Abstract
Fibrin plays a vital role in the coagulation process and fibrin fiber morphology can be studied using ultrastructural techniques. When studying the ultrastructure of fibrin networks, thrombin may be added to the plasma, ensuing fibrin network formation. The question that arises is whether there are differences in morphology when thrombin is added to plasma, versus morphology observed when plasma from citrated or recalcified citrated whole blood, is studied. The current study therefore aimed to compare ultrastructure of platelets and fibrin networks from these three techniques. Results indicated comparable platelet ultrastructure between smears formed from the plasma of citrated blood and that of the citrated recalcified blood. This method might give us further information regarding the ‘natural state’ fibrin assembly and association with platelets, when studying haemostasis. However, when studying the ultrastructure of fibrin networks, the addition of thrombin is necessary to form an expansive, fully coagulated layer of fibrin fibers.
Introduction
Fibrin plays an essential role in the clotting of blood during wound healing and also in the formation of pathogenic vascular thrombi.Citation1 Thrombin is the key activator of coagulation,Citation2 and allows fibrin networks to form. Fibrin assembly (through coagulation pathway and involvement of platelets) from fibrinogen proceeds in a highly ordered fashion. Under physiological conditions, thrombin catalyzes the hydrolytic removal of fibrinopeptides Aalpha and Bbeta from fibrinogen, converting the molecule to fibrin and revealing binding sites at its central domain that interact with complementary sites at the end domains of other fibrin molecules.Citation3 These noncovalent interactions cause fibrin monomers to assemble in a half-staggered manner into two-stranded protofibrils. Upon growing to sufficient length, the protofibrils aggregate laterally to form fibers that branch into a three-dimensional network.Citation3
To study the visco-elastic changes associated with fibrin polymerization, and thereby provide a global assessment of haemostatic functioning of a patient, the thromboelastography(TEG) technology has been widely used.Citation4 A TEG records the continuous profiles of whole blood coagulation by testing the efficiency of the coagulation process and provides information about platelet activation, fibrin formation, and clot retraction. TEG may either be performed immediately after blood collection or should immediate TEG processing not be possible, citrated blood may be used if recalcified after 1 to 8 hours of collection.Citation5 Citrate keeps the blood from forming clots, while the recalcification of citrate blood is performed by addition of CaCl2, and returns the blood to its ‘regular’ state. In various studies, the use of recalcified citrated whole blood in comparison with fresh native whole blood were investigated in TEG analysis where a concentration of 0·2M CaCl2 is used for the recalcification of citrated whole blood.Citation6Citation6,7
In our laboratory, we study the changes in ultrastructure of fibrin networks in different disease conditions. To do this, we draw blood in standard citrate tubes after which we centrifuge the blood to obtain platelet rich plasma (PRP). To study the ultrastructure of these fibrin fiber networks, thrombin may be added to assist in the polymerization process, with resulting fibrin network formation. However, the question that arises is whether an ‘artificial’ fibrin network is created that does not mimic the process that occurs in the body; and that with the addition of thrombin, an over-activated state of fibrin fibers are created. Therefore, the current research compares the ultrastructure of platelets and fibrin networks created from citrate blood using thrombin, recalcified citrate blood (without the addition of thrombin); and PRP from citrate whole blood.
Methods
Equipment used
A Zeiss ULTRA plus FEG-SEM with InLens capabilities were used to study surface morphology and micrographs were taken 1 kV. This instrument is located in the Microscopy and Microanalysis Unit of the University of Pretoria, Pretoria, South Africa.
Preparation of fibrin clots
Citrated whole blood with the addition of thrombin
Fresh PRP from a healthy donor was prepared by centrifuging citrated blood at 1 000 rpm (maximum RCF = 17·523×g; 1250 g) for 2 minutes. Human thrombin (provided by the South African National Blood Service) was used to prepare these fibrin clots from the donor. The thrombin solution was at a concentration of 20 U/ml and was made up in a biological buffer containing 0·2% human serum albumin. When thrombin is added to PRP, fibrinogen is converted to fibrin and intracellular platelet components, e.g. transforming growth factor, platelet-derived growth factor and fibroblastic growth factor are released into the coagulum. The PRP (10 μl) was mixed with 10 μl of human thrombin on a 0·2 μm millipore membrane to form the coagulum (fibrin clot).
Citrated whole blood with the addition CaCl2
The CaCl2 concentration of 0·2M was used and is according to standard procedures for TEG analysis.Citation6Citation6,7 Citrated whole blood (1 ml) was recalcified with 0·2M CaCl2 (60 μl) and centrifuged at 1000 rpm for one minute. This plasma (20 μl) was smeared onto a 0·2 μm millipore membrane.
