Abstract
The pathogenesis of acquired immunodeficiency syndrome-associated primary central nervous system lymphoma (AIDS-associated PCNSL) remains unclear. However, cell adhesion molecules have been reported to be strongly associated with PCNSL. In this study, we established Epstein–Barr virus (EBV)-transformed lymphoblastoid cell lines (LCLs) from HIV-positive patients (LCLHIV) and normal individuals (LCLN). The expression of CD18 antigen by LCLHIV was stronger than that by LCLN. We performed a cell adhesion assay using ISO-HAS, which is the human hemangiosarcoma cell line and expresses intercellular adhesion molecule 1 (CD54). The binding rates of LCLHIV and ISO-HAS without stimulation were higher than those of LCLN. Further, we demonstrated that azidothymidine or simvastatin inhibited the binding rates of LCLHIV and ISO-HAS more significantly than those of LCLN. Further, the levels of interleukin (IL)-8, a CD18 inducer, were higher in LCLHIV than in LCLN. We conclude that interaction between IL-8 and CD18 may be critical to AIDS-related PCNSL.
Introduction
Malignancy develops in approximately 30–40% of HIV-infected patients at some stage during their lifetime.Citation1 AIDS-associated primary central nervous system lymphoma (PCNSL) is associated with Epstein–Barr virus (EBV) infection and is classified as a diffuse large B-cell lymphoma. PCNSL represents 3.1% of all primary brain tumors and about 3% of non-Hodgkin's lymphoma,Citation2–Citation4 and usually develops in severely immunocompromised individuals with CD4 counts of less than 50/μl.Citation2–Citation4 Patients may present with confusion, memory loss, lethargy, or focal neurologic signs, and the median survival of patients presenting PCNSL is only 1–3 months without treatment.Citation2–Citation4 The incidence of AIDS-associated PCNSL has declined significantly with widespread use of highly active antiretroviral therapy (HAART), but AIDS-associated PCNSL occurs in 2–11% of HIV-infected patients, representing a 3600-fold higher incidence of AIDS-associated PCNSL than that of the general population.Citation5 However, the pathogenesis of AIDS-associated PCNSL remains unclear.Citation5 Two theories have been proposed on the basis of the course of AIDS-associated PCNSL. (1) Normal B lymphocytes in the central nervous system (CNS) may undergo clonal proliferation, which is supported by the presence of white matter brain lesions that herald brain lymphoma.Citation6 (2) Alternatively, a clone of malignant systemic lymphocytes expressing specific adhesion molecules might lodge in the CNS by a homing process.Citation7–Citation9 In this study, we proposed to study the latter theory. We established EBV-positive lymphoblastoid cell lines (LCLs) from HIV-infected patients (LCLHIV) and non-HIV-infected individuals (LCLN). Expression of lymphocyte function-associated antigen 1 (LFA-1, CD11a/CD18) by LCLHIV was higher than that by LCLN. In addition, we established an adhesion model between LCLs that expressed CD18 and a human hemangiosarcoma cell line, ISO-HAS,Citation10 which expressed intercellular adhesion molecule 1 (ICAM-1, CD54).Citation11 The binding rates between LCLs and ISO-HAS were higher in LCLHIV. Simvastatin, a 3-hydroxy-3-methylglutaryl (HMG) CoA reductase inhibitor, has been used as an antihypercholesterolemia drug.Citation12 Simvastatin binds to the inserted I domain of LFA-1,Citation13 thereby inhibiting the interaction between LFA-1 and ICAM-1.Citation14 In general, the most important aspect of AIDS-associated PCNSL treatment is the use of HAART.Citation15,Citation16 We focused on a reverse transcriptase inhibitor, azidothymidine (AZT).Citation17 Simvastatin or AZT decreased expression of CD18 by LCLHIV significantly. In a morphological study, simvastatin or AZT also blocked the binding between LCLs and ISO-HAS. In our study, interleukin (IL)-8 augmented CD18 expression resulting in high binding between LCLN and ISO-HAS. Finally, we suggested that LCLHIV produced large quantities of IL-8, which enhanced the expression of CD18 on the cell surface. In conclusion, IL-8 production induced by HIV infection could induce enhanced expression of CD18 on a clone of malignant lymphoma cells, resulting in CNS involvement by a homing process.
Materials and methods
Patients
Four HIV-infected patients (Is, Sh, and Ku following treatment by HAART, Ni was prior to HAART) were selected in this study. Four normal individuals were used as a control.
