Abstract
A total of 50 patients with chronic lymphocytic leukaemia (CLL), as well as the B-cell leukaemia cell lines MEC-1, JVM-3, and BV-173 were studied in order to assess the incidence of CD13/aminopeptidase N (APN) immunolabelling with a monoclonal antibody 7H5 compared to LeuM7 and to CD13 mRNA levels, and to correlate these data with the cytotoxic and apoptosis-induction activity of the natural phenolic APN inhibitor curcumin. CD13/APN was detected in a significant proportion of B-CLL patients (42/50, 84%), immunolabelled by 7H5 (42/50) ± LeuM7 (10/50). Molecular analysis for CD13 transcripts confirmed these data, resulting in a specific RT-PCR product in CD13 positive cases. Curcumin showed concentration-dependent cytoreductive efficacy and apoptosis-induction activity in all tested cell lines and primary cultures from CLL mononuclear cells. There was a clear tendency for a better response in CD13 positive cases. The incidence of CD13/APN in CLL suggests that the inhibition of APN/CD13 by curcumin may be an effective new molecular target for a more efficient therapy for these patients and warrants further investigations.
Introduction
Aminopeptidase N/CD13 (APN; gp150) is a membrane-bound glycoprotein, and a member of the M1 family of Zn2+-dependent metalloectopeptidases and aminopeptidases.Citation1,Citation2 It is widely expressed on the surface of various tissues and cell types as e.g. on renal, intestinal, central nervous, endothelial, and placental cells, as well as on haematopoietic cells such as myeloid cells, monocytes, subsets of human lymphocytes, etc. There is plenty of data that APN is overexpressed on tumour cells and it plays a crucial role in tumour angiogenesis, invasion, growth, and metastasis.Citation3 Therefore, APN inhibitors (APNI) may prove to be clinically efficacious for the treatment of these disorders. Some of the most intriguing APNI found to date are natural products of plant origin, which have been a part of traditional medicine for many centuries. Their use has been accepted as safe and efficacious. One of these molecules, curcumin, is a component of the culinary spice turmeric, which gives curry powder its yellow colour. Extensive research over the last decades has indicated that this polyphenol can both prevent and treat malignancies. Curcumin has been described to suppress tumour initiation, promotion, and metastasis.Citation4
In leukaemias, due to its myeloid-specific mode of expression, APN/CD13 significantly correlates with the myeloid lineage affiliation of neoplastic cells. However, it is rarely reported in mature lymphoid malignancies and is generally correlated to poor prognosis. Previous data showed that this might be due to the specific structure with 10 sites for N-linked glycosylation and abundant sites for O-glycosylation. Variations in glycosylation result in the formation of different epitopes, thus affecting recognition by monoclonal antibodies (Mab).Citation5 We developed a novel Mab 7H5, which was classified as specific for CD13 during the 6th Leukocyte Typing Workshop, Kobe, 1996 and suggested to recognize a new epitope of CD13, yielding reactivity with more than 80% of acute myeloid leukaemia cases. However, the reactivity with lymphoid malignancies has been poorly studied.Citation6,Citation7 B-cell chronic lymphocytic leukaemia (CLL) is the most common leukaemia in adults and, with the possible exception of allogeneic stem cell transplant, is incurable with current treatments. There is now great interest in identifying pharmacological agents that are able to modulate survival pathways in the hope of identifying potential novel therapies for treatment of CLL.Citation8
The aim of the study was to investigate the incidence of aminopeptidase N/CD13 in samples with CLL by immunophenotyping using 7H5 compared to the reference CD13 antibody LeuM7, as well as by molecular studies of CD13 mRNA expression. Further, these data were correlated with the cytotoxic and apoptosis-induction activity of curcumin in cell lines and in cultures of cells isolated from the bone marrow of CLL patients.