The millipore membranes were then placed in a Petri dish on filter paper dampened with phosphate buffered saline (PBS) to create a humid environment and placed at 37°C for 10 minutes. This was followed by a washing process where the millipore membranes with the coagula were placed in PBS and magnetically stirred for 20 minutes. This was done to remove any blood proteins trapped within the fibrin network.Citation8Citation8,9
Citrated whole blood without the addition of CaCl2 or thrombin
This PRP (20 μl) was placed on a 0·2 μm millipore membrane. The millipore membrane was then placed in a Petri dish on filter paper dampened with PBS to create a humid environment and placed at 37°C for 10 minutes. In this case, a PRP smear was therefore created to study platelets. This was followed by a washing process where the millipore membranes were placed in PBS and magnetically stirred for 20 minutes. This was done to remove any blood proteins trapped within the fibrin network.Citation8Citation8,9
Preparation of washed fibrin clots and PRP smears for SEM
Washed fibrin clots and PRP smears were fixed in a 2·5% glutaraldehyde/formaldehyde in Dulbecco’s Phosphate buffered saline solution with a pH of 7·4 for 30 minutes. Each clot/smear was rinsed three times in phosphate buffer for 5 minutes before being fixed for 30 minutes with 1% osmium tetraoxide (OsO4.) The samples were rinsed three times with PBS for 5 minutes and were dehydrated serially in 30, 50, 70, 90% and three times with 100% ethanol. The material was critical point dried and mounted.
Results and Discussion
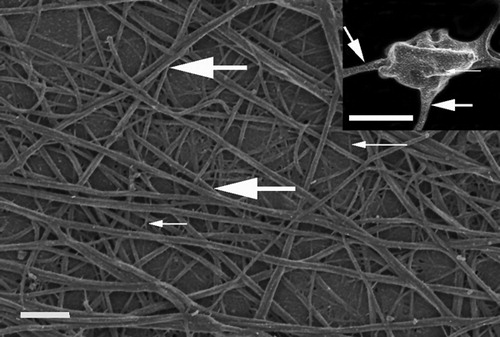
Previous research has shown that control fibrin networks in the presence of thrombin forms major, thick fibrin networks (thick, white arrows), as well as minor thin fibrin networks (thin, white arrows) (). Also, platelets with pseudopodia, a smooth membrane and fine open canalicular system pores are visible. Such a platelet is shown in the insert in . An open canalicular system pore is indicated with the thin, white arrow and thick fibrin fibers protruding from the platelet is shown with thick, white arrow ( – insert). This typical control fibrin and platelet morphology has been studied over the past 6 years and we believe that this is the ultrastructure of a typical fibrin clot during coagulation activation with thrombin.Citation10–Citation12 Typically, very few platelets are seen when a clot is prepared by adding thrombin to PRP obtained from citrated whole blood.
Figure 1. Fibrin network prepared by adding thrombin to platelet rich plasma. Thick, white arrow = major, thick fibers; Thin, white arrow = minor, thin fibers. Scale = 1 μm. Insert: platelet with thin, white arrow indicating open canalicular membrane pore; thick white arrow = major fibers leaving platelet. Scale = 1 μm.
During inflammation or changes in clotting potential, the addition of thrombin to PRP shows thickened masses of fibrin. This was also demonstrated recently in a group of healthy individuals who smoke. Their major, thick fibers formed a matted mass and attach longitudinally, and the term sticky fibrin phenomenon during smoking was coined.Citation13
Therefore, when studying changes in clotting in different diseases, the addition of thrombin may give us an idea of how fibrinogen and the other coagulation proteins will react in the human body, in the presence of thrombin. Because we compare the ultrastructure of the diseased clot to that of a control, and treat the control PRP the same as that of the diseased PRP, we believe that we have a good quality biological model. Although the activation of fibrin networks with thrombin seems a good idea, the question does arise whether we are not creating an artificially enhanced morphology. This research therefore compares the fibrin morphology created from thrombin to that of fibrin fibers created from citrate alone and from recalcified citrate whole blood.
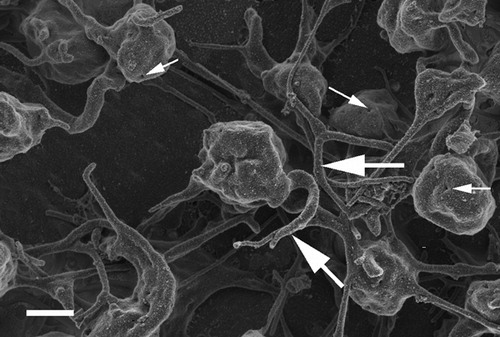
Since the development of the TEG technology, research as studied the effect of citrate blood and the recalcification of the blood to return it to its natural state before investigating the haemostatic functioning of a patients by looking at platelet activation, fibrin formation, and clot retraction.Citation4–Citation7Research has shown that addition of 0·2M CaCl2 is the ideal concentration to use in TEG procedures. Therefore, in the current study this CaCl2 concentration was also added to citrate control whole blood. shows the ultrastructure resulting from the addition of 0·2M CaCl2. When studying this PRP smear ultrastructure, it is clear that many platelets are present in the PRP and these platelets start to be associated with fibrin fibers (; thick, white arrow). Platelets also show open canalicular system pores (; thin, white arrows). Fibrin networks, similar to that of the thrombin activated coagulants do appear, but not as a typical spread-out layer. Here, these fibers are closely associated and are found only in the direct vicinity of the platelets. Also, only major, thick fibers are seen (; thick, white arrow), without the additional presence of minor, thin fibers as seen in the thrombin activated clot.
