Abstract
The objective of this single-center study was to determine the pretreatment risk factors and influence of comorbidity on outcome in patients with acute myeloid leukemia (AML). The research involved 145 patients with AML during a 58-month follow-up period. The results suggest that the most significant predictor of poor overall survival (OS) is an adverse karyotype (P = 0.007), while for poor rate of complete remission (CR) it is age ≥55 years, and for early death the most significant predictor is comorbidity, as scored by the Hematopoetic Cell Transplantation Comorbidity Index (HCT-CI), P = 0.001. When we divided the patients into two groups: aged ≥55 years and aged <55 years, these predictors differed. In the group aged ≥55 years the most significant predictor of OS (P = 0.013) and for early death (P = 0.003) was HCT-CI (P = 0.013), while in the younger group it was karyotype (P < 0.001). The most significant predictor of CR in the elderly was increased serum lactate dehydrogenase (LDH) level (P = 0.045). In the younger patients, the most significant predictor of CR was leukocytosis (P = 0.001) and for early death it was infection as the comorbidity (P = 0.007). We point out the importance of comorbidity for OS and early death, as well as the impact of infection in patients with AML.
Introduction
Acute leukemias are clonal malignant hematopoietic disorders that result from genetic alterations in normal hematopoietic stem cells. These changes induce differentiation arrest and/or excessive proliferation of abnormal leukemic cells or blasts.Citation1 Acute myeloid leukemia (AML) is the most common type of leukemia in adults, yet continues to have the lowest survival rate of all leukemias.Citation2 The incidence of AML increases with advancing age. At age 40 years it is 1:100 000, but the annual rate increases to 15:100 000 at age >75 years.Citation3–Citation6 The median age at presentation is approximately 65 years.Citation1
The prognosis and clinical course of AML differs among patients depending on specific features of the disease, such as cytogenetic/molecular genetic characteristics, white blood cell (WBC) count and serum lactate dehydrogenase (LDH) level. Moreover, patient-related factors including age, performance status, and pre- or co-existing diseases (comorbidity) are of prognostic importance, too.Citation7–Citation9 Together, these factors have a significant influence on the outcome for AML patients.
Comorbidity is any distinct additional clinical entity that pre-exists or occurs during the clinical course of a primary disease of a patient.Citation10 An important influence of comorbidity on patient outcome has been demonstrated in several malignancies.Citation11 The Hematopoetic Cell Transplantation Comorbidity Index (HCT-CI) was recently developed by Sorror et al.Citation12 for patients who were candidates for allogeneic stem cell transplantation. The HCT-CI tested successfully in older patients with AML,Citation11–Citation14 but, there are few data about the impact of comorbidity on outcome of younger patients with AML.
The objectives of this single-center study were to determine pretreatment risk factors for overall survival (OS), rate of complete remission (CR), and early death in patients with AML, as well as to estimate the influence of comorbidity on patient outcome.
Materials and methods
Patients
This study included 145 patients with previously untreated non-promyelocytic AML monitored for 58 months. OS was defined as the time from diagnosis to death or date of last follow up. Early death was defined as death occurring before response evaluation unless evidence of resistant disease was provided at least 7 days after conclusion of the chemotherapy that was within 60 days after start of treatment.Citation15 Response criteria for CR were recommended by the International Working Group.Citation16 The following parameters were recorded: age, sex, WBC and platelet counts, hemoglobin level, serum LDH level, expression of CD34 antigen on leukemic blasts, AML subtype classification by the French-American-British (FAB) Cooperative Group,Citation17 karyotype risk group using the European LeukemiaNet (ELN) recommendation,Citation18 PS using the Eastern Cooperative Oncology Group (ECOG) scale, and the comorbidity score using HCT-CI.
Treatment
Patients were treated with the Medical Research Council (MRC) 12 regimen. Induction chemotherapy consisted of daunorubicin at a daily dosage of 45 mg/m2 on days 1–3, in combination with cytarabine at 200 mg/m2 daily as a continuous intravenous infusion for 7 days (3 + 7 regimen). Patients who achieved CR after 1 or 2 cycles of induction chemotherapy received two cycles of consolidation chemotherapy: mitoxantrone at a daily dosage of 10 mg/m2 for 5 days with cytarabine 1000 mg/m2 for 3 days (MiDAC regimen). In the group of patients (≥55 years and HCT-CI ≥3, 25 received the MRC 12 regimen with 50% reduced doses of daunorubicin in induction chemotherapy, 20 patients were treated by palliative cytoreductive therapy with hydroxyurea or etoposide, applied per OS and nine patients were only on supportive therapy with periodical blood transfusions All patients received prophylactic antibiotic therapy with ciprofloxacin (500 mg twice a day) and fluconazole (150 mg) or itraconazole (200 mg) daily per OS.