Materials and methods
Patients and cell lines
In total, 50 CLL patients (mean age 62 ± 11 years, ranging 37–89 years) diagnosed according to the morphological and immunophenotypic criteria of the WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th edition were included in the present study after local ethics committee approval.Citation9 Immunophenotyping and molecular studies were performed on lysed bone marrow and peripheral blood samples. Primary cell cultures of cells isolated from the bone marrow of CLL patients were subjected to further cytotoxicity and apoptosis studies. The following three B-cell leukaemia cell lines were also included in the study: MEC-1 (DSMZ catalogue code: ACC 497) – a human chronic B-cell leukaemia cell line derived from the peripheral blood of a patient with B-CLL in prolymphocytoid transformation; JVM-3 (DSMZ catalogue code: ACC 18) – a human chronic B-cell leukemia cell line, established from the peripheral blood of a 73-year-old man with B-prolymphocytic leukaemia at diagnosis; BV-173 (DSMZ catalogue code: ACC 20) – a human B-cell precursor leukaemia cell line, established from the peripheral blood of a 45-year-old man with chronic myeloid leukaemia in blast crisis in 1980, expressing CD10+, CD13+, and CD19+, and containing the t(9;22) b2-a2 fusion gene.
Immunophenotypic studies
Direct immunostaining was performed according to the protocol of the manufacturer using the following fluorochrome-conjugated monoclonal antibodies: (1) anti-CD13 mouse monoclonal antibodies 7H5-PE (IgG2a) (BulBio NCIPD Ltd, Sofia, Bulgaria); LeuM7-PE (clone: L138) (Becton Dickinson (B-D), Cat N 347837); (2) myeloid: CD11c, CD14, CD15, CD33, CD64; (3) B-lymphoid: CD10, CD19, CD20, CD21, CD22, CD23, IgM; (4) T-lymphoid: CD1a, CD2, CD3, CD4, CD5, CD7, CD8 (B-D); (5) others: CD45, CD34, CD56, CD38, HLA-DR, CD36 (B-D). Stained samples were collected and analysed on a FACSCalibur or FACS Canto II flow cytometers (B-D) and analysed by ‘CellQuest Pro’ or ‘BD FACSDiva’ version 6.1.2. software (B-D), respectively, as previously described. At least 10 000 events for each sample were collected and analysed.Citation7 A 20% threshold for ‘positivity’ of the neoplastic population was accepted.
Molecular studies of mRNA expression of APN/CD13 by RT-PCR
Total RNA was extracted from mononuclear cells isolated from bone marrow and/or peripheral blood of a selected group of 10 CLL patients with ≥90% lymphocytes in the sample, as well as from the cell lines (MEC-1, JVM-3, BV-173), using column ready to use kit (NucleoSpin® RNA II, MACHEREY-NAGEL or SV Total RNA Isolation System, Promega Corporation, Madison, WI 53711-5399 U.S.A.). Complementary DNA (cDNA) was synthesized by reverse transcription of 1 µg of RNA using 5 µM random hexamers (Roche Applied Science, Mannheim, Germany), 1× first strand buffer and 200 U Moloney murine leukemia virus reverse transcriptase (M-MLV RT) (Promega) in a final volume of 20 µl and consecutive incubation of the samples at 37°C for 1 hour and at 99°C for 3 minutes.Citation10 CD13 gene expression was determined by a semi-quantitative multiplex PCR with co-amplification of β2-microglobulin (B2-M) or beta actin mRNA as an internal control of the quality of mRNA and efficiency of reverse transcription. Briefly, 5 µl of cDNA was amplified in 25 µl medium containing 1× PCR buffer, 2.5 mM MgCl2, 1 U Taq polymerase (Invitrogen, Carlsbad, CA, USA), 200 µM each of dNTPs (Promega), and 1 pM of each of upstream and downstream primers: 5′-gtccagggtccaggttccag-3′/5′-tgacagtgcgatgattgtgcac-3′ (CD13), and 5′-acccccactgaaaaagatga-3′/5′-atcttcaaacctccatgatg-3′ (B2-M), respectively.Citation11 The mixture was incubated in a Mastercycler Gradient (Eppendorf, Hamburg, Germany) firstly at 95°C for 9 minutes, followed by 30 cycles of amplification: 95°C for 30 seconds; 61°C for 30 seconds; 72°C for 60 seconds; and terminated at 72°C for 10 minutes. The amplification conditions were chosen after serial experiments with a different number of cycles leading to a negative result for CD13 mRNA using cDNA from patients with T-cell acute lymphoblastic leukemia (T-ALL) with >98% lymphoblasts, that were CD13 negative by immunophenotyping. Amplification products were run on a 3% agarose gel and, after staining with ethidium bromide, visualized by UV irradiation and photographed.
