Abstract
Chronic myeloid leukemia is a disorder that develops when a hematopoietic stem cell acquires the Philadelphia chromosome carrying the chimeric BCR/ABL oncogene leading to a deregulated cell proliferation and a decreased apoptosis in response to mutagenic stimuli. Therefore, it has been considered that BCR/ABL oncogene is a potential attractive target for anticancer agents. Antisense strategies aiming to suppress the expression of BCR/ABL in chronic myeloid leukemia cells have been studied by several research groups over the last decade. In the present study, the effect of Morpholino Oligo Antisense in BCR/ABL oncogene silencing was evaluated. To examine the hypothesis, K562 was used as a BCR/ABL fusion gene positive cell line using a Jurkat cell line as a control. The capacity of Morpholino Oligo Antisense in inhibiting the translation of p210bcr-abl protein by a western blotting technique, inhibition of cell proliferation, and stimulation of apoptosis by flow cytometric analysis after 24 and 48 hours was studied. Prolonged exposure of K562 cell line to Morpholino Oligo Antisense targeted against BCR-ABL showed proliferation inhibition as the main feature. Following western blotting, we found that complete silencing of BCR-ABL had been achieved but flow cytometric analysis showed no significant apoptosis. The results indicate that Morpholino Oligo Antisense was able to inhibit p210bcr-abl, but did not induce apoptosis due to co-silencing of BCR.
Introduction
It is generally believed that chronic myeloid leukemia (CML) develops when a single, pluripotential, hematopoietic stem cell acquires a Philadelphia chromosome (Ph chromosome) carrying the BCR-ABL fusion gene, which confers on its progeny a proliferative advantage over normal hematopoietic elements and thus allows the Ph-positive clone gradually to displace residual normal hematopoiesis.Citation1,Citation2 Moreover, CML cells seem to survive longer than their normal counterparts, as a result of a defective apoptotic response to stimuli that would otherwise lead to physiologic cell death.Citation3 The fusion of BCR and ABL on the Ph chromosome occurs in a head-to-tail manner, with the 3′ end of ABL in chromosome 9 joined to the 5′ end of BCR in chromosome 22.Citation4 This configuration places the fusion gene under the control of the BCR promoter and translated to p210Bcr-Abl and p190Bcr-Abl proteins. The Abl protein is a non-receptor tyrosine kinase, which normally has very little constitutive kinase activity.Citation5,Citation6 However, both the p210Bcr-Abl and the p190Bcr-Abl proteins have constitutively activated tyrosine kinase enzymatic activity, with higher levels of activity in the p190 protein. Tyrosine kinase activity is essential to cellular signaling and growth, and constitutively elevated kinase activity has been associated with oncogenic changes in several systems.Citation7
Recent advances in our understanding of the genetic changes that accompany cell transformation have provided new approaches for the development of cancer therapeutic agents that can potentially target specific genes that are expressed in a cancer cell. As the BCR/ABL oncogene plays a critical role in the pathogenesis of leukemia, it is considered that the BCR/ABL oncogene is an attractive target for cancer therapy. Antisense strategies aiming to suppress the expression of BCR/ABL in CML cells have received the attention of several research groups over the last decade. This is a promising approach, and several antisense oligonucleotides have already reached the stage of clinical trials.Citation8 We describe an overview of the design, preparation, and properties of Morpholino oligos, a novel antisense structural type that deals with sequence specificity and provides high and predictable activity in cells. Morpholino oligos also exhibit little or no non-antisense activity, afford good water solubility, are immune to nucleases, and are designed to have low production costs.Citation9,Citation10 Moreover, because of the excellent RNA binding affinity of Morpholino oligo, it seemed likely that they would be effective in blocking translation of their targeted mRNAs in both the presence and absence of RNase H.Citation9 As Morpholino oligos have more subunits in length and are independent of RNase H, they appear to be one of the more attractive antisense therapeutics in their exquisite specificity for the targeted genetic sequences.Citation8,Citation9
In this present study, we examined the effect of Morpholino Oligo Antisense, designed according to AMOD a web-based program on BCR/ABL silencing by inhibiting the translation of p210bcr-abl protein. In order to achieve this, K562 was used as a BCR/ABL fusion gene positive cell line and Jurkat cell line as a control. We assessed appropriate Morpholino Oligo Antisense doses delivered to the cytosol of the cell through an Endo-Porter system, a non-toxic delivery system of Morpholino. The translation of p210bcr-abl protein and p160bcr, p83bcr-related protein as control were evaluated by western blotting, in addition, cell proliferation, and stimulation of apoptosis were analyzed by flow cytometry after 24 and 48 hours.