Methods
A leukocyte count ≥30 × 109/l was considered as leukocytosis. A serum LDH concentration more than 1.5 times the upper limit of normal values (>600 U/l) was taken as high. A cut-off >10% was used for CD34 antigen expression, as detected by flow-cytometry. Cytogenetics risk group categories were stratified by ELN classification, as follows: favorable, intermediate I, intermediate II, and adverse. ECOG PS ranged from 0 to 4, and scores <2 vs. ≥2 were compared. Comorbidity values <3 vs. ≥3, as graded by HCT-CI, were also compared. All recorded parameters were examined as risk factors in all patients included in the study, as well as in the subgroup of patients aged ≥55 years and the subgroup of patients <55 years old. The impact of infection as the comorbidity in the HCT-CI score and its role as the risk factor for OS, CR, and early death, was particularly explored in the younger patients. Infection as the comorbidity was defined according to the HCT-CI definition: documented infection or fever of unknown etiology requiring antimicrobial treatment before, during, and after the start of the induction regimen.
Statistical analysis
Statistical associations were assessed using Pearson's xCitation2 test and the Fisher exact test. Survival rates were estimated by the Kaplan–Meier method and the log-rank test was employed for comparison of survival curves between different groups of patients. Univariate and multivariate COX proportional regression models were employed for identification of risk factors. Hazard ratios were calculated with a 95% confidence interval (CI). Parameters that were statistically significant in the univariate analyses were included in the multivariate regression procedure. All statistical analyses were performed with SPSS, version 18 (SPSS).
Results
The main characteristics of the patients are shown in . Their mean age was 55 years (range 18–79 years). There were 76 (52%) women and 69 (48%) men. Eighty-one patients (56%) were aged ≥55 years and 64 (44%) were <55 years old.
Table 1. Patient characteristics
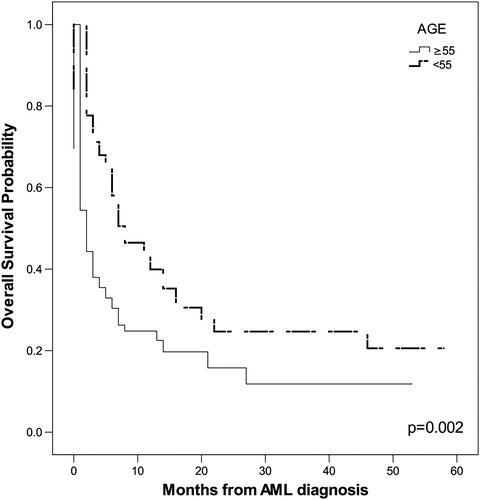
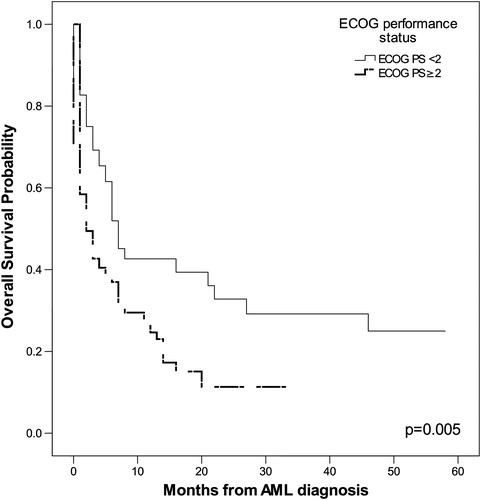
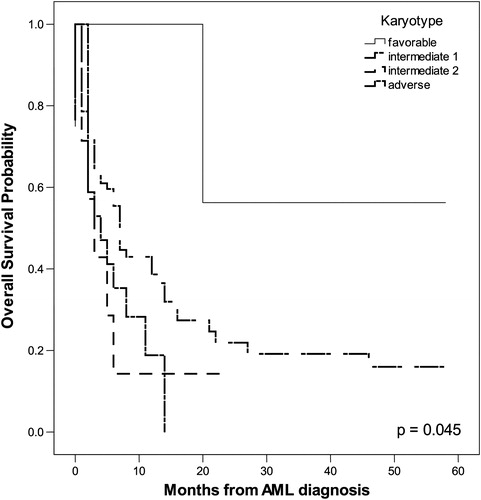
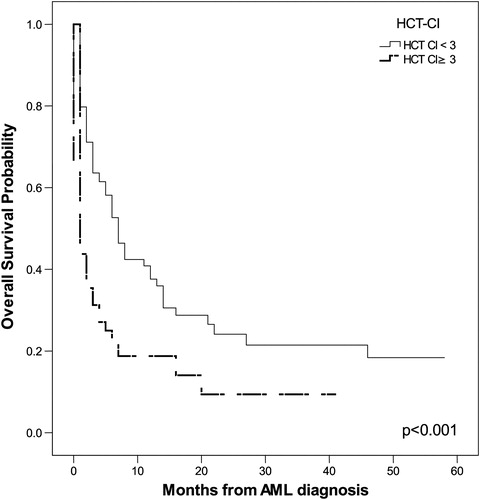
The probability of OS in months, as tested by the Kaplan–Meier method, correlated positively with the following parameters: age ≥55 years (P = 0.002), leukocytosis (P = 0.011), serum LDH level (P = 0.003), ECOG PS ≥2 (P = 0.005), adverse karyotype (P = 0.045), and HCT-CI ≥ 3 (P < 0.001). The impact of these parameters on OS is shown in .