MTT-dye reduction assay
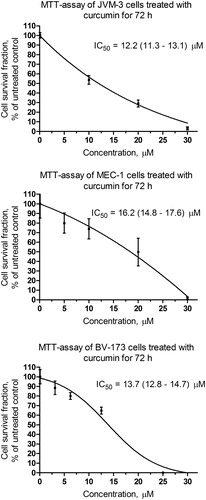
The cytotoxic activity of curcumin was evaluated in cell cultures of MEC-1, JVM-3, and BV-173 cell lines using the MTT-dye reduction assay. Logarithmically growing cells were seeded into 96-well microplates (100 µl/well at a density of 1 × 105 cells/ml) and exposed to various concentrations of curcumin (C1386, Sigma-Aldrich, Zwijndrecht, the Netherlands) for 48 and 72 hours. The cell survival fraction was determined by the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetra-zoliumbromide) dye-reduction assay as described by Mosmann et al. with some modifications.Citation12 Briefly, after incubation with the test compound, MTT-solution (10 mg/ml in phosphate-buffered saline (PBS)) was added (10 µl/well). Plates were further incubated for 4 hours at 37°C and the formazan crystals formed were dissolved by adding 100 µl/well of 5% formic acid in 2-propanol. Absorption was measured by an automated microtiter plate spectrophotometer (Labexim LMR-1, Lengau, Austria) at 550 nm. For each concentration at least eight wells were used. A mixture of RPMI-1640 (100 μl), MTT-solution (10 μl) and 5% formic acid in 2-propanol (110 μl) was used as blank solution.
Flow cytometry (FCM) analysis for sub-G0 fraction
FCM analysis was performed as described by Watson et al.Citation13 Briefly, control or compound-treated cells were pelleted, washed with cold PBS, and resuspended in 100 μl PBS and 300 μl 96% ethanol. The cells were kept at −20°C. At the day of FCM measurements the cells were centrifuged and resuspended in 500 μl PBS, containing 20 μg/ml RNAase and 20 μg/ml propidium iodide at room temperature. The tubes were protected from light, incubated at 4°C for 1 hour, and the red fluorescence emitted from the PI–DNA complex analysed after laser excitation of the fluorescent dye at 488 nm by FACS Canto II flow cytometer (B-D). DNA QC particles (B-D) and FACS Diva (B-D) were used to set instrument photomultiplier tube voltages and amplifier gains, check instrument resolution and linearity, and verify instrument alignment. At least 20 000 events were collected for each sample at a resolution of 262 144 linear channels using linear amplification of all signals. Apoptotic cells (cells with fractional DNA content; sub-G0 cells) were defined on histograms and expressed as percentages by ModFit LT ver 3.0 software.
Statistical analysis
The distribution of discrete clinical and biological features among patient subgroups was compared by the Fischer's exact test. For continuous variables, the one-way analysis of variance (ANOVA) was used to determine whether there were any significant differences between the means of two independent (unrelated) samples. The computations were carried out with SPSS 13.0 for Windows (USA).
