Abstract
Fasting serum prolactin (PRL) levels in response to metoclopramide (MCP) and lymphocyte cytokine profiles was studied in patients given allografts and their donors. Thirty normoprolactinemic volunteers (12–59 years) were studied: group 1, 10 healthy men; group 2, 8 males and 2 females with various hematologic diseases; and group 3, 3 males and 7 females HLA-identical sibling donors: PRL and cytokines were measured. Four surviving recipients developed acute graft-versus-host disease (GVHD) (+), and six did not.
Before transplant
Fasting PRL concentrations were higher in ‘future’ GVHD(+) recipients than in their donors (P < 0.001). The opposite was seen in response to MCP (P = 0.01). Donors had a predominant T-helper type 1 (Th1) cytokine profile compared with recipients (P ≤ 0.02), and GVHD(+) recipients had a greater tumor necrosis factor (TNF) value than GVHD(−) (P = 0.05).
After transplant
On days +30 and +100, a mild sustained rise in fasting PRL levels occurred only in GVHD(+) recipients (P ≤ 0.05) simultaneously with a transient rise in Th1 cytokines. GVHD(−) recipients had no changes. Donors with a Th1 cytokine profile might be more prone to induce GVHD in their recipients, and a mild sustained rise in PRL concentrations after transplantation in recipients GVHD(+) might participate in the amelioration of the severity of GVHD.
Introduction
The etiopathogenic mechanism of graft-versus-host disease (GVHD) is still not fully understood.Citation1,Citation2 GVHD has been arbitrarily separated into two categories: acute and chronic GVHD with a predominant T-helper type 1 (Th1) cytokine profile in the former and a predominance of a Th2 cytokine profile in the latter.Citation3,Citation4
There is limited information on the possible participation of PRL in the occurrence of GVHD after allogeneic hematopoietic stem cell transplantation (allo-HSCT) in longitudinal studies. The presence of high serum prolactin (PRL) concentrations in patients after allo-HSCT complicated by chronic GVHD has been previously reported.Citation5 This observation may well have clinical relevance since PRL – whether of pituitary or extrapituitary origin – acts not only as a hormone, but also as a Th1 type of cytokine, which functions as a co-mitogen with interleukin-2 (IL-2) in the activation and proliferation of T cells and natural killer (NK) cells providing that PRL concentrations are within physiological range.Citation6,Citation7 Moreover, PRL influences restoration of the hematopoietic homeostasis under dysregulated conditions,Citation8,Citation9 and recombinant human PRL stimulates hematopoiesisCitation9,Citation10 and lymphoid and myeloid reconstitutionCitation11 in animal models. At present, it is well accepted that PRL has a key role in both the humoral and cellular immune responses in humans, acting through endocrine, paracrine, and autocrine mechanisms;Citation12,Citation13 however, either too little or too much PRL, may be immunologically deleteriousCitation14 especially under prolonged conditions.Citation6,Citation12
Basal pituitary PRL is under the control of a tonic inhibitory mechanism mediated by dopamine (DA)Citation15 and both basal serum PRL concentrations and their response to intravenous (i.v.) metoclopramide (MCP) (DA-blocking agent) have been considered as a good indicator for evaluating the functional status of the hypothalamic DA tone in different clinical settings.Citation16,Citation17 A previous report in a small group of patients with leukemia studied after allo-HSCT, and following MCP infusion, showed a greater area under the PRL curve (AUC-PRL) in patients with chronic GVHD than in patients without GVHD or normal controls.Citation18 This finding suggested the existence of an increased functional level of their hypothalamic dopaminergic (DA) tone – as an adaptive mechanism – aiming to decrease the release of pituitary PRL and maintain the circulating PRL concentrations within a physiological range.
The aims of the present study were: (1) to explore PRL concentrations both basal and after MCP administration in patients who will receive an allo-HSCT and their donors before and at various intervals after the allograft transplant and (2) to investigate a possible association between the cytokine profile, serum PRL concentrations, and the subsequent clinical course after the allograft transplant.
Materials and methods
The study protocol was approved by the Internal Review Board of the Human Ethical Committee of the Centro de Hematología y Medicina Interna de Puebla (Puebla, Mexico) and the Hospital Universitario, Universidad Autónoma de Nuevo León (UANL) (Nuevo León, Mexico). Written informed consent was obtained from all volunteers. The study was conducted according to the Declaration of Helsinki (as amended October 2000).