Cells and cell culture
ISO-HAS, which is a human hemangiosarcoma cell line,Citation10 was cultured on a collagen disc at 37°C in a humid atmosphere containing 5% CO2 in RPMI medium with 10% fetal calf serum (FCS). ISO-HAS was subcultured by adding 0.05% trypsin–0.53 mM EDTA for 15 minutes and rinsing with phosphate-buffered saline (PBS) without Mg and Ca. B95-8 is an EBV-transformed leukemia cell line. 8E5 is an HIV-1-positive cell line. B95-8 and 8E5 were cultured at 37°C in a humid atmosphere containing 5% CO2 in RPMI with 10% FCS. LCLs were cultured at 37°C in a humid atmosphere containing 5% CO2 in RPMI with 10% FCS.
Establishment and characteristics of LCL cells
Peripheral blood mononuclear cells (PBMCs) of the HIV-infected patients and normal subjects were separated from the peripheral blood by using Ficoll-Paque Plus (Amersham Pharmacia Biotech Inc., Piscataway, NJ, USA). PBMCs were incubated with supernatant from B95-8, and LCLs were obtained from each patient by transformation with EBV (B95-8). A fluorescence-activated cell sorting (FACS) analysis revealed the cells to be CD3−, CD4−, CD8−, CD5−, CD19+, CD20+, and HLA-class I+ and II+.
Reagents
Simvastatin was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA) and dissolved in dimethyl sulfoxide. AZT was purchased from Sigma-Aldrich and dissolved in distilled water. PKH67 Green Fluorescent Cell Linker Kit was purchased from Sigma-Aldrich.
FACS analysis
LCL suspensions (1 × 106 cells/ml) were stained with anti-CD11a antibody, anti-CD11b antibody, anti-CD18 antibody, anti-CD50 antibody, and anti-CD54 antibody (PE Conjugate Antibody (BD Immunocytometry Systems, San Jose, CA, USA) at 1:100 dilution for 15 minutes at 4°C. The cells were then washed twice with PBS and subjected to flow cytometry analysis on a FACScaliber with the Cell Quest Software (BD Immunocytometry Systems, San Jose, CA, USA).
DNA PCR for EBV
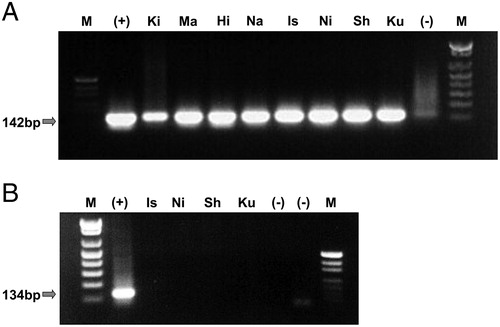
Total DNA was extracted from LCLs by using standard methods. EBV PCR was performed using the forward primer (5′-CAAGAACCCAGACGAGTCCGTAGAA-3′) and the reverse primer (5′-AAGAAGCATGTATACTAAGCCTCCC-3′).
Reverse transcriptase-polymerase chain reaction for HIV
Total RNA was extracted from LCLs. The RT reaction was carried out with 1 µg of total RNA, oligo (dT), 200 U of Moloney murine leukemia virus reverse transcriptase, and deoxynucleotides (dNTP) in a total volume of 20 µl. After cDNA synthesis, 1 µl of Taq polymerase was added to each reaction aliquot, and a reaction volume of 50 µl was used. Reverse transcriptase-polymerase chain reaction (RT-PCR) for HIV was performed using the forward primer SK145 (5′-AGTGGGGGGACATCAAGCAGCCATGCAAAT-3′) and the reverse primer SK431, (5′-TGCTATGTCAGTTCCCCTTGGTTCTCT-3′). A total of 35 cycles was carried out, each cycle consisted of denaturation (94°C for 1 minute), annealing (55°C for 1 minute), and elongation (72°C for 2 minutes).