Figure 2. Leukocytes count ≥ 30 × 109/l had significant impact on OS (P = 0.011 by Kaplan–Meier method).
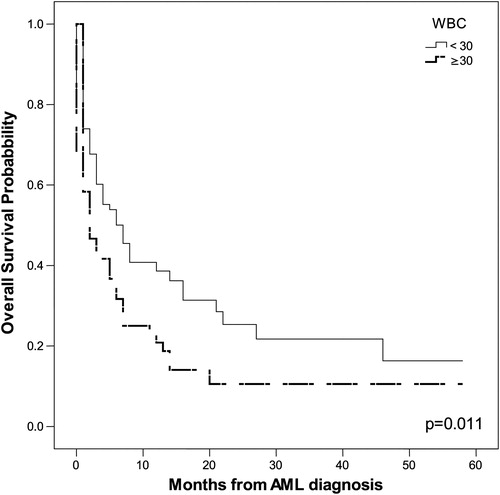
Univariate COX proportional regression analysis indicated that the following tested features were significant predictors for poor OS: age ≥55 years (P = 0.002), adverse karyotype (P = 0.008), and HCT-CI ≥3 (P = 0.035). The multivariate COX proportional regression method pointed to adverse karyotype (P = 0.007, relative risk (RR) = 1.417, 95% CI 1.101–1.823) as the most significant predictor for poor OS.
In the univariate analysis, age ≥55 years (P < 0.001), leukocytosis (0.029), ECOG PS ≥2 (P = 0.006), and HCT-CI ≥3 (P = 0.010) were found to be significantly correlated with a poor rate of CR. The most significant factor associated with poor CR rate in the multivariate analysis, was age ≥55 years (P < 0.001, RR = 0.229, 95% CI 0.108–0.508).
Univariate analysis showed that significant factors for early death were increased serum LDH level (P = 0.009) and HCT-CI ≥ 3 (P = 0.001). The most significant risk factor for early death using the multivariate method was HCT-CI ≥ 3 (P = 0.001, RR = 0.357, 95% CI 0.202–0.630).
In the subgroup of patients aged ≥55 years, 41 patients (51%) had HCT-CI ≥3, while only 8 patients (12%) younger than 55 years had HCT-CI ≥3 . Thus, the incidence of HCT-CI ≥ 3 was significantly higher in the older group (P < 0.001). Moreover, in that group 30 patients (47%) had HCT-CI = 0 (without any comorbidity), 14 (22%) had HCT-CI = 1, 12 (19%) had HCT-CI = 2, and 8 patients (12%) had HCT-CI ≥3. Infection as a comorbidity had an impact on the HCT-CI score (according to the HCT-CI definition) in most patients (94%) >55 years old with HCT ≥1. The distribution of comorbidities in the younger patients is shown in .
Table 2. Distribution of comorbidity scored in the HCT-CI in patients <55 years
Because the significant cut-off age for poor OS and CR was an age ≥55 years, as well as the increased incidence of HCT-CI ≥3, we estimated the risk factors separately for both groups. The most significant risk factor for OS in the group <55 years old was adverse cytogenetics (P < 0.001, RR = 1.731, 95% CI 1.239–2.342), while in the older group it was HCT-CI ≥ 3 (P = 0.013, RR = 1.893, 95% CI 1.142–3.137). In the group aged <55 years, the most significant factor for poor CR rate was leukocytosis (P = 0.001, RR = 4.000, 95% CI 1.747–9.117) and for early death it was infection as the comorbidity (P = 0.007, RR = 0.063, 95% CI 0.008–0.471). In the group of patients aged ≥55 years, the most significant risk factor for poor CR rate was elevated serum LDH level (P = 0.045, RR = 3.070, 95% CI 1.026–9.184) and HCT-CI ≥3: P = 0.003, RR = 0.627 (95% CI 0.463–0.849) for early death.
