Abstract
The first bone marrow transplantation in Latin America was performed more than 30 years ago and since then several countries have started transplant programs. The Center for International Blood and Marrow Transplant Research captured information on 13 473 transplants performed in Latin America from 1981 to 2009. The current report summarizes this activity. Argentina, Mexico, and Brazil have the largest activity in the region. Despite increase in the annual number of transplants, the activity is limited to sibling donor and autologous transplants. Indications are similar to other regions with a proportionally higher number of pediatric transplants for treatment of non-malignant diseases. Unrelated donor transplant activity is also increasing through collaborations with international donor registries and the development of the first national donor registry in Brazil. Umbilical cord transplants were also reported in Latin American centers, mainly in Brazil and most commonly used for treatment of children with malignant diseases. In conclusion, hematopoietic cell transplantation is routinely performed in several centers in Latin America. However, the activity is low compared to the population in need. Challenges with costs of transplantation, donor availability, number of centers of excellence, and trained personnel need to be addressed for further development of this field in the region. Additionally, more integration between countries and transplant centers is an important next step and can assist in improving awareness for the field and maximizing the transplant activity in Latin America.
Keywords:
Introduction
Hematopoietic cell transplantation (HCT) is a complex and costly procedure that requires an established infrastructure, trained personnel, availability of medications, and laboratory and ancillary services support. The number of transplants performed annually worldwide has increased in the last two decades;Citation1 however, this growth has disproportionally been observed in countries classified by the World Bank as high and intermediate high gross national income.Citation2 This observation is mostly related to the difficulty in implementing HCT programs in developing healthcare systems, which accentuates the need of this curative procedure in these countries.
Latin America has a long experience with transplantation starting in 1981. However, the current activity remains proportionally low compared to the region’s population and many challenges to the advancement in transplantation in the region remain.Citation3
Data present in this report are a compile of all first transplants performed in Latin American centers from 1982 to 2009 and registered with the Center for International Blood and Marrow Transplant ResearchCitation1 since 1981.
Overall Activity
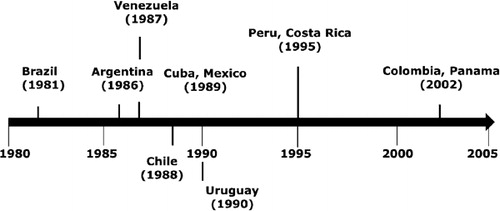
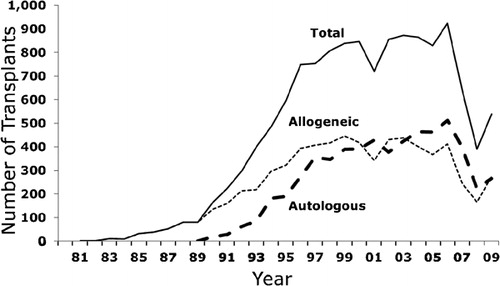
Brazil and Argentina were the first countries in the region to initiate transplant programs in the 1980s (). Presently, there are a total of 55 centers in Latin America that report to the Center for International Blood and Marrow Transplant Research (CIBMTR) and the largest numbers of centers are in Brazil (n = 20), Argentina (n = 14), and Mexico (n = 7). According to the Brazilian Society of Bone Marrow Transplantation, there are more than 55 activate centers that are registered and authorized by the Brazilian government to perform transplants. However, the Brazilian Society of Bone Marrow Transplantation estimates that the activity from centers participating in the CIBMTR accounts for ∼70% of the overall activity of allogeneic HCT in Brazil, as most of the autologous transplants are not routinely reported and the activity from non-reporting centers is low. outlines the growth in the annual number of transplants. Latin American centers reported a total of 13 473 (7008 allogeneic and 6465 autologous transplants) from 1981 to 2009. The overall activity is increasing, albeit not as steep as in Europe or US. Interestingly, the number of allogeneic is higher than autologous in contrast to what is observed in the US,Citation1 which might be a result in of under-reporting autologous HCT. In addition, there is a fluctuation in the number of cases reported annually, which outlines the difficulty in reporting transplant cases. In the US, Canada, and Europe, most transplant data are collected and reported by data managers or clinical research coordinators, in contrast to Latin American centers, where these activities are performed by physicians. Some of the largest centers in Brazil have implemented this professional position in their transplant programs to accommodate the requirements of data reporting and developing local databases. The drop in transplant numbers in 2007 reflects a change in the data collection system from paper-based to electronic-based and data collection outside the US is still being updated.