Study population
The study involved 30 volunteers, aged 12–59 years with a body mass index (BMI) between 18.7 and 37.1 kg/m2. None drank alcohol regularly or ingested any medication known to increase serum PRL concentrationsCitation19 4 months prior to the study. All volunteers were euthyroid (serum thyroid stimulating hormone [TSH] <2.5 µU/ml and free T4 between 0.8 and 2.0 ng/dl) and were seronegative for both hepatitis C virus and the human immunodeficiency virus. The volunteers were classified into three categories: group 1, 10 clinically healthy men (aged 20–52 years; described in a previous report);Citation18 group 2, 10 consecutive patients (2 females and 8 males, aged 14–59 years) candidates for allo-HSCT: 2 with severe aplastic anemia (SAA), 2 with chronic myelogenous leukemia (CML), 2 with acute myelogenous leukemia (AML), 2 with multiple myeloma (MM), 1 with non-Hodgkin's lymphoma (NHL), and 1 with mycosis fungoides (MF); and group 3, 10 healthy human leukocyte antigen (HLA)-identical sibling donors (7 females and 3 males, aged 12–53 years).
Patients and donors
All patients allografted in the Centro de Hematología y Medicina Interna de Puebla, and the Hospital Universitario, UANL were prospectively accrued onto the study. Classes I and II HLA antigens were studied by molecular biology. All patients with acute leukemia underwent transplantation after achieving a complete remission and had a Karnofsky status of 100% when the procedure was performed. The donor was an HLA-compatible sibling in all instances.
HSC mobilization and apheresis
Granulocyte colony stimulating factor (10 µg/kg/day) was delivered to the sibling donors on day −5 to +2. The apheresis procedures were performed on days 0, +1, and +2, according to the cell counts, by means of a Haemonetics V-50 PLUS machine (Haemonetics Corporation, Braintree, MA, USA) or a Baxter C-3000 PLUS machine (Baxter Healthcare, Deerfield, IL, USA), using the Spin–Nebraska protocol.Citation20 A total of 5000–7000 ml of blood/m2 was processed in each of the apheresis procedures, to obtain a minimum of 5 × 108 mononuclear cells and/or 2–6 × 106 viable CD34 cells/kg of the recipient. Enumeration of the total white blood, mononuclear and CD34+ positive cells was done by flow-cytometryCitation21,Citation22 in an EPICS Elite ESP apparatus (Coulter Electronics, Hialeah, FL, USA), using the anti-CD34 monoclonal antibody HPCA-222 (Becton Dickinson, San José, CA, USA). No purging procedures were performed.
Conditioning and grafting
The ‘Mexican’ reduced intensity stem cell transplantation conditioning regimen was used:Citation23,Citation24 Oral busulphan, 4 mg/kg was delivered on days −6 and −5; i.v. cyclophosphamide, 350 mg/m2 on days −4, −3, and −2; i.v. fludarabine, 30 mg/m2 on days −4, −3, and −2; oral cyclosporin A (CyA) 5 mg/kg was started on day −1; and i.v. methotrexate 5 mg/m2 was delivered on days +1, +3, +5, and +11. Oral CyA was continued through day 100 with adjustments according to the levels of whole blood CyA. Ondansetron (1 mg orally every 12 hours during 2 days after i.v. chemotherapy), ciprofloxacin (250 mg bid), and itraconazole (100 mg bid) were used in all patients; antibiotics and antimycotics were used until more than 500 granulocytes/μl were present. The products of the peripheral blood stem cell (PBSC) apheresis were re-infused as outpatients on days 0–2, according to the CD34 cell yield (vide supra). Acute and chronic GVHD were defined according to conventional criteria: acute GVHD was defined as that appearing within 100 days after allo-HSCT.
Chimerism studies
In cases with a sex mismatch, a fluorescent in situ hybridization technique to demonstrate the X and Y chromosomes was carried out,Citation25 whereas restriction fragment length polymorphismCitation26 studies in the peripheral blood lymphocytes were used in the other cases.