Discussion
The aim of the present study was to determine which factors are associated with poor prognosis in patients with AML. We also focused on the influence of comorbidities on patient outcome, particularly on the impact of infection as a comorbidity in AML patients <55 years. The mean age of the patients in our study was 55 years, while other authors reported a median age of 65 years in patients with AML at presentation.Citation1,Citation2 Thus, our average patient newly diagnosed with AML is approximately 10 years younger than those in published epidemiological data. Epidemiological studies have shown the importance of ethnicity and socioeconomic status on the incidence and survival of patients with AML,Citation2,Citation19 but the observed age difference in our study remains unclear and requires further investigation.
We have shown that significant predictors of poor OS in patients with AML are age, leukocytosis, increased serum level of LDH, adverse karyotype, poor ECOG PS, and high HCT-CI. In our group of patients, the cut-off for age of patients with significantly shorter OS was 55 years. Ages ranging from 60 to 75 years for cut-off for poor OS were found in other studies.Citation6,Citation20,Citation21 Thus, our AML patients also had a lower age cut-off for OS compared with patients described in other studies. Kristinsson et al.Citation19 demonstrated that socioeconomic status is significantly associated with survival in patients with AML, as lower mortality was observed among the highest socioeconomic group and differences in comorbidity and lifestyle were likely factors to explain these findings. The lower socioeconomic status and poorer living conditions of our patients could be a reason for the younger age cut-off for OS. The significant influences of leukocytosis, serum LDH level, adverse karyotype, ECOG PS ≥2, and HCT-CI ≥3 on OS is in accordance with published findings.Citation3,Citation5–Citation7,Citation13,Citation15,Citation20,Citation22,Citation23 The most significant independent predictor for poor OS in our investigation was adverse karyotype and this agrees with the findings of other authors.Citation5,Citation7,Citation18,Citation20
Our research demonstrated significant correlations between the rate of CR and: leukocytosis, serum LDH level, ECOG PS ≥2, HCT-CI ≥3, and age ≥55, which was the most significant factor for a poor rate of CR in the multivariate analysis. The significant factors for early death were serum LDH level and HCT-CI ≥3. Multivariate analysis indicated HCT-CI ≥3 to be the most important factor for early death. Other recent studies also found significant associations of these parameters with lower CR rate and early death rates.Citation3,Citation5,Citation6,Citation13
The significant age cut-off for poor OS and CR was 55 years. Also, the incidence of a high HCT-CI was greatly increased in patients aged ≥55 years compared with the younger patients. This was the reason we divided our patients into two groups according to age for separate assessment of risk factors for OS, CR, and early death. Because infection had the most frequent impact on HCT-CI score in patients younger than 55 years old, we estimated infection as a separate risk factor for outcome in this group. The most significant predictor for poor OS in this group was adverse karyotype, while in the group aged ≥55 years it was HCT-CI ≥ 3. The data regarding the significance of adverse karyotype for OS, as well as the influence of comorbidity on OS in elderly patients with AML support many recently published findings.Citation1,Citation5–Citation7,Citation9,Citation10,Citation13–Citation15,Citation17,Citation18,Citation20 In the group aged ≥55 years, the most significant predictor of poor CR rate was serum LDH level, while the most important predictor of early death was also comorbidity. These results are in accordance with the published findings.Citation5,Citation13,Citation15 Nevertheless, in the group <55 years old, the most significant risk factor for poor CR rate was leukocytosis and for early death it was infection as a comorbidity. Infections are an important cause of morbidity and mortality for patients with AML.Citation24 These patients are at a particularly high risk of infection, probably related to the intensity of their therapy, which leads to prolonged and profound neutropenia. Although it is known that infections are common in patients with AML, little is understood about the prediction value of infection in this population.
Conclusion
We have determined the most significant pretreatment risk factors for outcome of patients with AML. Adverse karyotype was the most significant risk factor for poor OS, while age ≥55 years was the most significant risk factor for poor CR rate and comorbidity the most significant risk factor for early death. More exactly, in elderly patients, aged 55 years, the most significant predictor of poor OS and early death was comorbidity, while serum LDH level was the most significant predictor of poor CR rate. In younger patients with AML, aged <55 years, the most significant predictor of poor OS was adverse karyotype, while the most significant predictor of poor CR rate was leukocytosis. We particularly emphasize that this study confirmed that infection as a comorbidity was the most significant predictor of early death in younger patients with AML.