The age of transplant recipients in Latin America is also in contrast to what is observed in the US. The median patient ages for allogeneic and autologous transplants are 30 and 45 years, respectively, compared to 45 and 55 years in the US. There are proportionally higher numbers of pediatric transplants performed in the region. Among allogeneic transplants however, the number of transplants performed in patients in the fifth and sixth decades of life has increased in the last decade in parallel to what is observed in other countries. The advent and increase in utilization of reduced intensity conditioning regimens is the main reason for this trend.
Transplant Indications
The main indications for allogeneic HCT were leukemias (48%), severe aplastic anemia (19%), myelodysplasia (7%), and inherited red cell disorders (5%). The most frequent malignant disease for an allogeneic HCT was chronic myelogenous leukemia (CML; n = 1769; 25%), although the frequency of HCT for this indication declined since 2001 with availability of tyrosine kinase inhibitors in the region. The debate over transplantation for CML in Latin America outlines an important issue related to cost-effectiveness of treatment. As tyrosine kinase inhibitors are available in these countries, the costs of chronic use can easily exceed the cost of transplantation. Groups in Mexico developed programs for early transplant referral for patients with CML and used non-myeloablative conditioning intensity to minimize early transplant toxicity.Citation4Citation4,5 The most frequent non-malignant disease is severe aplastic anemia (n = 1389; 19%). The estimated incidence of aplastic anemia in the region is variable,Citation6–Citation8 and the high proportion of transplant for this disorder is a result of transplant centers specialized in such transplants that serve as referring center for the region. Similarly, there is a respectful activity of transplants for Fanconi Anemia in BrazilCitation9 which contributed to the identification of a novel mutation only seen in patients from this region.Citation10
Among autologous HCT, the most frequent indication was lymphoma (n = 3155; 48%); however, it can be estimated that the number of autologous HCT performed in Latin America is two- to three-fold higher than this number.
Graft Type
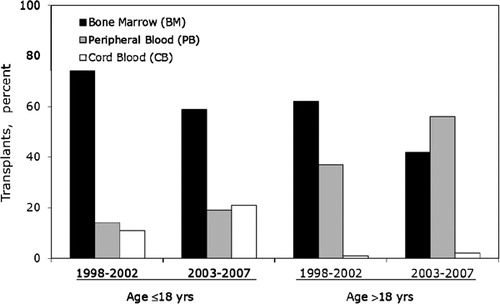
Bone marrow is the most common graft type utilized in children (). Utilization of umbilical cord blood is also increasing in Latin America. During the period of this report, 358 umbilical cord blood transplants were registered with the CIBMTR, and 98% were performed in patients younger than 18 years. The majority of these transplants were performed at Brazilian centers and the most common indications were acute leukemias and non-malignant diseases. Cord blood units were initially obtained through Eurocord and most recently the development of the BrasilCord Network of public umbilical cord blood banks in Brazil, allowed for the processing and distribution of this graft source regionally. Among adults, the most common graft type was bone marrow and after 2002, mobilized peripheral blood became the graft of choice. However, compared to practices in the US, bone marrow continues to be used in proportionally higher number of transplants in Latin America.
Donor Type
Utilization of unrelated donor transplants is infrequently performed in Latin America, and 15 centers are affiliated with US National Marrow Donor Program to perform unrelated donor transplants facilitated by this donor registry. During the period, 9% (n = 681) of all allogeneic transplants used an unrelated donor in Latin America. Unrelated donor HCTs in the US are now the most common allogeneic transplant. The development of local donor registries, including the Brazilian Donor Registry (REDOME), may facilitate the search of unrelated donors with similar racial and ethnic background as the recipients.