Results
APN/CD13 incidence in CLL
Our previous studies in haematopoietic malignanciesCitation7 prompted us to study 7H5 and LeuM7 expression in chronic lymphoid malignancies. A total of 50 CLL patients at diagnosis were included in the present study. Leukocyte number in the peripheral blood was 40 × 109/l ± 39 × 109/l, B-lymphocytes accounted for >5 × 109/l and showed CLL-phenotype CD19(+) CD20low(+) CD22low(+) CD5(+) CD23(+) FMC7(−) CD10(−). Interestingly, a significant proportion of the CLL cases stained positive for 7H5 (42/50, 84%) (). At the same time, LeuM7 was found positive above the accepted threshold of positivity in 10/50 (20%) CLL cases, all but one of these cases being positive for both APN/CD13 Mabs. Only 7/50 (14%) CLL cases were double negative. Thus, the introduction of 7H5 Mab in the phenotyping panel revealed an elevated percentage of APN/CD13(+) CLL cases that would otherwise have been underestimated.
Figure 1. Expression of APN/CD13 as defined by 7H5 and Leu M7 epitopes in B-CLL. Peripheral blood samples from B-CLL patients were stained as described in Materials and methods section. Histogram A – a patient with a discordant APN/CD13 phenotype (7H5 + LeuM7−); histogram B – a patient with a concordant positive APN/CD13 phenotype (7H5 + LeuM7+) of CLL B-cells.
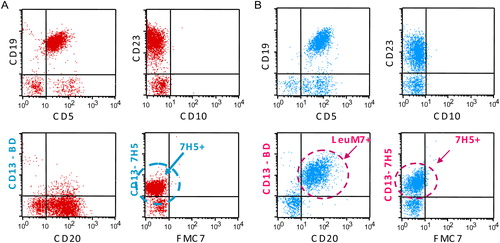
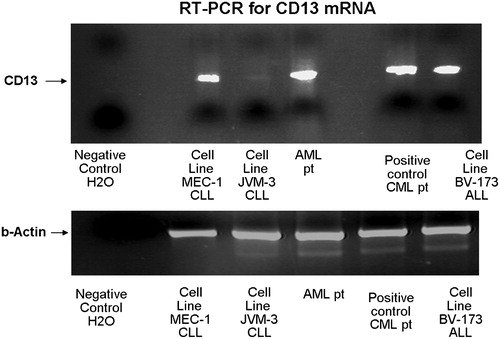
FCM performed on the cell lines using the two Mabs confirmed the presence of both epitopes of APN/CD13 on BV-173 cells, as specified in the characteristics of the cell line. None of the Mabs could identify surface expression of the molecule on JVM-3 and MEC-1 cells.
The multiplex RT-PCR for APN/CD13 mRNA produced results that showed good correlation with immunophenotypic data. PCR positivity was in concordance with either 7H5 or LeuM7 positivity in CLL patients (). The reaction was adapted and carrie out using 30 cycles, when amplification proceeds exponentially at a constant efficiency and the yield of the RT-PCR product is proportional to the starting amount of the template thus preventing a PCR signal from the low proportion of myeloid cells in the selected samples. Interestingly, the molecular study revealed positive amplification of APN/CD13 mRNA not only in BV-173 samples, where this was expected, but also in MEC-1 samples, while JVM-3 showed negative results ().
Figure 2. RT-PCR for APN/CD13 mRNA in leukaemias. The multiplex RT-PCR showed good concordance with the immunophenotypic pattern of APN/CD13 in haematological malignancies. Abreviations: AML – acute myeloid leukaemia; ALL – acute lymphoblastic leukaemia; CLL – chronic lymphocytic leukaemia.
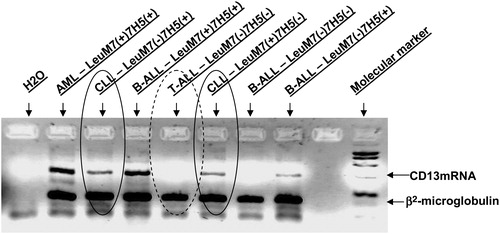
Figure 3. RT-PCR for APN/CD13 mRNA in cell lines. The RT-PCR showed positive results for APN/CD13 mRNA in the MEC-1 and BV-173 cell lines and negative in the JVM-3 cell line. Samples from patients with a CD13(+) acute myeloid leukaemia (AML) and a chronic myeloid leukaemia, respectively, were used as positive controls.
