Abstract
Sensitized recipients are at a high risk of graft rejection in hematopoietic stem cell transplantation. To explore the trace of donor cells, we tried to explore homing and engraftment of bone marrow cells (BMCs) derived from different donors in a murine model of sensitization. Sensitized BALB/c mice were used as transplanted recipients, which received BMCs derived from C57BL/6 or BALB/c donors after lethal irradiation. The homing study showed that the donor cells decreased along with time in recipients of the C57BL/6 donor group, but the donor cells increased along with time in recipients of the BALB/c donor group. For the engraftment assay, all the sensitized recipients transplanted with BMCs derived from C57BL/6 donors died after lethal irradiation. In contrast, all the recipients transplanted with BMCs derived from BALB/c donors got long-term survival. Our results suggest that it is crucial to have human leukocyte antigen identical donors for sensitized recipients during hematopoietic stem cell transplantation.
Introduction
Many hematological diseases, such as thalassemia major, sickle cell disease, and aplastic anemia, require life-long transfusion support.Citation1–Citation5 Given that these patients received repeated transfusions from human leukocyte antigen (HLA)-mismatched donors before transplantation, they are highly likely to be sensitized and produce high levels of donor-reactive antibodies.Citation6–Citation8 In our center, serum samples from children with β-thalassemia major were analyzed for panel reactive antibody and the results showed that 7 of 20 samples were antibody-positive, with the intensity of positivity ranging from 30 to 75%.Citation9 High level of serum antibody is considered to contribute to low engraftment of hematopoietic stem cells following infusion in sensitized recipients with subsequent failure of the transplant.Citation10–Citation12 Approximately 25–30% of patients may have an HLA-identical sibling donor.Citation13,Citation14 Therefore, it is important to understand the fate of hematopoietic stem cells derived from different donors in sensitized recipients.
The level of sensitization associated with transfusions is due to white blood cells present in allogeneic blood products.Citation8,Citation15,Citation16 Sensitized recipients are at a high risk of graft rejection following hematopoietic stem cell transplantation. However, the homing and engraftment of donor cells in sensitized recipients is not clear. In our study, a murine model of sensitization was established by repeated transfusions of allogeneic spleen cells, which was considered representative of highly sensitized patients in the clinic. Using this sensitized model, we explored the homing and engraftment of bone marrow cells (BMCs) derived from fully major histocompatibility complex disparate and identical donors in vivo.
Materials and methods
Animals
Male C57BL/6 and BALB/c mice, aged 6–8 weeks and weighed 18–20 g, were purchased from the Experimental Animal Center of Sun Yat-sen University (Guangzhou, China). All the animals were handled and housed in accordance with the guidelines of the Sun Yat-sen University Animal Care and Use Committee.
A murine model of sensitization
BALB/c mice were sensitized by repeated transfusions of allogeneic spleen cells from C57BL/6 mice. Briefly, C57BL/6 mice were killed, and their spleens were removed and teased into single-cell suspensions. A total of 1 × 106 nucleated splenocytes (0.1 ml) were transfused to BALB/c mice via their tail vein weekly for 2 weeks (on day −14 and day −7, respectively). The primed mice were used as sensitized recipients on day 0.
Anti-donor antibodies
Sera were obtained from sensitized BALB/c mice for anti-donor antibodies detection. Sera obtained from naive BALB/c mice were used as control. Anti-donor antibodies were measured by flow cross-match assay. Briefly, splenocytes (1 × 106, 100 µl) from C57BL/6 mice were incubated with 10 µl sera for 30 minutes. Cells were washed and incubated with fluorescein isothiocyanate (FITC)-conjugated polyclonal goat anti-mouse immunoglobulin (BD PharMingen, San Diego, CA, USA). Levels of anti-donor antibodies were determined by flow cytometry (Becton Dickinson, San Jose, CA, USA).
Bone marrow transplantation
Bone marrow transplantation was performed on day 0. Sensitized BALB/c mice were used as recipients, and donor BMCs were derived from C57BL/6 or BALB/c mice. Sensitized BALB/c mice underwent total body irradiation with 800 cGy using cobalt-60 gamma rays before transplantation. A total of 1 × 107 (0.2 ml) of BMCs obtained from femurs and tibias of donor mice were injected into the recipients. Sensitized BALB/c mice receiving irradiation alone were used as control group.
Homing trace
For homing studies, BMCs were labeled with the cell tracker, 5- and 6-carboxyfluorescein diacetate succinimidyl ester (CFSE, Invitrogen, Carlsbad, CA, USA), according to standard protocols before transplantation.Citation17 Sensitized recipients were sacrificed at specific time points (2, 12, and 48 hours) post-transplantation. Cell suspensions collected from the femur and spleen, and retro-orbital sinus underwent red cell depletion by treatment with a lysing buffer. The percentage of donor cells homing to the respective organ was tested by monitoring CFSE+ cells using flow cytometry.