Experimental protocol
The protocol has been previously published.Citation18 In brief, between 8:00 and 8:30 hours and after a 10–12-hour overnight fast an indwelling catheter was placed in a forearm vein and kept patent with a slow i.v. drip of 0.15 M NaCl solution. After a 30-minute rest, three basal non-heparinized blood samples were obtained at 15-minutes interval (−30, −15, and 0 minutes) and thereafter at 30, 60, 90, and 120 minutes following a 10-mg i.v. bolus of MCP (Carnotprim, Laboratorios Carnot, Mexico City). Duplicate determinations of serum PRL were performed. The serum PRL concentrations at −30, −15, and 0 minutes in each patient were pooled and expressed as mean fasting serum PRL concentration. The AUC-PRL was calculated by the trapezoid method.
Volunteers in group 1 were studied only once.Citation18 In group 2 the MCP test was performed the day before the non-ablative stem cell transplantation conditioning regimen was initiated, and in group 3 a week before the administration of granulocyte colony-stimulating factor. An additional blood sample was obtained in the patients and their donors for the determination of cytokines. Thereafter, three non-heparinized fasting blood samples were again obtained at 15-minute intervals only in the patients on days +14, +30, and +100, at which time the MCP test was repeated as in the basal study. Blood samples for the determination of cytokines were also obtained on days +14, +30, and +100.
Hormonal assays
The PRL determinations were performed in duplicate by a quimioluminiscent immunoassay using the Immulite 1000 equipment and commercially available kits (Siemens Medial Solutions Diagnostics Limited, Llanberis, UK). The intra- and interassay coefficients of variation over the range 0–150 ng/ml were ≤5.0 and ≤5.7%, respectively. The kits were calibrated against the World Health Organization 3rd International Reference Preparation 84/500. Serum samples were analyzed in four assays, and the samples were distributed equally relative to each group studied. The sensitivity of the assay was 0.5 ng/ml.
Determination of cytokines in culture supernatants
This was done with an amplified fluorescence bead array by flow cytometry, using the BD CBA Human Th1/Th2 Cytokine Kit® (BD Biosciences, San Jose, CA, USA). Briefly, each bead in a cytometric bead array provides a capture solid face for a given protein or peptide and is analogous to a coated well in an enzyme immunoassay. The admixture of several beads allows the simultaneous detection of multiple analytes in a small volume of sample. The broad dynamic range of fluorescence detection of flow cytometers is used to measure a very wide range of protein/peptide concentrations in a single dilution of the samples. The aforementioned kit allows the quantitation of IL-2, IL-4, IL-5, IL-10, tumor necrosis factor (TNF) and interferon-γ (IFNγ) protein levels in a single sample, and the performance of the assay has been optimized for analysis of tissue/cell culture supernatants. Detection limits were as follows (pg/ml): 2.6 for IL-2 and IL-4, 2.4 for IL-5, 2.8 for IL-10 and TNF, and 5.1 for IFNγ. Values below the detection limits were considered as the value for the detection limit.
Statistical analysis
Descriptive statistics were used for the general characteristics of each group. The AUC-PRL during the i.v. MCP test was calculated by the trapezoid method.Citation27 Kruskal–Wallis, Wilcoxon signed and Yan, and the U Mann–Whitney tests were used to analyze intra- and intergroup differences, with Bonferroni's correction test when needed. Spearman's correlation coefficient between continuous variables was performed as needed. The statistical analysis was performed using SPSS Windows version 15.0 (SPSS Inc., Chicago, IL, USA). A P value ≤0.05 was considered significant.
Results
Chronological age and BMI were similar among the three groups (). All patients engrafted successfully and became full chimeras after the allograft. Four patients developed acute GVHD (GVHD+): patients 1, 2, 4, and 5, and the GVHD was a limited (grades I and II) form of the condition; two had cutaneous forms of GVHD and mild liver damage. After a follow-up range of 100 days post-allograft, four patients were alive and two of them (4 and 5) in complete remission. There were six patients (3, 6–10) who did not develop acute GVHD (GVHD−): three of them are alive in good health conditions (3, 6, and 10), three died on day +15 (9, hemorrhagic dengue), and +30 (7, leukemic transformation and 8, relapsed leukemia) ().