Flow cytometric analysis of curcumin influence on apoptosis in cultured CLL cells
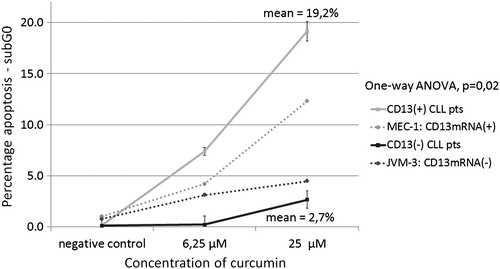
Screening for apoptotic cells in samples from cell cultures developed from isolated mononuclear cells from patients with B-lymphocytic leukaemias was performed by FCM using PI staining. Curcumin caused a concentration-dependent increase in the apoptotic population of freshly isolated mononuclear cells from CLL patients (Wilcoxon exact ranks test, P = 0.028). An increase in the number of apoptotic cells was observed after incubation of APN/CD13(+) CLL cells with curcumin from a mean 8.35 ± 2.38% at 6.25 µM to 19.17 ± 5.07% apoptotic cells at 25 µM. In contrast, APN/CD13(−) cells showed significantly lower levels of apoptosis in the presence of the same amounts of curcumin – 0.15 ± 0.25% at 6.25 µM and 2.7 ± 1.07% at 25 µM (one-way ANOVA, P = 0.02 at 25 µM). The FCM analysis of the two CLL cell lines showed comparable results. The JVM-3 cell line, which was APN/CD13 negative both by immunolabelling and RT-PCR, showed low levels of apoptosis induction after incubation with curcumin: 3.12% at 6.25 µM and 4.47% at 25 µM curcumin concentration, respectively, while the MEC-1 cell line that appeared to be CD13 positive by RT-PCR showed similar results to the APN/CD13(+) patients’ samples: 4.2% at 6.25 µM reaching 12.3% at 25 µM (; ).
Figure 4. FCM analysis of the sub-G0 fraction after 72 hours incubation with curcumin in: (А) CD13 mRNA(+) MEC-1 cell line; (B) JVM-3 cell line.
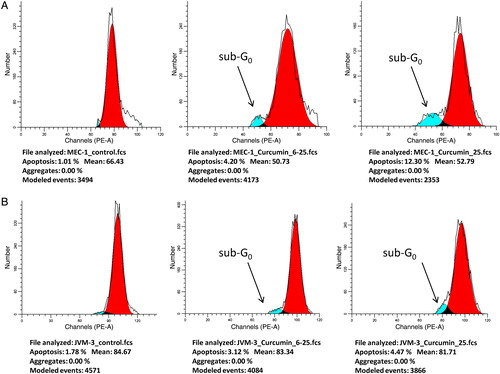
Figure 5. Comparison of the sub-G0 fraction of curcumin-treated CD13/APN positive and negative CLL cells. Curcumin caused a concentration-dependent induction of apoptosis as determined by FCM on freshly isolated and cultured mononuclear cells from CLL patients and cell lines after 72 hours. A considerable difference was observed between CD13/APN(+) vs. (−) cases.
MTT-dye reduction assay for curcumin in B-cell chronic leukaemia cell lines
Curcumin caused concentration-dependent cytotoxic effects after 72 hours of incubation in all leukaemic cell lines investigated (). The concentration–effect curves allowed intrapolation of the corresponding IC50 values; however, failed to demonstrate significant differences between APN/CD13 positive and negative cell lines – IC50 13.7 (12.8–14.7) µM for BV-173 and 16.2 (14.8–17.6) µM for MEC-1 compared to IC50 12.2 (11.3–13.1) µM for JVM-3.
