetfor cancer therapy. Biochim Biophys Acta 2006;1765:25–37.
- Bechard D, Meignin V, Scherpereel A, Oudin S, Kervoaze G, Bertheau P, et al.. Characterization of the secreted formof endothelial-cell-specific molecule 1 by specific monoclonal antibodies. JVasc Res 2000;37:417–25.
- Dallett M, O’Hagan KA, Koyler HA, Mills KI. Identification of gene networks associatedwith acute myeloid leukemia by comparative molecular methylation and expressingprofiling. Biomark Cancer 2010;2:43–5.
- Hatfield K, Øyan AM, Ersvaer E, Kalland KH, Lassalle P, Gjertsen BT, et al.. Primary human acute myeloid leukaemiacells increase the proliferation of microvascular endothelial cells throughthe release of soluble mediators. Br J Haematol 2009;44:53–68.
- Ryningen A, Stapnes C, Lassalle P, Corbascio M, Gjertsen BT, Bruserud Ø. A subset of patients with high-risk acutemyelogenous leukemia shows improved peripheral blood cell counts when treatedwith the combination of valproic acid, theophylline and all-trans retinoicacid. Leuk Res 2009;33:779–87.
- Bruserud Ø, Halstensen A, Peen E, Solberg CO. Serum levels of adhesion molecules and cytokinesin patients with acute leukaemia. Leuk Lymphoma 1996;23:423–30.
- Scherpereel A, Depontieu F, Grigoriu B, Cavestri B, Tsicopoulos A, Gentina T, et al.. Endocan, a new endothelial marker inhuman sepsis. Crit Care Med 2006;34:532–7.