Figure 2. Platelet rich plasma from a citrated whole blood sample recalcified with addition of 0·2M CaCl2. Thick, white arrows = fibrin fibers associated with platelets; thin, white arrows = open canalicular membrane pores. Scale = 1 μm.
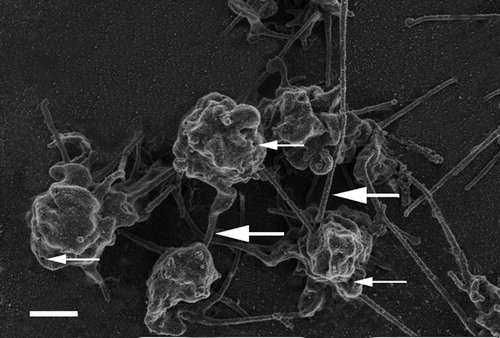
shows a PRP smear from citrated whole blood. In this smear, platelets were also associated with thick, major fibers. These ultrastructural characteristics of the platelets are comparable to those seen after recalcification of whole citrate blood (). No ultrastructural changes in platelets could therefore be distinguished between these two techniques (recalcified citrated whole blood versus PRP smears from citrated whole blood).
Conclusion
We conclude by suggesting that, when research into the ultrastructure of fibrin networks are required, the addition of thrombin is necessary to form a spread-out, fully coagulated layer of fibrin fibers. However, in addition to this, platelet morphology and associated fibrin fibers can be studied without the addition of thrombin, as this might give additional information. We did not see ultrastructural differences between citrated and citrate recalcified PRP smears. It is therefore suggested that, in the study of ultrastructure, in order to keep chemical alterations to the minimum, PRP from citrated whole blood should be studied. We conclude that this method might give us further information regarding the ‘natural state’ fibrin assembly and association with platelets, when studying haemostasis.
References
- O’Brien ET, Falvo MR, Millard D, Eastwood B, Taylor RM, Superfine R. Ultrathin self-assembled fibrin sheets. ProcNatl Acad Sci 2008;105(49):19438–43.
- Stief TW. Specific determination of plasmatic thrombinactivity. Clin Appl Thromb Hemost 2006;12(3):324–9.
- Ryan EA, Mockros LF, Weisel JW, Lorand L. Structural origins of fibrin clot rheology. BiophysJ 1999;77(5):2813–26.
- Reikvam H, Steien E, Hauge B, Liseth K, Hagen KG, Størkson R, et al.. Thrombelastography. TransfusApher Sci 2009;40(2):119–23.
- Camenzind V, Bombeli T, Seifert B, Jamnicki M, Popovic D, Pasch T, et al.. Citrate storage affects Thrombelastographanalysis. Anesthesiology 2000;92(5):1242–9.
- Bowbrick VA, Mikhailidis DP, Stansby G. The use of citrated whole blood in thromboelastography. AnesthAnalg 2000;90:1086–8.
- Rajwal S, Richards M, O’Meara M. The use of recalcified citrated whole blood –a pragmatic approach for thromboelastography in children. PaediatrAnaesth 2004;14:656–60.
- Pretorius E, Briedenhann S, Marx J, Smit E, van der Merwe CF, Pieters M, et al.. Ultra-structural comparison of themorphology of three different platelet and fibrin fibre preparations. AnatRecord 2007;290:188–98.
- Pretorius E, Oberholzer HM, Smit E, Steyn E, Briedenhann S, Franz RC. Ultrastructural changes in platelet aggregatesof HIV patients: a scanning electron microscopical study. UltrastructPathol 2008;32:75–9.
- Pretorius E. The role of platelet and fibrin ultrastructurein identifying disease patterns. Pathophysiol HaemostThromb 2008;36(5):251–8.
- Pretorius E, Oberholzer HM, van der Spuy WJ, Meiring JH. Macrothrombocytopenia: investigating the ultrastructureof platelets and fibrin networks using scanning and transmission electronmicroscopy. Ultrastruct Pathol 2009;33(5):216–21.
- Pretorius E, Oberholzer HM, van der Spuy WJ, Meiring JH. Age-related changes in fibrin networks andplatelets of individuals over 75: a scanning electron microscopy study showing ‘thromboticpreparedness’. J Thromb Thrombolysis 2010;29(3):271–5.
- Pretorius E, Oberholzer HM, van der Spuy WJ, Meiring JH. Smoking and coagulation: the sticky fibrinphenomenon. Ultrastruct Pathol 2010;34(4):236–9.