Materials and Methods
Cell culture
K562 cells, a chronic myelogenous leukemia line, and Jurkat cells were maintained in RPMI 1640 medium (Sigma, St Louis, MO, USA) supplemented with 10% fetal bovine serum (Gibco; Invitrogen, Carlsbad, CA, USA) and 1 unit/ml penicillin–streptomycin (Gibco) ().Citation11 Fetal bovine serum was heat inactivated at 56°C for 20 minutes. K562 cell line was used as a BCR/ABL fusion gene positive and Jurkat cell line as a control. Both cell lines were incubated in humidified air at 37°C with 5%CO2 for 192 hours and half of the medium was changed every 48 hours. Cell viability assessed by trypan blue was >95%, following standard procedures.
Morpholino Oligo Antisense
The nucleotide sequences in Morpholino Oligo Antisense were designed by AMOD, a transparent, versatile, and effective tool for short oligonucleotide and primer design. It is a web-based program that aids in the functional evaluation of nucleotide sequences through sequence characterization and antisense Morpholino oligonucleotide (target site) selection. Submitted sequences are analyzed by translation initiation site prediction algorithms and sequence-to-sequence comparisons; results are used to characterize sequence features required for Morpholino design.Citation12
Experimental Morpholino with the sequences of 5′CGC GAA GCC CAC CGG GTC CAC CAT G3′ and negative control of Morpholino with the sequences of 5′-CCT CTT ACC TCA GTT ACA ATT TAT A 3′ were from Gene Tools, LLC (Philomath, OR, USA) in the form of lyophilized compounds. Negative control is the Morpholino with special sequences whose complementary does not exist in the K562 and Jurkat cells genomes. The lyophilized compounds were dissolved in diethylpyrocarbonate treated water, and every 100μmol/l of the solution were stored at −80°C. The Morpholinos were heated at 95°C for 5 minutes and quenched in ice before being transferred in K562 cells.Citation8
Delivery of Morpholino Oligo Antisense into cell cytosol
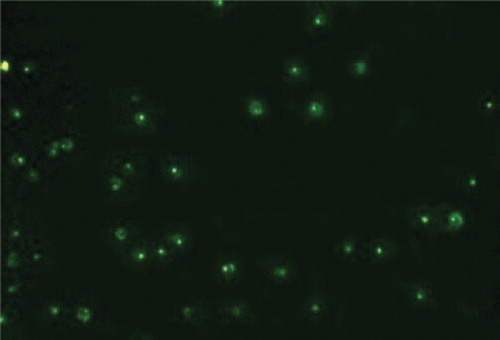
In order for a Morpholino Oligo Antisense to down-regulate gene expression, it must penetrate into the targeted cells. It was delivered to the cytosol of cell through Endo-Porter (Gene Tools, LLC) which is simple to use and shows little or no-toxicity to the cells even after continuous contact for 30 or 40 hours.Citation10 In order to calculate the appropriate doses of Morpholino-specific Endo-Porter required for successful delivery of the antisense graded experiments were performed. For this purpose, K562 cells (105/ml) were suspended and split up in volumes of 1 mm into 24-well plate along with 10 μM/ml fluorescent experimental Morpholino Oligo Antisense. Then, different concentrations of Endo-Porter from 2 to 8 μM/ml were added to cell suspension into consecutive seven wells in three rows. The delivered Endo-Porter led to rapid absorption of Morpholino into the cytosol. Delivery was assessed under inverted fluorescence microscopy after 48 hours. The fluorescence intensity and scattered pattern into the cytosol reflected suitable delivery and low fluorescence intensity indicated inadequate delivery for the various doses of Endo-Porter (). In some cases, both fluorescent patterns could be observed which indicates appropriate delivery doses. Following these experiments, the concentration of 7 μM/ml of Endo-Porter was selected as suitable for the ensuing studies.
Optimization of Morpholino Oligo Antisense
The principal aim was to optimize the amount of the Morpholino necessary to decrease cell proliferation and increase cell apoptosis. The Morpholinos were optimized for use at 37°C. When used at much lower temperatures, few Morpholinos have been reported to inhibit some non-targeted genes.Citation10 For this purpose, K562 cells at 1010/ml were suspended by volume of 1 mm into 24-well plate along with 7 μM/ml Endo-Porter as described previously. Different concentrations of Morpholino from 2 to 7 μM/ml were added to cell suspension into consecutive six wells in three rows. After 24 and 48 hours, the rates of cell proliferation and cell apoptosis were evaluated by flow cytometry.