Conclusions
HCT is an underutilized therapeutic option for many malignant and non-malignant diseases in Latin America. According to the CIBMTR, the annual number of registered transplants is ∼1000, even if this represents a third of the actual activity, the transplant rate for the region is less than 50 per 10 million people. Transplant rates in the US only are >300 per 10 million people.Citation3 This outlines the need for advancement of transplantation in Latin America as the number of patients in need for a curative treatment is high. One common argument against advancement of transplantation in developing health care systems is related to prioritizing treatment for communicable diseases, improvements in infant mortality, and other basic health indexes before investment in highly complex procedures. Implementation of a transplant program has an important local impact and often results in improvement in the quality of transfusion products, overall laboratory, and consultant and ancillary services. HCT is the only curative therapy for several non-malignant and malignant diseases; however, access to transplantation for these patients in Latin America is limited. With many decades of experience, HCT in Latin America remains with a slow growth due to challenges in infrastructure, irregular distribution of important antibiotic and immune suppressant medications. Despite these challenges, advances such as the development of REDOME and BarsilCord offer necessary structure to expand the number of unrelated donor transplants. One important next step for advancement of transplants in Latin America is further integration among countries, as there is a large discrepancy related to transplant availabilities across the region. Many countries have no transplant activity or small autologous transplant programs. Training and twinning between Latin American centers would be facilitated by more integration. Additionally, there is a need for a regional registry to improve capture of transplant activity, to report post-transplant outcomes and understand areas that need improvement. Ultimately this integration will take transplantation in the region to the next level of development and improve access to transplantation for patients.
References
- Pasquini MC, Wang Z, Horowitz M, Gale RP. 2010 report from the Center for International Blood and Marrow Transplant Research (CIBMTR): current uses and outcomes of hematopoietic cell transplant for blood and bone marrow disorders. In: , Cecka J M, Terazaki P I, ed, editors. Clinical transplants 2010. Los Angeles (CA): The Terasaki Foundation Laboratory; 2011. p. 87–105.
- Gratwohl A, Passweg J, Baldomero H, Horisberger B, Urbano-Ispizua A. Economics, health care systems and utilization of haematopoietic stem cell transplants in Europe. Br J Haematol. 2002;117(2):451–68.
- Gratwohl A, Baldomero H, Aljurf M, Pasquini MC, Bouzas LF, Yoshimi A, et al.. Hematopoietic stem cell transplantation: a global perspective. JAMA. 2010;303(16):1617–24.
- Ruiz-Arguelles GJ. Allogeneic stem cell transplantation using non-myeloablative conditioning regimens: results of the Mexican approach. Int J Hematol. 2002;76 Suppl 1:376–9.
- Ruiz-Arguelles GJ, Gomez-Almaguer D, Morales-Toquero A, Gutierrez-Aguirre CH, Vela-Ojeda J, Garcia-Ruiz-Esparza MA, et al.. The early referral for reduced-intensity stem cell transplantation in patients with Ph1 (+) chronic myelogenous leukemia in chronic phase in the imatinib era: results of the Latin American Cooperative Oncohematology Group (LACOHG) prospective, multicenter study. Bone Marrow Transplant. 2005;36(12):1043–7.
- Hamerschlak N, Maluf E, Pasquini R, Eluf-Neto J, Moreira FR, Cavalcanti AB, et al.. Incidence of aplastic anemia and agranulocytosis in Latin America — the LATIN study. Sao Paulo Med J. 2005;123(3):101–4.
- Maluf EM, Pasquini R, Eluf JN, Kelly J, Kaufman DW. Aplastic anemia in Brazil: incidence and risk factors. Am J Hematol. 2002;71(4):268–74.
- Maluf E, Hamerschlak N, Cavalcanti AB, Junior AA, Eluf-Neto J, Falcao RP, et al.. Incidence and risk factors of aplastic anemia in Latin American countries: the LATIN case-control study. Haematologica. 2009;94(9):1220–6.
- Bonfim CM, de Medeiros CR, Bitencourt MA, Zanis-Neto J, Funke VA, Setubal DC, et al.. HLA-matched related donor hematopoietic cell transplantation in 43 patients with Fanconi anemia conditioned with 60 mg/kg of cyclophosphamide. Biol Blood Marrow Transplant. 2007;13(12):1455–60.
- Yates J, Keeble W, Pals G, Ameziane N, van Spaendonk R, Olson S, et al.. Novel inactivating mutations of FANCC in Brazilian patients with Fanconi anemia. Hum Mutat. 2006;27(2):214.