Table 1. Baseline clinical and relevant hematological data in healthy volunteers (group 1) and in patients with allo-HSCT (group 2) with and without graft-versus-host disease (GVHD+ and GVHD−) and in their donors (group 3)
During the MCP test, none of the 30 volunteers experienced any side-effects, including extrapyramidal symptoms, as the result of an exaggerated sensitivity to the DA antagonist used.
Serum PRL before transplant
| 1. | Considering the three initial groups (10 individuals each), basal serum PRL concentrations were higher in patients than in their donors and in controls (P ≤ 0.01). No differences existed between donors and controls. Patients who subsequently developed GVHD (‘future’ GVHD+) had basal PRL levels higher than their corresponding donors (P < 0.001), whereas patients who did not develop GVHD (‘future’ GVHD−) had similar PRL levels than their respective donors. Patients with ‘future’ GVHD(+) tended to have higher serum PRL concentrations than ‘future’ GVHD(−), but did not reach statistical significance due to the small sample size. Donors of recipients with ‘future’ GVHD(+) had lower serum PRL concentrations than donors of ‘future’ GVHD(−) (P = 0.01). | ||||
| 2. | AUC-PRL: The greatest AUC-PRL was seen in donors and the lowest in the control group (P = 0.01) whereas the patients had a middle value (P = 0.05). Patients with ‘future GVHD(+) had a tendency to lower values than their corresponding donors, but did not reach statistical significance due to the small sample size, whereas patients with ‘future’ GVHD(−) had an AUC-PRL lower than their respective donors (P = 0.05). There were no differences in AUC-PRL neither between patients with ‘future’ GVHD(+) and patients with ‘future’ GVHD(−) nor among their corresponding donors. | ||||
Serum PRL after transplant (days +14, +30, and +100)
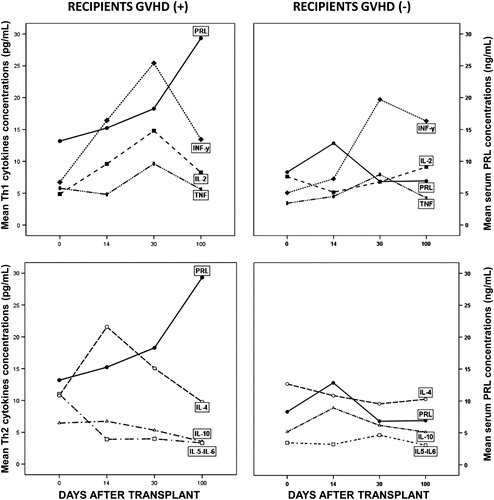
| 1. | Basal serum PRL: A progressive rise in PRL levels was seen in the group of GVHD+ as compared with GVHD− patients on days +14 (P = 0.05), +30 (P = 0.02), and +100 (P = 0.05) post-allograft. At no time during follow-up, GVHD− patients had a mean serum PRL concentration above 7.4 ng/ml whereas GVHD+ patients always had mean serum PRL concentrations ≥15.2 ng/ml. | ||||
| 2. | AUC-PRL (day +100): There were no significant differences between GVHD+ and GVHD− patients. | ||||
| 3. | Correlation analysis (). | ||||
Table 2. Serum PRL concentrations both basal and during the metoclopramide test (AUC-PRL) in the pre- and post-transplant period in healthy controls (group 1), patients (group 2), and their donors (group 3)
In GVHD+ patients, but not in GVHD− patients, a significant linear positive correlation was observed between the time elapsed from basal pre-transplant to day +100 of the study and total serum PRL concentrations (rho = 0.361; P < 0.001) (data not shown).
Basal cytokine profiles
Basal concentrations of Th1 cytokines (IFNγ, TNF, and IL-2) showed a tendency to be higher among donors of patients who later on developed GVHD than those who did not (mainly in TNF), but the small sample size and the large individual variations precluded any statistical significance ().
Figure 1. Basal Th1 type of cytokines before transplant in donors of patients who later on developed GVHD(+) and those who did not (−), as well as their corresponding recipients (mean ± SEM).