Cell preparing for flow cytometry technique
After 24 and 48 hours exposure of cells to Morpholino, the cells were rinsed by phosphate-buffered saline (PBS) and suspended again by buffer binding. Then 195 μl of cell suspension were incubated with 5 μl Annexin V (AbD Serotec, Raleigh, NC, USA) conjugated with fluorescein isothiocyanate (Gene Tools, LLC) for 10 minutes at room temperature. After washing with PBS, the cells were prepared with 190 μl buffer binding and 24 μg/ml propidium iodide for flow cytometry.
Western blot analysis
After 24 and 48 hours exposure of the cells to Morpholino, the cells were harvested for western blot analysis. Total protein extracts from cells were obtained as indicated; cells were rinsed with PBS three times, and lysed in 25 mM lysis buffer (Cell Signaling, Danvers, MA, USA) with 100 mM phenylmethylsulphonyl fluoride (Sigma) and protease inhibitor mixture (Roche Diagnostics, Basel, Switzerland). After high speed centrifugation of 14 000 rev/minute for 10 minutes at 4°C, supernatant was collected and whole cell extracts were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis. The resolved proteins were transferred to nitrocellulose membranes or polyvinyllidene difluorid membranes (Millipore, Billerica, MA, USA) and probed with primary polyclonal antibody against BCR (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and secondary polyclonal antibody conjugated with biotin (AnaSpec, Fremont, CA, USA) against FC portion of primary antibody. Proteins were visualized by using radiographic film.
Measurement of cell viability
Viability of K562 with or without exposure to Morpholino was performed by trypan blue staining. Then cell population was counted by Neubauer lamella.
Statistical analysis
Significance levels were determined by Kruskal–Wallis and Mann–Whitney for comparison of the groups.
Results
Effect of Morpholino Oligo Antisense on cell apoptosis
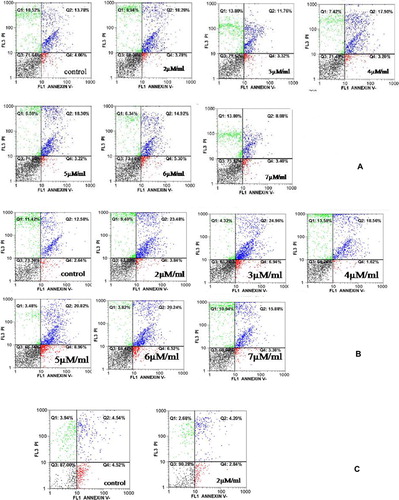
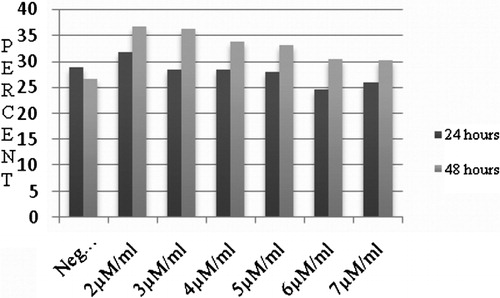
Incubation of K562 cells for 24 and 48 hours with different concentrations of Morpholino (2, 3, 4, 5, 6, and 7 μM/ml) and 7 μM/ml Endo-Porter resulted in changes in the rates of cell apoptosis and proliferation (). Although all Morpholino concentrations increased cell apoptosis, these were not statistically significant. In , cell apoptosis under culture conditions with different concentrations of Morpholino Oligo Antisense is summarized. The highest rate of cell apoptosis was in the sample treated with 2 μM/ml of Morpholino which was 39·72%±3·6. The negative control group was 26·64%±6·1.
Effect of Morpholino Oligo Antisense on cell proliferation and viability
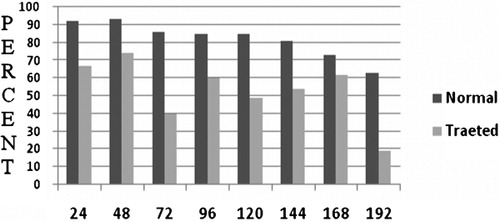
In contrast to our hypothesis, increasing the Morpholino concentration did not alter cell proliferation. Although all Morpholino concentrations reduced cell proliferation, the highest level of cell proliferation occurred when a limiting concentration of Morpholino (2 μM/ml) was applied. Consequently, 2 μM/ml Morpholino was selected to continue the study. shows that the trend for cell viability in the control group was gradually downwards, with 92% remaining after 24 hours culture to 63% by 192 hours. In treated group, by comparison, viability was much more erratic. In the first 48 hours culture, cell viability was approximately 70%, and at 72 hours it had declined to only 40%. Continued follow-up showed that cell viability fluctuated between 60 and 19% over 192 hours culturing. However, overall there were significant differences between control and treated groups in term of cell viability.