Binding assay of LCLs and ISO-HAS
Labeling of ISO-HAS was performed with PKH-26 membrane dye (Sigma-Aldrich Co. St. Louis, MO, USA). Briefly, cells were suspended in 0.5 ml of diluent and immediately mixed with an equal volume of 4 mM PKH-26 for 5 minutes at room temperature. The labeling reaction was stopped by the addition of an equal volume of neat FCS for 1 minute, and cells were then washed twice in PBS. ISO-HAS labeled with PKH-26 were used as target cells. The labeled ISO-HAS cells (1 × 105) were incubated with LCLs (1 × 105)for 48 hours at 37°C in 5% CO2 using 100-mm collagen type I-coated dishes (Iwaki, Tokyo, Japan). To observe binding of LCLs and ISO-HAS, a confocal laser scanning microscope, LSM5Pascal-V3.0 (Carl Zeiss Co. Ltd., Jena, Germany) was used.
Enzyme-linked immunosorbent assay assay
Cytokine levels of IL-8, monocyte chemoattractant protein, and tumor necrosis factor in culture supernatants were determined with sandwich enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN, USA).
Data analysis
Data were presented as the mean ± standard error. Differences with P values less than 0.05 were considered statistically significant.
Results
Establishment of LCLs from the HIV-infected patients
EBV-positive LCLs were established from four HIV-infected patients. As shown in , EBV-transformation was confirmed in LCLHIV (Is, Sh, Ku, Ni) and LCLN (Ki, Ma, Hi, Na) by PCR. However, notably, no HIV infection was confirmed by RT-PCR in either LCL.
Expression of CD18 by LCLs derived from HIV patients
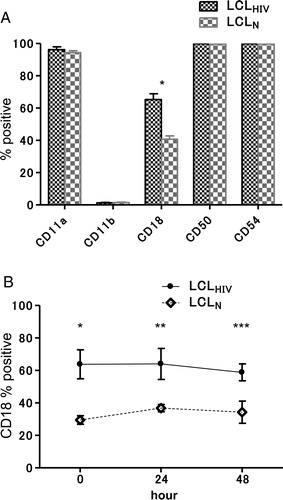
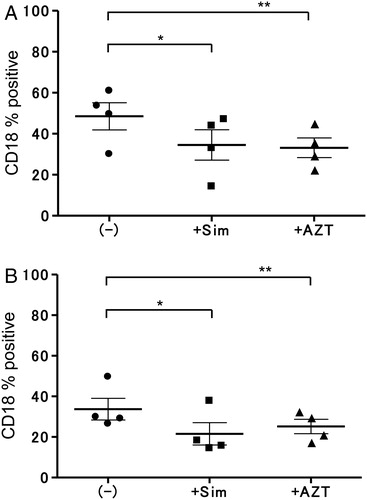
A shows the expression of adhesion molecules, including CD11a, CD11b, CD18, CD50, and CD54, by the LCLs by FACS. Expression of CD18 by LCLHIV was significantly higher than that by LCLN. On the other hand, there were no significant differences in the expression of the other molecules between LCLHIV and LCLN. The degree of CD18 expression did not change with culture time (B). After obtaining the above results, we established an adhesion system using ISO-HAS, which is a human hemangiosarcoma cell line.
Figure 2. Expression of adhesion molecules in LCLs by FACS. (A) Expressions of CD11a, CD11b, CD18, CD50, and CD54 were determined by FACS. The expression of CD18 by LCLHIV was significantly greater than that by LCLN. (B) Expressions of CD18 by LCLHIV and LCLN at several culture stages. The expression of CD18 in LCLHIV (Is, Sh, Ku, Ni) was significantly greater than that in LCLN (Ki, Ma, Hi, Na) by FACS at 0 hour (*P = 0.0102), 24 hours (**P = 0.0320), and 48 hours (***P = 0.0291).
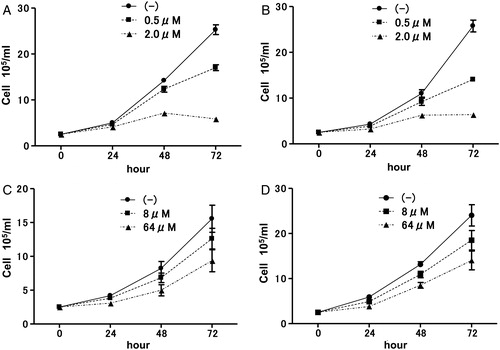
Effects of simvastatin or AZT on proliferation of LCLs
It has been reported that simvastatin inhibited nuclear factor-kappa B (NF-κB) activation, resulting in apoptosis of EBV-transformed LCLs in vitro. Simvastatin induced inhibition of cell proliferation of both LCLHIV and LCLN (A and 3B, respectively). In the treatment of AIDS-associated PCNS, anti-HIV drugs are usually used with chemotherapy; therefore, we examined the effects of AZT on proliferation of LCLs. Similarly to simvastatin, there was no significant difference in growth inhibition between LCLHIV (C) and LCLN (D) with AZT.