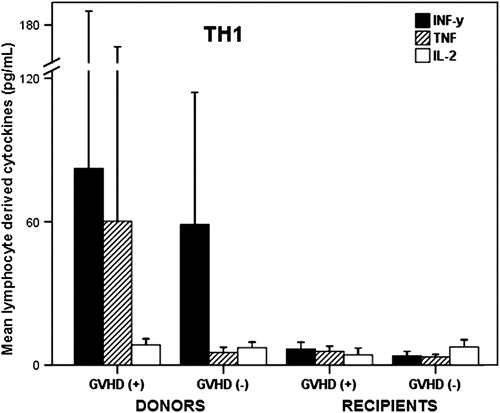
Comparing the dyad donor–recipient with future GVHD(+), IFNγ, and IL-2 were greater in donors than in recipients (P = 0.01 and 0.02, respectively) and TNF although also higher in donors than in recipients did not reach statistical significance due to the great individual variability. In the dyad donor–recipient with future GVHD(−), IFNγ, and TNF were significantly higher in donors than in recipients (P = 0.004 and 0.01, respectively). A greater TNF concentrations in patients with future GVHD(+) than in those with future GVHD(−) was the only difference (P = 0.05) seen between both groups of patients. Th2 cytokines (IL-4, IL-5, IL-6, and IL-10) were very similar between both groups of donors and their corresponding recipients (data not shown).
Cytokine profiles after transplant
In recipients with GVHD(+) (all of whom survived), there was a clear coincidence in the simultaneous and significant rise in PRL on day +30 compared to basal (P < 0.05) with a tendency to rise in Th1 cytokines that reached statistical significance for IFNγ (P = 0.05) and TNF (P = 0.04) and near significance for IL-2 (P = 0.07) (). Interestingly, on day +100 serum PRL had its peak increase simultaneously with a significant decrease in all Th1 cytokine levels. In Th2 cytokines, only IL-4 had a rise on day +14 without statistical significance due to the large individual variations. In GVHD(−) patients (three of whom died on ≤day 30 after transplant) the only noticeable rise was seen on day +30 for IFNγ but neither in PRL, TNF, IL-2 nor in any of the Th2 cytokines.
Discussion
The present study in a small group of patients who received an allo-HSCT due to different hematological malignancies and their respective donors disclosed several points: fasting PRL concentrations and its response to MCP are a good index for evaluating the functional status of the hypothalamic DA tone; the higher the fasting PRL and the lower the AUC-PRL response to MCP, the lower the functional level of the hypothalamic DA tone.Citation16–Citation18 The opposite is interpreted as an increased DA tone. Consequently, our finding that before transplant patients had a greater fasting PRL level and lower MCP-induced AUC-PRL than donors suggests the existence of a decreased DA tone in the former, but an increased DA tone in the latter. The overall effect in the patients would be to maintain PRL synthesis and release as high as possible but near physiological levels, as an adaptive mechanism to up-regulate their battered cellular immune milieu (PRL acting as a Th1 cytokine) consecutive to the intense pre-transplant immune suppressive regimen. On the contrary, in donors the high DA tone would tend to maintain under control a possible latent or transient (stress-induced?) hyperprolactinemiaCitation28,Citation29 that could up-regulate their immune milieu. Interestingly, the four GVHD(+) patients (all of whom survived), but none of the GVHD(−) (three of which died), had a significant progressive mild rise (15–40 ng/ml) in serum PRL levels through the first 100 days after transplant. This difference could not be ascribed to any medication known to increase serum PRL levels, since none of the patients had ingested this type of drugs in 4 months prior to the study. Also, it is known that PRL is a competitive inhibitor of cyclosporine at therapeutic levels;Citation30 however, we did not find any correlation between serum PRL concentrations and levels of whole blood CyA, neither in GVHD(+) nor in GVHD(−) patients. The aforementioned difference is in accord with the known fact that endocrine-derived signals (specifically PRL) participate in the regulation of immune development and function,Citation31 mainly when the body is under stress conditions to counteract the negative effects of glucocorticoidsCitation32–Citation34 and with clear antiapoptotic activity.Citation34–Citation37
The interpretation of post-transplant cytokine patterns in recipients is complicated by two facts: First, the uniform pre-transplant antineoplastic or conditioning regimes may affect differently the individual cytokine responses and secondly, early (<15 days post-transplant) venous blood sample drawn from the recipients contain basically recipient's cells, while late samples (>15 days from successful grafts) should contain variable proportions of recipient's and donor's cells depending on the degree of chimerism. Nevertheless, the post-transplant cytokine patterns in recipients showed clear differences. Although no discernible changes were observed in GVHD(−) patients neither in the Th1 nor in the Th2 type of cytokines, in GVHD(+) patients, a steady rise of variable degree was observed in Th1 cytokines, with a peak value on day +30 simultaneously with the first significant rise in fasting PRL levels.