Effect of Morpholino Oligo Antisense on BCR/ABL
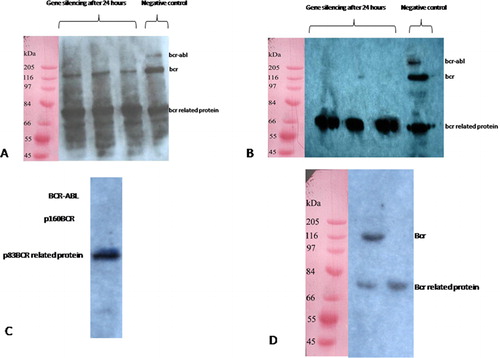
Morpholino Oligo Antisense was tested in Ph+ K562 cells and Jurkat cells as a control cell line expressing p210bcr-abl protein, p83bcr related protein, and p160bcr under the control of 7 μM/ml Endo-Porter penetration. The BCR-ABL protein levels were determined by western blotting at 24 and 48 hours following treatment with either 2 or 5 μM/ml Morpholino. These results show an elimination of p210bcr-abl protein in K562 cells treated with 2 μM/ml Morpholino compared with negative control treated after 24 hours (); meanwhile, level of p160bcr expression became weaker and after 48 hours the band disappeared (). In contrast, neither 2 nor 5 μM/ml concentration of Morpholino affected p83bcr-related protein expression. Compared with 2 μM/ml concentration of Morpholino, expressions of both p210bcr-abl and p160bcr in K562 cells were suppressed by 5 μM/ml Morpholino after 24 hours and almost no binding was detectable (). However, treated Jurkat cells with Morpholino lead to a suppression of p160bcr after 24 hours with no effect on p83bcr-related protein ().
Figure 6. (A) Western blotting 24 hours following 2 μM/ml Morpholino Oligo Antisens treatment showing that p210bcr-abl suppressed and the level of p160bcr became weaker, (B) by 48 hours, bond of p210bcr-abl and p160bcr was not observed. The expression of p83bcr-related protein was considered as internal control group. (C) In the case of K562 treated with 5 μM/ml Morpholino, no p210bcr-abl and p160bcr band was detected after 24 hours. (D) Jurkat, the control group included cells treated with 2 μM/ml Morpholino.
Discussion
Based on the hypothesis that antisense-locked nucleic acids could effectively suppress BCR/ABL, Morpholino Oligo Antisense was tested.Citation8 In our study, it was demonstrated that Morpholino could induce BCR/ABL gene silencing by inhibition of p210bcr-abl expression after 24 hours and that cell proliferation declined. However, cells apoptosis was not significantly altered even when p160bcr was suppressed for 48 hours following K562 exposure to Morpholino.
There have been major advances in the treatment of CML and the introduction of imatinib which specifically targets BCR-ABL tyrosine kinaseCitation13 has been a great success. However, it is becoming clear that a significant portion of patients and long-term treatment with imatinib develop resistance because of the acquisition of mutations in the kinase domain of BCR-ABL, which leads to escape from treatment response.Citation11 Therefore, the development of other BCR-ABL inhibitors has continued and one promising modality is gene therapy via siRNA and Antisenses.Citation14 The approach using siRNA is potentially flawed as it is exposed enzymatic degradation in the cytosol following which the cells regain their proliferative potential.Citation15 The application of antisense for therapeutic purposes is an alternate approach. Because of the excellent Morpholino binding and affinity to RNA structure, it seemed likely that these oligos would be effective in blocking the translation of their targeted mRNAs, and this has been found to be the case. Morpholino oligo, in both presence and absence of RNase H, inhibits its targeted mRNA better than the corresponding S-DNA oligo in the presence of added RNase H.Citation9,Citation16 However, one limitation of Morpholinos application for gene silencing is their restriction on start codon sequences, that is, Morpholinos are complementary of start codon of specific gene. It is therefore possible that using Morpholino for BCR/ABL gene silencing may lead to switching off of the normal BCR gene. The BCR gene silencing occurs due to lack of allelic exclusion in the Philadelphia translocation; therefore, all of the gene fusions associated with this translocation, such as b2/a2, b3/a2, are blocked since they have a same start codon.Citation17,Citation18 Morpholinos also can affect all the mutations leading to imatinib resistance which happens in ABL kinase domain.Citation13,Citation19 The purpose of our study was to try to inhibit the intensive cell proliferation and to induce cells apoptosis. Cell proliferation decreased as a result of p210bcr-abl suppression, but cell apoptosis did not significantly increase. It is possible that this may be due normal BCR gene silencing. We found that increasing Morpholino Antisense concentration decreased apoptosis, demonstrating the major role of BCR in apoptosis induction, since BCR was suppressed at an earlier time in vitro. However, using lower concentrations of Morpholino restricted cell proliferation and the cells survived in vitro without significant proliferation rate until cells death. Stimulation of K562 cells by Morpholino Antisense may maintain cells in a quiescent state.