Discussion
Remarkably, the 7H5 epitope was detected in a significant proportion of B-cell CLL, thus allowing for the identification of CD13, which otherwise might have remained underestimated in a total of 86% of CLL cases. Our data showed that the Mab clones used to detect the CD13 antigen have variable immunoreactivity against cells with B-cell phenotype, which may partly explain the conflicting reports concerning the incidence of myeloid antigen expression in lymphomas.Citation14,Citation15 Several series have been published on the expression of CD13Citation16–Citation18 in cases of CLL. However, in these studies, the frequency of CD13 expression ranged from 1.9 to 50%. The discrepancy among different studies may be due to technical factors such as the antibody clone, the sensitivity of individual methods, Fc receptor binding that may produce artefact data, contamination of myelomonocytic cells in the gate, and lack of assessment for co-expression with CD19. In our hands, RT-PCR for CD13 mRNA in a selected group of patients with a high proportion of more than 90% lymphocytes in the examined material showed good correlation with immunophenotypic data allowing for specific identification of APN/CD13 transcripts in LeuM7(+) and/or 7H5(+) leukaemic cell populations, which has not been reported so far in CLL.
The demonstrated expression of APN/CD13 across lineage boundaries is not unique in haematological malignancies. However, the important and discordant expression of CD13 epitopes in lymphoid malignancies raises the question about the biological basis and clinical relevance of this phenomenon. Variations in the O-glycosylation of the molecule or discrete differences in the oligosaccharide composition may lead to variable masking of protein epitopes.Citation19 The location of O-glycosylation sites is essential for the functional characteristics of the glycoprotein, and might be of physiological relevance. In fact, the clinical relevance of CD13 should be looked for in the functional characteristics of the molecule rather than in its lineage-specific expression.
In addition, the incidence of CD13/APN in B-cell CLL suggests that inhibition of APN/CD13 may be an effective new molecular target therapy for these patients. Natural and synthetic inhibitors of APN activity have been characterized so far and revealed that APN is able to modulate major biological events (cell proliferation, invasion, angiogenesis).Citation20–Citation22
Curcumin appears to be a promising compound with APN inhibitory capacity and has already been shown to have antioxidant, chemopreventive, chemotherapeutic, and chemosensitizing activities.Citation23,Citation24 Our previous studies also provided data indicating that curcumin has a promising antitumour potential, which is accompanied by chemopreventive and chemoprotective properties. Curcumin is a relatively non-toxic polyphenolic natural product isolated from the leafy plant Curcuma longa (turmeric). It has been used in India as a household anti-inflammatory remedy for centuries. For the last decade there has been a rapidly growing interest in curcumin's ability to combat various types of cancer because it is safe and can be taken orally. Interestingly, curcumin is also a powerful immune system booster.Citation25 Therefore we addressed the hypothesis that curcumin might be an effective agent particularly in CLL patients, who are further immunosuppressed, and also that APN/CD13 may play a role.
Using the MTT-dye reduction assay we demonstrated a dose-dependent antileukaemic activity of curcumin on freshly isolated and cultured mononuclear cells from CLL patients which is in line with other published studies that show also a dose-dependent apoptosis induction activity.Citation26,Citation27 In addition, the induction of apoptosis as shown by the sub-G0 fraction in FCM after incubation with curcumin was significantly higher in APN/CD13(+) cases compared to the negative. The net cytotoxic efficacy was very similar which could be explained by the involvement of various curcumin-induced signal transduction modulations, e.g. inhibition of prosurvival factors such as NF-κB, STAT3 and Akt, SYK, and AID inhibition.Citation28–Citation30 Therefore, additional molecular changes should be investigated in order to characterize curcumin's mode of action against CLL. It seems that curcumin exerts very complex cellular events thus contributing to apoptosis induction and manifestation of its hallmarks: poly(ADP-ribose) polymerase cleavage in primary B-CLL cellsCitation26,Citation31 and release of cytochrome C from the mitochondrial membrane.Citation32 Evidence has been provided that curcumin inhibits prosurvival pathways in CLL B-cells and may overcome their stromal protection in combination with other natural extracts.Citation26 Recent observations suggested that modulation of miRNA expression may be another important mechanism underlying the pharmacological effects of curcumin.Citation33
In conclusion, our data demonstrated a dose-dependent anti-leukaemic effect of curcumin on B-CLL cells and also that APN/CD13 may play a role. The incidence of CD13/APN in B-cell neoplasms and CLL, particularly, suggests that inhibition of APN/CD13 by curcumin may be an effective new molecular target for a more efficient therapy for these patients. Our data suggest that additional detailed evaluation of curcumin as a potential therapeutic agent for the treatment of CLL is warranted.