Acknowledgement
This study was supported by the Ministry of Science and Technological Development of Republic of Serbia, N0 41004.
References
- Jabbour EJ, Estey E, Kantarjian HM. Adult acute myeloid leukemia. Mayo Clin Proc. 2006;81(2):247–60.
- Deschler B, Lubbert M. Acute myeloid leukemia: epidemiology and etiology. Cancer. 2006;107(9):2099–107.
- Erba HP. Prognostic factors in elderly patients with AML and the implications for treatment. Hematol Am Soc Hematol Educ Program. 2007;1:420–8.
- Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–5.
- Kantarjian H, O'Brien S, Cortes J, Slovak ML, Willman CL, Godwin JE, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high risk myelodisplastic syndrome: predictive prognostic models for outcome. Cancer. 2006;106:1090–8.
- Giles FJ, Borthakur G, Ravandi F, Faderl S, Verstovsek S, Thomas D, et al. The haematopoetic cell transplantation comorbidity index score predictive of early death and survival in patients over 60 years of age receiving induction therapy for acute myeloid leukemia. Br J Haematol. 2007;136:624–7.
- Grimwade D, Hills RK. Independent prognostic factors for AML outcome. Hematol Am Soc Hematol Educ Program. 2009:385–95.
- Schellongowski P, Staudinger T, Kundi M, Laczika K, Locker GJ, Bojic A, et al. Prognostic factors for intensive care unit admission, intensive care outcome, and post-intensive care survival in patients with de novo acute myeloid leukemia: a single center expiriance. Haematologica. 2011;96(2):231–7.
- Blaise D, Vey N, Faucher C, Mohty M. Current status of reduced intensity conditioning allogenic stem cell transplantation for acute myeloid leukemia. Haematologica. 2007;92(4):533–41.
- Feinstein AR. The pretherapeutic classification of co-morbidity in chronic disease. J Clin Dis. 1970;23:455–68.
- Birim O, Kappetein AP, Bogers AJJC. Charlson comorbidity index as a predictor of long term outcome after surgery for non-small cell lung cancer. Eur J Cardiothorac Surg. 2005;28:759–62.
- Sorror ML, Marris MB, Storb R, Fonatsch C, Heinecke A, Wormann B, et al. Hematopoetic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assasment before alogenic HCT. Blood. 2005;106:2912–9.
- Hiddeman W, Kern W, Schoch C, Mozziconacci MJ, Arnoulet C, Coso D, et al. Management of acute myeloid leukemia in elderly patients. J Clin Oncol. 1999;17:3569–76.
- Etienne A, Esterni B, Charbonnier A, Baron F, Sandmaier BM, Maloney DG, et al. Comorbidity is an independent predictor of complete remission in elderly patients receiving induction chemotherapy for acute myeloid leukemia. Cancer. 2007;109:1376–83.
- Malfuson JV, Etienne A, Turlure P, de Revel T, Tomas X, Contentin S, et al. Risk factors and decision criteria for intensive chemotherapy in older patients with acute myeloid leukemia. Haematologica. 2008;93:1806–13.
- Cheson BD, Bennet JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, et al. International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. Revised recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcome, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol. 2003;21:4642–9.
- Bennet JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al. Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group. Br J Haematol. 1976;33:219–28.
- Donner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:454–74.
- Kristinsson SY, Derolf AR, Edgren G, Dickman PW, Bjorkkholm M. Socioeconomic differences in patient survival are increasing for acute myeloid leukemia and multiple myeloma in Sweden. J Clin Oncol. 2009;27(12):2073–80.
- Breccia M, Frustaci AM, Cannella L, Stefanizzi C, Latagliata R, Cartoni C, et al. Comorbidities and FLT3-ITD abnormalities as independent prognostic indicators of survival in elderly acute myeloid leukemia patients. Haematol Oncol. 2009;27:148–53.
- Klepin HD, Baldalucci L. Acute myelogenous leukemia in older adults. Onncologist. 2009;14:222–32.
- Ferrnandez HF. New trends in the standard of care for initial therapy of acute myeloid leukemia. Hematol Am Soc Hematol Educ Program. 2010;2010:56–61.
- Lowenberg B. Acute myeloid leukemia: the challenge of capturing disease variety. Hematol Am Soc Hematol Educ Program. 2008;1:1–11.
- Sung L, Lange BJ, Gerbing RB, Alonzo TA, Feusner J. Microbiologically documented infections and infection-related mortality in children with acute myeloid leukemia. Blood. 2007;110(10):3532–9.