Thus, in summary, we have shown that Morpholino Oligo Antisense is able to inhibit K562 cells proliferation and cell longevity but without inducing apoptosis. Understanding the effects of Morpholino Oligo Antisense on Bcr/Abl gene may provide important insights for novel anticancer therapies.
References
- Eaves CJ, Eaves AC. Stem cell kinetics. Baillieres Clin Haematol 1997;10:233–57.
- Carella A M, Daley G Q, Eaves C J, Goldman J M, Hehlmann R, editors. Chronic myeloid leukaemia: biology and treatment. London: Martin Dunitz; 2001. p. 73–100.
- Bedi A, Zehnbauer BA, Barber JP, Sharkis SJ, Jones RJ. Inhibition of apoptosis by BCR-ABL in chronic myeloid leukemia. Blood 1994;83:2038–44.
- Heisterkamp N, Stam K, Groffen J, de Klein A, Grosveld G. Structural organization of the BCR gene and its role in the Ph9 translocation. Nature (Lond) 1985;315:758–61.
- Deininger MW, Goldman JM, Melo JV. The molecular biology of chronic myeloid leukemia. Blood 2000;96:3343–56.
- Kurzrock R, Gutterman JU, Taplaz M. The molecular genetics of Philadelphia positive leukemias. N Engl J Med 1988;319:990–8.
- Shepherd P, Suffolk R, Halsey J, Allan N. Analysis of molecular breakpoint and m-RNA transcripts in a prospective randomized trial of interferon in chronic myeloid leukaemia: no correlation with clinical features, cytogenetic response, duration of chronic phase, or survival. Br J Haematol 1995;89:546–54.
- Rapozzi V, Cogoi S, Xodo LE. Antisense locked nucleic acids efficiently suppress BCR/ABL and induce cell growth decline and apoptosis in leukemic cells. Mol Cancer Ther 2006;5:1683–92.
- Summerton J, Weller D. Morpholino antisense oligomers: design, preparation, and properties. Antisense Nucleic Acid Drug Dev 1997;7:187–95.
- Summerton J. Morpholino antisence oligos: applications in biopharmaceutical research. IPT 2005;17:32–7.
- Gumireddy K, Baker SJ, Cosenza SC, John P, Kang AD, Robell KA, et al.. A non-ATP-competitive inhibitor of BCR-ABL overrides imatinib resistance. Natl Acad Sci USA 2005;102:1992–8.
- Klee EW, Shim KJ, Pickart MA, Ekker SC, Ellis LB. AMOD: a morpholino oligonucleotide selection tool. Nucleic Acids Res 2005;33:506–11.
- Apperley J. Mechanisms of resistance to imatinib in chronic myeloid leukemia. Lancet Oncol 2007;8:1018–29.
- Koldehoff M, Steckel NK, Beelen DW, Elmaagacli AH. Synthetic small interfering RNAs reduce bcr-abl gene expression in leukaemic cells of de novo Philadelphia(+) acute myeloid leukaemia. Clin Exp Med 2006;6:45–7.
- Withey JME, Harvey AJ, Crompton MR. RNA interference targeting of BCR-ABL increases chronic myeloid leukemia cell killing by 17-allylamino-17-demethoxygeldanamycin. Leuk Res 2006;30:553–60.
- Taylor M, Paulauskis J, Weller D, Kobzik L. In vitro efficacy of Morpholino-modified antiscrise oligomers directed against tumor necrosis factor-alpha mRNA. J Biol Chem 1996;22:17445–52.
- Collins S, Coleman H, Groudine M. Expression of bcr and bcr-abl fusion transcription in normal and leukemic cells. Mol Cell Biol 1987;7:2870–6.
- Laurent E, Talpaz M, Kantarjian H, Kurzrock R. The BCR gene and Philadelphia chromosome-positive leukemogenesis. Cancer Res 2001;61:2343–55.
- Melo J, Chuah C. Resistance to imatinib mesylate in chronic myeloid leukemia. Cancer Lett 2007;249:121–32.