Effects of simvastatin or AZT on the expression of CD18 by LCLs
The effect of simvasatin or AZT on CD18 expression of both LCLs was observed by FACS. The LCLs were incubated with simvastatin (0.5 µM final conc.) or AZT (8.0 µM final conc.) for 24 hours. As shown in A, the expression of CD18 by LCLHIV was significantly reduced by treatment with simvastatin or AZT (P = 0.0208, P = 0.0113). The expressions of the other adhesion molecules (CD11a, CD54) showed no significant differences in the presence or absence of reagents (data not shown). However, as shown in B, the expression of CD18 by LCLN was downregulated significantly by simvastatin (P = 0.0015) but not by AZT (P = 0.0887). Because simvastatin or AZT reduced CD18 expression, we established a binding model using these reagents.
Figure 4. Effects of simvastatin or AZT on CD18 expression of LCLHIV or LCLN. (A) Expression of CD18 by LCLHIV was decreased significantly with simvastatin (*P = 0.0208) or AZT (**P = 0.0113). (B) Simvastatin induced downregulation of CD18 in LCLN (*P = 0.0015), but AZT did not induce downregulation (**P = 0.0887).
The effects of reagents on the adhesion model between LFA-1 and ICAM-1
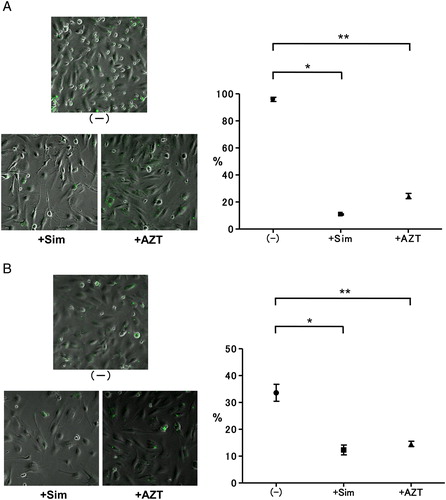
As shown in , expression of CD54 by ISO-HAS was detected; however, there was no expression of CD11a or CD18. As the expression of CD54 occurred on ISO-HAS, we established a binding system between ISO-HAS and LCLs. Binding rates of LCLHIV and ISO-HAS in spontaneous culture were 97–99%. As shown in A, significant inhibition of binding between LCLHIV and ISO-HAS by simvastatin or AZT was observed (P < 0.0001). In contrast, the binding rates of LCLN and ISO-HAS without reagents were 30–40%. The binding rates of LCLN and ISO-HAS were decreased by simvastatin (P = 0.0115) or AZT (P = 0.0115) (B).
Figure 5. Expression of CD54 and CD18 by ISO-HAS. Expressions of adhesion molecules in ISO-HAS by FACS. ISO-HAS mostly expressed ICAM-1 (CD54, 99.59%). No expression of LFA-1 (CD11a/CD18, 1.82%/1.39%) was detected.
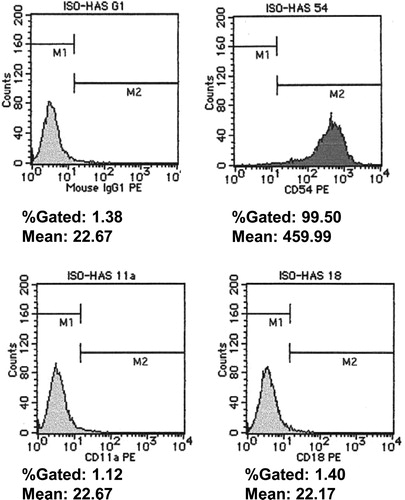
Figure 6. Inhibition of binding between LCLs and ISO-HAS by simvastatin or AZT. (A) The binding rates of LCLHIV and ISO-HAS without reagents were 97–99%. Binding rates were significantly decreased by simvastatin (0.5 µM final conc.) (*P < 0.0001) or AZT (8.0 µM final conc.) (**P < 0.0001). (B) The binding rates between ISO-HAS and LCLN were 30–40% in spontaneous culture. Simvastatin (0.5 µM final conc.) (*P < 0.0001) or AZT (8.0 µM final conc.) induced significantly decreased binding (**P < 0.0001).