Acknowledgement
The study was supported by the National Science Fund of the Bulgarian Ministry of Education, Youth and Science (grants VU-L-309/07, CVP01/0119-ДО02-35/2009).
References
- Taylor A. Aminopeptidases: structure and function. FASEB J. 1993;7:290–8.
- Antczak C, De Meester I, Bauvois B. Ectopeptidases in pathophysiology. Bioessays. 2001;3:251–60.
- Sato Y. Role of aminopeptidase in angiogenesis. Biol Pharm Bull. 2004;27(6):772–6.
- Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23(1A):363–98.
- Asmun R, Holmes K, Shapiro L. CD13 (aminopeptidase N) cluster workshop report. In: , Stuart F, Schlossman SF, Boumsell L, Gilks W, Harlan JM, Kishimoto T, Morimoto C, Ritz J, Shaw S, Silverstein R, (eds.) Leucocyte typing V. New York: Oxford University Press; 1995. p. 771–5.
- Kishimoto T, Kikutani H, van dem Borne AEG, Goyert SM, Mason DY, Miyasaka M, et al. (eds.) Leucocyte typing VI. White cell differentiation antigens. New York: Garland Publishing Inc.; 1997. p. 962–3.
- Michova А, Guenova M, Nikolova M, Taskov H. CD13 epitope 7H5 exhibits a particular expression pattern in haematological malignancies. Haema. 2003;6(3):336–43.
- O'Brien S, Kay NE. Maintenance therapy for B-chronic lymphocytic leukaemia. Clin Adv Hematol Oncol. 2011;9(1):22–31.
- Muller-Hermelink HK, Montserrat E, Catowsky D, Campo E, Harris NL, Stein H. Chronic lymphocytic leukemia/small lymphocytic lymphoma. In: , Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, (eds.) WHO classification of tumours of haematopoietic and lymphoid tissues, 4th ed. Lyon: IARC; 2008. p. 180–2.
- van Dongen JJ, Macintyre EA, Gabert JA, Delabesse E, Rossi V, Saglio G, et al. Standardized RT-PCR analysis of fusion gene transcripts from chromosome aberrations in acute leukemia for detection of minimal residual disease. Report of the BIOMED-1 concerted action: investigation of minimal residual disease in acute leukemia. Leukemia. 1999;13(12):1901–28.
- Dybkaer K, Olesen G, Pedersen FS, Kristensen JS. Stromal-mediated down-regulation of CD13 in bone marrow cells originating from acute myeloid leukaemia patients. Eur J Haematol. 2001;66(3):168–77.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63.
- Watson J, Erta E. Flow cytometry. In: , Freshley R, (ed.) Animal cell culture. Oxford: IRL Press; 1992. p. 165–21.
- Nakase K, Kita K, Shiku H, Tanaka I, Nasu K, Dohy H, et al. Myeloid antigen, CD13, CD14, and/or CD33 expression is restricted to certain lymphoid neoplasms. Am J Clin Pathol. 1996;105(6):761–8.
- Cocco AE, Osei ES, Thut DM, Edinger AK, Powers JJ, Fu P, et al. Bimodal cell populations are common in chronic lymphocytic leukaemia but do not impact overall survival. Am J Clin Pathol. 2005;123(6):818–25.