These observations are in accordance with the pre-transplant findings and follows the same line of thought that a rise in PRL levels near physiological levels might be playing a favorable role and contributing with other factors to mount an ‘early’ limited immune reconstitution (Th1-like) to ameliorate the severity of a GVHD, attempting to survive, as in other classes of immune-suppressed conditions.Citation35,Citation38 This suggestion is supported by the fact that pituitary PRL at physiologic concentrations enhance NK-cell activity and are requisite for the IL-2-driven T-lymphocyte proliferation by its action at the nucleus levelCitation39,Citation40 and may directly stimulate IL-2 and IFNγ production (Th1 phenotype).Citation7,Citation13,Citation41 However, sustained and marked hyperprolactinemia might be deleterious.Citation7,Citation13
Donors of patients who subsequently developed GVHD (GVHD+) showed predominantly Th1 responses during the pre-transplant period. This finding suggests that a donor's cell population biased toward a cellular proinflamatory stage is more capable of mediating an acute GVHD in a similar fashion that this type of response is highly effective to mediate graft rejection.Citation42,Citation43 We could hypothesize that the opposite might prove true for those donors prone to develop strong Th2 responses that could be viewed as a potential treatment to improve outcome.Citation42,Citation43
The limitation of our study is the small number of patients studied which precluded a more detailed statistical analysis and thus, a definitive conclusion. Nevertheless, the differences observed in PRL and cytokine profiles both in donors and recipients whether GVHD+ or GVHD− if proved constant in a larger number of patients might bear a useful predictive value.
There is a great need for new treatment approaches for the prevention of acute and chronic GVHD.Citation43,Citation44 Considering PRL functions as a potent Th1 cytokine along with its pleiotrophic effects (including its immune hematopoietic effects after bone marrow transplantation in animals) and the limited toxicity following its systemic administration the use of recombinant human PRL could be of therapeutic value.Citation8–Citation11 In addition, we would like to propose that the pharmacological modulation of endogenous PRL concentrations (using drugs already in clinical use for many years)Citation45 both in donors and recipients could represent an attractive therapeutic approach. This would raise the possibility that endogenous PRL may participate to rebuild the battered immune system of the transplanted patient from inside the body and in a more physiological manner.
References
- Thomas ED. Hematopoietic stem cell transplantation. Sci Am. 1995;272:38–47.
- Reddy P. Pathophysiology of acute graft-versus-host disease. Hematol Oncol. 2003;21:149–61.
- Lunn RA, Sumar N, Bansal AS, Treleaven J. Cytokine profiles in stem cell transplantation: possible use as a predictor of graft-versus-host disease. Hematology. 2005;10:107–14.
- Krenger W, Ferrara JL. Graft-versus-host disease and the Th1/Th2 paradigm. Immunol Res. 1996;15:50–73.
- Hinterberger-Fischer M, Kier P, Spona J, Sebesta C, Tiefengraber E, Habertheuer KH, et al. Prolactin: a possible mediator of graft-versus-host disease following allogeneic bone marrow transplantation in humans. Bone Marrow Transplant. 1994;14:403–6.
- Matera L. Action of pituitary and lymphocyte prolactin. Neuroimmunomodulation. 1997;4:171–80.
- Matera L, Mori M. Cooperation of pituitary hormone prolactin with interleukin-2 and interleukin-12 on production of interferon-gamma by natural killer and T cells. Ann NY Acad Sci. 2000;917:505–13.
- Richards SM, Murphy WJ. Use of human prolactin as a therapeutic protein to potentiate immunohematopoietic function. J Neuroimmunol. 2000;109:56–62.
- Welniak LA, Richards SM, Murphy WJ. Effects of prolactin on hematopoiesis. Lupus. 2001;10:700–5.