Effects of IL-8 on LCLs
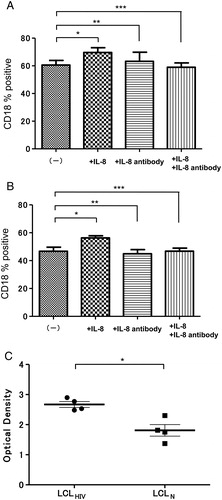
It has been reported that IL-8 augmented CD18 expression in the immune system; therefore, we examined the effects of IL-8 on expression of CD18 by both LCLs. As shown in A and 7B, CD18 expression was significantly increased in LCLN (P = 0.0333) but not in LCLHIV (P = 0.0073). This discrepancy remains unresolved. We hypothesized that LCL derived from HIV patients produced large quantities of IL-8, and the sensitivity of IL-8 was, therefore, lower than that of LCL derived from normal individuals. Further, we measured the IL-8 level in the supernatant of LCLs. As shown in C, the concentration of IL-8 in the supernatant of LCLHIV was higher than that in the supernatant of LCLN.
Figure 7. Effects of IL-8 on CD18 expression of LCLHIV or LCLN. Expression of CD18 by LCLHIV (A) or LCLN (B) increased with IL-8 (100 ng/ml final conc.) (LCLHIV, *P = 0.0333; LCLN, *P = 0.0073). (C) IL-8 level in culture supernatant from LCLHIV was higher than that in supernatant from LCLN (*P = 0.0171).
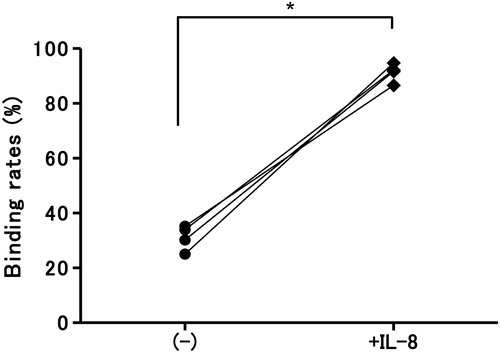
Effects of IL-8 on binding between LCLN and ISO-HAS
We next examined the effects of IL-8 on the binding rates of LCLN and ISO-HAS. Notably, as shown in , IL-8 enhanced binding rates to high levels, similar to those of LCLHIV in spontaneous culture.
Discussion
AIDS-associated primary CNS lymphoma (PCNSL) is associated with EBVinfection.Citation18 In this study, we established EBV-transformed lymphoblastoid cell lines (LCLs) from both HIV-infected patients (LCLHIV) and non-HIV-infected individuals (LCLN) to investigate the pathogenesis of PCNSL. There were no significant differences in cell growth between the LCLHIV and the LCLN. To study CNS involvement in AIDS-associated PCNSL, we focused on the expression of cell adhesion molecules. Notably, the expression of CD18 by the LCLHIV was significantly higher than that by the LCLN. These results suggested that AIDS-associated PCNSL possibly develops via the CD18 and CD54 interaction pathway. It has been reported that EBV-infected lymphocytes migrate to the CNS by adhesion of LFA-1 and ICAM-1.Citation19 Therefore, a cell-attachment assay using the human hemangiosarcoma cell line, ISO-HAS, which expresses only CD54, was carried out. Notably, cell binding rates of LCLHIV were higher than those of LCLN. These results indicated that PCNSL possibly develops via adhesion processes between CD18 and CD54. It has been reported that simvastatin, a 3-hydroxy-3-methylglutaryl (HMG) CoA reductase inhibitor, binds to the inserted I domain of LFA-1 and inhibits the interaction between LFA-1 and ICAM-1. Moreover, simvastatin dissociated the latent membrane protein 1 from lipid rafts and inhibited NF-κB activation, which induced apoptosis of EBV-transformed LCLs in vitro.Citation20 Consequently, the expression of CD18 on LCLs was significantly reduced by treatment with simvastatin, and simvastatin significantly reduced adhesion rates between LCLs and ISO-HAS. Additionally, we found that AZT, which is one of the classical nucleic reverse transcriptase inhibitors, induced growth inhibition of LCLs and reduced CD18 expression by LCLs. It was suggested that simvastatin or AZT may inhibit the growth of lymphoma cellsCitation21 and prevent CNS involvement via the LFA-1/ICAM-1 pathway. In fact, antiretroviral therapy has been used for AIDS-associated PCNSL.Citation22,Citation23 Moreover, it was suggested that simvastatin should be combined with antiviral therapy. CD11b is a potential critical marker of lymphoma and leukemia and may be useful as a minimal residual disease (MRD) specific