- Pinto A, Del Vecchio L, Carbone A, Roncadin M, Volpe R, Serraino D, et al. Expression of myelomonocytic antigens is associated with unfavourable clinicoprognostic factors in B-cell chronic lymphocytic leukaemia. Ann Oncol. 1991;2(Suppl 2):107–13.
- Tàssies D, Montserrat E, Reverter JC, Villamor N, Rovira M, Rozman C. Myelomonocytic antigens in B-cell chronic lymphocytic leukaemia. Leuk Res. 1995;19(11):841–8.
- Bal K, Barcos MP, Stewart C. Phenotypic heterogeneity of B cells in patients with chronic lymphocytic leukaemia/small lymphocytic lymphoma. Am J Clin Pathol. 2003;119(6):824–32.
- O'Connell P, Gerkis V, D'Aprice A. Variable O-glycosylation of CD13 (aminopeptidase N). J Biol Chem. 1991;266/7:4593–7.
- Bauvois B, Dauzonne D. Aminopeptidase-N/CD13 (EC 3.4.11.2) inhibitors: chemistry, biological evaluations, and therapeutic prospects. Med Res Rev. 2006;26(1):88–130.
- Luan Y, Xu W. The structure and main functions of aminopeptidase N. Curr Med Chem. 2007;14(6):639–47.
- Zhang X, Xu W. Aminopeptidase N (APN/CD13) as a target for anti-cancer agent design. Curr Med Chem. 2008;15(27):2850–65.
- Alaikov T, Konstantinov SM, Tzanova T, Dinev K, Topashka-Ancheva M, Berger MR. Antineoplastic and anticlastogenic properties of curcumin. Ann N Y Acad Sci. 2007;1095:355–70.
- Sreekanth CN, Bava SV, Sreekumar E, Anto RJ. Molecular evidences for the chemosensitizing efficacy of liposomal curcumin in paclitaxel chemotherapy in mouse models of cervical cancer. Oncogene. 2011;30(28):3139–52.
- Varalakshmi Ch, Ali AM, Pardhasaradhi BV, Srivastava RM, Singh S, Khar A. Immunomodulatory effects of curcumin: in-vivo. Int Immunopharmacol. 2008;8(5):688–700.
- Hayun R, Okun E, Berrebi A, Shvidel L, Bassous L, Sredni B, et al. Rapamycin and curcumin induce apoptosis in primary resting B chronic lymphocytic leukaemia cells. Leuk Lymphoma. 2009;50(4):625–32.
- Ghosh AK, Kay NE, Secreto CR, Shanafelt TD. Curcumin inhibits prosurvival pathways in chronic lymphocytic leukaemia B cells and may overcome their stromal protection in combination with EGCG. Clin Cancer Res. 2009;15(4):1250–8.
- Aggarwal S, Ichikawa H, Takada Y, Sandur SK, Shishodia S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IkappaBalpha kinase and Akt activation. Mol Pharmacol. 2006;69(1):195–206.
- Saydmohammed M, Joseph D, Syed V. Curcumin suppresses constitutive activation of STAT-3 by up-regulating protein inhibitor of activated STAT-3 (PIAS-3) in ovarian and endometrial cancer cells. J Cell Biochem. 2010;110(2):447–56.
- Haque S, Lee H, Waqas B, Chiorazzi N, Mongini P. Anti-inflammatory curcumin inhibits AID expression within cycling human B cells. J Immunol. 2010;184 (Meeting Abstract Supplement):96.21.
- Angelo LS, Kurzrock R. Turmeric and green tea: a recipe for the treatment of B-chronic lymphocytic leukaemia. Clin Cancer Res. 2009;15(4):1123–5.
- Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008;269:199–225.
- Sun M, Estrov Z, Ji Y, Coombes KR, Harris DH, Kurzrock R. Curcumin (diferuloylmethane) alters the expression profiles of microRNAs in human pancreatic cancer cells. Mol Cancer Ther. 2008;7(3):464–73.