- Sun R, Gault RA, Welniak LA, Tian ZG, Richards S, Murphy WJ. Immunologic and hematopoietic effects of recombinant human prolactin after syngeneic bone marrow transplantation in mice. Biol Blood Marrow Transplant. 2003;9:426–34.
- Sun R, Zhang J, Zhang C, Zhang I, Liang S, Sun A, et al. Human prolactin improves engraftment and reconstitution of human peripheral blood lymphocytes in SCID mice. Cell Mol Biol. 2004;1:129–36.
- Matera L. Endocrine, paracrine and autocrine actions of prolactin on immune cells. Life Sci. 1996;59:599–614.
- Chuang E, Molitch ME. Prolactin and autoimmune diseases in humans. Acta Biomed. 2007;78 (Suppl. 1):255–61.
- Reber PM. Prolactin and immunomodulation. Am J Med. 1993;95:637–44.
- Ben-Jonathan N. Dopamine: a prolactin inhibiting hormone. Endocr Rev. 1985;6:564–89.
- Quigley ME, Judd SJ, Gilliland GB, Yen SSC. Effects of a dopamine antagonist on the release of gonadotropin and prolactin in normal women and in women with hyperprolactinemic anovulation. J Clin Endocrinol Metab. 1979;98:718–20.
- Parra A, Ramírez-Peredo J, Larrea F, Cabrera V, Coutiño B, Torres I, et al. Decreased dopaminergic tone and increased basal bioactive prolactin in men with immunodeficiency virus infection. Clin Endocrinol (Oxf). 2001;54:731–8.
- Parra A, Ramírez-Peredo J, Hidalgo R, Morales-Toquero A, Velásquez-Ramírez G, Ruiz-Argüelles A, et al. Altered functional status of the hypothalamic dopaminergic tone in patients with chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation: a pilot study. Biol Blood Marrow Transplant. 2006;12:566–72.
- Yen SSC. Prolactin in human reproduction. In: , Yen SSC, Jaffe RB, (eds.) Reproductive endocrinology. 3rd ed. Philadelphia: Saunders; 1991. p. 357–88.
- Kessinger A, Armitage JO, Landmark JD, Smith DM, Weisenburger DD. Autologous peripheral hematopoietic stem cell transplantation restores hemopoietic function following marrow ablative therapy. Blood. 1998;71:723–7.
- Ruiz-Argüelles A. Flow cytometry in the clinical laboratory. Principles, applications and problems. Ann Biol Clin. 1992;50:735–43.
- Ruiz-Argüelles A, Orfao A. Caracterización y evaluación de células totipotenciales en sangre periférica y médula ósea. In: , Ruiz-Argüelles GJ, San-Miguel JF, (eds.) Actualización en Leucemias. 1st ed. México City: Editorial Médica Panamericana; 1996. p. 79–82.
- Ruiz-Argüelles GJ, Gómez-Almaguer D, López-Martínez B, Cantú-Rodríguez OG, Jaime-Pérez JC, González-Llano O. Results of an allogeneic non-myeloablative stem cell transplantation program in patients with chronic myelogenous leukemia. Haematologica. 2002;87:894–6.
- Ruiz-Argüelles GJ, Gómez-Almaguer D, Morales-Toquero A, Gutiérrez-Aguirre CH, Vela-Ojeda J, García-Ruiz-Esparza MA, et al. Latin American Cooperative Oncohematology Group. The early referral for reduced-intensity stem cell transplantation in patients with Ph1 (+) chronic myelogenous leukemia in chronic phase in the imatinib era: results of the Latin American Cooperative Oncohematology Group (LACOHG) prospective, multicenter study. Bone Marrow Transplant. 2005;36:1043–7.
- Pinkel D, Straume T, Gray JW. Cytogenetic analysis using quantitative, high sensitivity fluorescence hybridization. Proc Natl Acad Sci USA. 1996;83:2934–8.
- Yam P, Petz L, Knowlton R, Wallace R, Stock A, deLange G, et al. Use of DNA restriction fragment length polymorphisms to document marrow engraftment and mixed hematopoietic chimerism following bone marrow transplantation. Transplantation. 1987;43:399–407.