marker in PBC-ALL.Citation24 Therefore, we concluded that CD18 may be a critical marker of MRD in PCNSL. Furthermore, considering the high expression of CD18 by LCLHIV, we focused on production of IL-8, which augmented the CD18 molecule. It has been reported that IL-8 production was higher in HIV patients.Citation25–Citation28 In our experiment, the IL-8 level in the cell-free supernatant of LCLHIV was higher than that in the supernatant of LCLN. In fact, EBV infection induced several cytokines, including IL-8.Citation29 However, EBV infection was detected in both LCLHIV and LCLN by DNA analysis (). These results suggested that HIV infection possibly augmented IL-8 production in association with EBV infection. It has been reported that EBV- and HIV-infected B-cell lines were the major secretors of IL-8.Citation28 However, because IL-8 receptors were not detected in any cell lines secreting IL-8 protein constitutively,Citation28 it was concluded that there was no apparent evidence of IL-8 autocrine loops. Our results corroborated that chemokine-induced CD18 was a critical parameter in the development of AIDS-associated PCNSL. IL-8 has been reported to enhance the adherence of neutrophils to endothelial and subendothelial matrix proteins by inducing the expression of CD11b/CD18.Citation30 Cytokines, particularly IL-8, play an important role in the development of AIDS-associated B-cell lymphomagenesis. Furthermore, expression of CD18 and serum level of IL-8 may be used as clinical indicators of risk status.
Acknowledgements
Author thanks to Mrs K. Furukawa and Mrs K. Niki for technical assistance and Ms S. Yoshida and Ms S. Nagayama for preparing the manuscript.
The authors declare no competing financial interests.
References
- Spano JP, Atlan D, Breau JL, Farge D. AIDS and non-AIDS-related malignancies: a new vexing challenge in HIV-positive patients, part I: Kaposi's sarcoma, non-Hodgkin's lymphoma, and Hodgkin's lymphoma. Eur J Intern Med. 2002;13:170–9.
- Raez LE, Patel P, Feun L, Restrepo A , Raub WA, Cassileth PA. Natural history and prognostic factors for survival in patients with acquired immune deficiency syndrome (AIDS)-related primary central nervous system lymphoma (PCNSL). Crit Rev Oncog. 1998;9:199.
- Herrlinger U, Schabet M, Bitzer M, Petersen D, Krauseneck P. Primary central nervous system lymphoma: from clinical presentation to diagnosis. J Neurosurg. 2000;92:261–6.
- Fitzsimmons A, Upchurch K, Batchelor T. Clinical features and diagnosis of primary central nervous system lymphoma. Hematol Oncol Clin North Am. 2005;19:689–703.
- Anthony IC, Crawford DH, Bell JE. B lymphocytes in the normal brain: contrasts with HIV-associated lymphoid infiltrates and lymphomas. Brain. 2003;126:1058.
- Alderson L, Fetell MR, Sisti M, Hochberg F, Cohen M, Louis DN. Sentinel lesions of primary CNS lymphoma. J Neurol Neurosurg Psychiatry. 1996;60:102–5.
- Drillenburg P, Pals ST. Cell adhesion receptors in lymphoma dissemination. Blood. 2000;95:1900–10.
- Smith JR, Braziel RM, Paoletti S, Lipp M, Uguccioni M, Rosenbaum JT. Expression of B-cell-attracting chemokine 1 (CXCL 13) by malignant lymphocytes and vascular endothelium in primary central nervous system lymphoma. Blood. 2003;101:815–21.
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–14.
- Masuzawa M, Fujita Y, Hara H, Nishiyama S, et al. Establishment of a human hemangiosarcoma cell line (ISO-HAS). Int J Cancer. 1999;81:305–8.
- Carrasco YR, Fleire SJ, Cameron T, Dustin ML, Batista FD. LFA-1/ICAM-1 interaction lowers the threshold of B cell activation by facilitating B cell adhesion and synapse formation. Immunity. 2004;20:589–99.
- Szkodziński J, Romanowski W, Hudzik B, Kaszuba A , Nowakowska-Zajdel E, Szkilnik R, et al. Effect of HMG-CoA (3-hydroxy-3-methylglutaryl-CoA) reductase inhibitors on the concentration of insulin-like growth factor-1 (IGF-1) in hypercholesterolemic patients. Pharmacol Rep. 2009;61:654–64.
- Li S, Wang H, Peng B, Zhang M, Zhang D, Hou S, et al. Efalizumab binding to the LFA-1 alphaL I domain blocks ICAM-1 binding via steric hindrance. Proc Natl Acad Sci USA. 2009;106:4349–54.
- Schramm R, Menger MD, Harder Y, Schmits R, Adam O, Weitz-Schmidt G, et al. Statins inhibit lymphocyte homing to peripheral lymph nodes. Immunology. 2007;120:315–24.
- Gerstner E, Batchelor T. Primary CNS lymphoma. Expert Rev Anticancer Ther. 2007;7:689–700.
- Portegies P, Solod L, Cinque P, Chaudhuri A, Begovac J, Everall I, et al. Guidelines for the diagnosis and management of neurological complications of HIV infection. Eur J Neurol. 2004;11:297–304.
- Purcet S, Minuesa G, Molina-Arcas M, Erkizia I, Casado FJ, Clotet B, et al. 3′-Azido-2′, 3′-dideoxythymidine (zidovudine) uptake mechanisms in T lymphocytes. Antivir Ther. 2006;11:803–11.
- Knowles DM. Etiology and pathogenesis of AIDS-related non-Hodgkin's lymphoma. Hematol Oncol Clin North Am. 2003;17:785–820.
- Bashir R, Coakham H, Hochberg F. Expression of LFA-1/ICAM-1 in CNS lymphomas: possible mechanism for lymphoma homing into the brain. J Neurooncol. 1992;12:103–10.
- Katano H, Pesnicak L, Cohen JI. Simvastatin induces apoptosis of Epstein-Barr virus (EBV)-transformed lymphoblastoid cell lines and delays development of EBV lymphomas. Proc Natl Acad Sci USA. 2004;101:4960–5.
- Kurokawa M, Ghosh SK, Ramos JC, Mian AM, Toomey NL, Cabral L, et al. Azidothymidine inhibits NF-kappaB and induces Epstein-Barr virus gene expression in Burkitt lymphoma. Blood. 2005;106:235–40.
- Diamond C, Taylor TH, Im T, Miradi M, Wallace M, Anton-Culver H. Highly active antiretroviral therapy is associated with improved survival among patients with AIDS-related primary central nervous system non-Hodgkin's lymphoma. Curr HIV Res. 2006;4:375–8.
- Skiest DJ, Crosby C. Survival is prolonged by highly active antiretroviral therapy in AIDS patients with primary central nervous system lymphoma. AIDS. 2003;17:1787–93.
- Rhein P, Mitlohner R, Basso G, Gaipa G, Dworzak MN, Kirschner-Schwabe R, et al. CD11b is a therapy resistance- and minimal residual disease-specific marker in precursor B-cell acute lymphoblastic leukemia. Blood. 2010;115:3763–71.
- Matsumoto T, Mike T, Nelson RP, Trudeau WL, Lockey RF, Yodoi J. Elavated serum level of IL-8 in patients with HIV infection. Clin Exp Immunol. 1993;93:149–51.
- Cota M, KLeinschmidt A, Ceccherini-Silberstein F, Aloisi F, Mengozzi M, Mantovani A, et al. Upregulated expression of interleukin-8, RAMTES and chemokine receptors in human astrocytic cells infected with HIV-1. J Neurovirol. 2000;6:75–83.
- Kedzierska K, Crowe SM. Cytokines and HIV: interactions and clinical implications. Antiviral Chem Chemother. 2001;12:133–50.
- Sharma V, Zhang L. Interleukin-8 expression in AIDS-associated lymphoma B-cell lines. Biochem Biophys Res Commun. 2001;282:369–75.
- Klein SC, Kube D, Abts H, Diehl V, Tesch H. Promotion of IL-8, IL-10, TNFα and TNFb production by EBV infection. Leukemia Res. 1996;20:633–6.
- Rot A. Endothelial cell binding of NAL-1/IL-8: Role in neutrophil emigration. Immunol Today. 1992;13:291–4.