- Tai MM. A mathematical model for the determination of total area under glucose tolerance and oter metabolic curves. Diabetes Care. 1994;17:152–4.
- Suginami H, Hamada K, Yano K, Kurada G, Matsuura S. Ovulation induction with bromocriptine in normoprolactinemic anovulatory women. J Clin Endocrinol Metab. 1996;62:899–903.
- Reinthaller A, Neunteufel W, Bieglmayer C, Fischl F. The metoclopramide-provocation test for prediction of transient hyperprolactinemia during cycle stimulation. Fertil Steril. 1990;53:368–71.
- Kast R. Blocking of cyclosporine immunosupression by neuroleptics. Transplantation. 1989;47:1095–6.
- Clevenger CV, Freier DO, Kline JB. Prolactin receptor signal transduction in cells of the immune system. J Endocrinol. 1998;157:187–97.
- Gala RR. The physiology and mechanisms of stress-induced changes in prolactin secretion in the rat. Life Sci. 1990;46:1407–20.
- Kant GJ, Bauman RA, Anderson SM, Mongey EH. Effects of controllable and uncontrollable chronic stress on stress-responsive plasma hormones. Physiol Behav. 1992;51:1285–8.
- Weimann E, Baixeras E, Zamzani M, Kelly P. Prolactin blocks glucocorticoid-induced cell death by inhibiting the disruption of the mitocondrial membrane. Leuk Res. 1999;23:751–62.
- Parra A, Ramírez-Peredo J, Larrea F, Pérez-Romano B, Cabrera V, Torres I, et al. Serum prolactin is associated with apoptosis in men with immunodeficiency virus infection. Immunol Cell Biol. 2001;79:285–90.
- Leff MA, Buckley DJ, Krumenacker JS, Reed JC, Miyashita T, Buckley AR. Rapid modulation of the apoptosis regulatory genes, bcl-2 and bax by prolactin in rat Nb2 lymphoma cells. Endocrinology. 1996;137:5456–62.
- Krumenacker JS, Buckley DJ, Leff MA, Mc Cormack JT, De Jong G, Gout PW, et al. Prolactin regulated apoptosis of Nb2 lymphoma cells: pim-1, bcl-2, and bax expression. Endocrine. 1998;9:163–70.
- Parra A, Reyes-Terán G, Ramírez-Peredo J, Benedict J, Quiroz V, Cárdenas M, et al. Differences in nocturnal basal and rhythmic prolactin secretion in untreated compared to treated HIV-infected men are associated with CD4+ T-lymphocytes. Immunol Cell Biol. 2004;82:24–31.
- Clevenger CV, Rusell DA, Appasamy PM, Pristowsky MB. Regulation of interleukin-2-driven T-lymphocyte proliferation by prolactin. Proc Natl Acad Sci USA. 1990;87:6460–4.
- Clevenger CV, Sillman AL, Hanley-Hyde J, Pristowsky MB. Requirement for prolactin during cycle regulated gene expression in cloned T-lymphocytes. Endocrinology. 1992;130:3216–22.
- Matera L, Cesano A, Bellone G, Oberholtzer E. Modulatory effect of prolactin on the resting and mitogen-induced activity of T, B, and NK lymphocytes. Brain Behav Immun. 1992;6:409–17.
- Fowler DH, Gress RE. Th2 and Tc cells in the regulation of GVHD, GVL, and graft rejection: considerations for the allogeneic transplantation therapy of leukemia and lymphoma. Leuk Lymphoma. 2000;38:221–34.
- Mariotti J, Foley J, Ryan K, Buxhoeveden N, Kapoor V, Amarnath S, et al. Graft rejection as a Th1-type process amenable to regulation by donor Th2-type cells through an interleukin-4/STAT6 pathway. Blood. 2008;112:4765–75.
- Hinterberger-Fisher M, Hinterberger W. Blood stem cell transplantation for breast cancer: new approaches using pre-peri-post transplant immunotherapy. Expert Opin Biol Ther. 2001;1:1029–48.
- Hernández-Ruiz J, Farfán B, Córdoba J, Parra A, Ramírez J, Kershenobich D, et al. Effect of prolactin in-vitro and in-vivo on perforin and trail expression in human NK lymphocytes. Eur J Immunol. 2009;39 (Suppl. 1):S-345.