Engraftment assay
Mortality was scored daily. The survival rate was determined on 28 days post-transplantation. White blood cells in peripheral blood and BMCs in the femur were counted weekly post-transplantation. BMCs from femurs of recipients were cultured in methylcellulose medium (Methocult™GFM3434; Stem Cell Technologies Inc, Vancouver, Canada) for 7 days of incubation. Colony-forming units for granulocyte/monocyte (CFU-GM) were calculated under an inverted microscope (×100).Citation18 Femurs of recipients were collected 7 days post-transplantation. Histological sections were stained with hematoxylin and eosin and imaged at ×100 magnification.Citation19
Statistical analysis
Results are expressed as mean ± standard error of the mean, and the data were analyzed by SPSS 16.0 statistical software. Comparisons between experimental results were made using one-way analysis of variance test analysis. Values of log-rank P were determined using the Kaplan–Meier method comparing survival curves. P < 0.05 was considered statistically significant.
Results
A murine model of sensitization was established by repeated transfusions of allogeneic splenocytes
Sera from sensitized or naive BALB/c mice were incubated with FITC-conjugated goat anti-mouse to determine the level of anti-donor antibodies that had bound to the donor splenocytes. Our results showed that the percentage binding rates for sensitized sera and naive sera were (90.32 ± 5.07)% and (5.15 ± 2.38)%, respectively, and the differences were statistically significant (P < 0.001). These results indicated that high levels of antibody were present in the serum of primed mice. A murine model of sensitization was successfully established by repeated transfusions of allogeneic splenocytes.
Cells from fully identical donors succeeded in homing to sensitized recipients
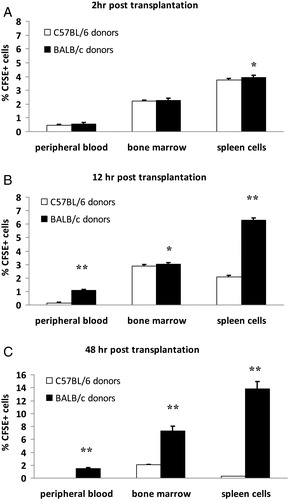
To perform homing trace, BMCs labeled with fluorescent dye CFSE were injected into the sensitized recipients. Our experiments showed that after labeling with CFSE in vitro, the intensity of fluorescence for BMCs did not attenuate 48 hours later (data not given). As shown in , 2 hours after BMCs infusion, the percentage of donor CFSE+ cells in the spleen of the C57BL/6 donor group and BALB/c donor group was (3.73 ± 0.13)% and (3.93 ± 0.16)%, respectively (P < 0.05). No significant difference was found in peripheral blood and bone marrow between these two groups (P > 0.05). However, 12 hours after BMCs infusion, the percentage of CFSE+ cells in peripheral blood, bone marrow, and the spleen of the BALB/c donor group was significantly more than C57BL/6 donor group by 6.2-fold (P < 0.001), 0.05-fold (P < 0.05), 1.99-fold (P < 0.001), respectively. At 48 hours post-BMCs infusion, the different numbers of CFSE+ cells in peripheral blood, bone marrow, and spleen between the BALB/c and C57BL/6 donor group differed by 57-fold, 2.57-fold, and 55-fold, respectively (P < 0.001). The results suggested that BMCs from fully identical donors succeeded in homing to sensitized recipients.
Figure 1. Homing trace in different tissues of recipients at special time points after bone marrow transplantation. BMCs of different donors labeled with fluorescent dye CFSE were injected into sensitized recipients, then homing to different tissues was evaluated. The percentage of CFSE+ donor cells in peripheral blood, bone marrow, and spleen cells of recipients were analyzed by flow cytometry at various time points. (A) Two hours post-transplantation. (B) Twelve hours post-transplantation (C) Forty-eight hours post-transplantation. Values are given as mean ± SD of five mice per time point. *P < 0.05 C57BL/6 donor group vs. BALB/c donor group, **P < 0.001 C57BL/6 donor group vs. BALB/c donor group.
Cells from fully identical donors rescued irradiated mice of sensitization
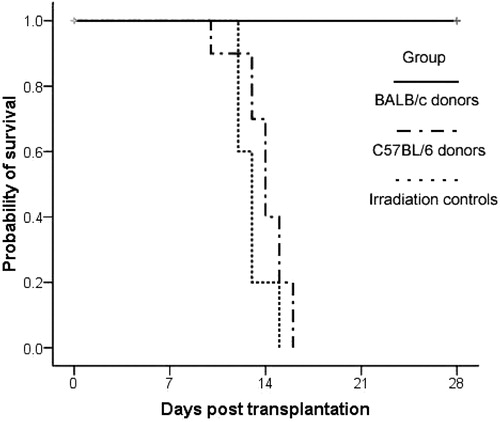
To test engraftment of cells from different donors in sensitized recipients, mortality was scored daily after bone marrow transplantation. As expected, without transplantation, the mice died at 10–16 days after irradiation, with a median of 14 days (). The sensitized BALB/c mice receiving BMCs of C57BL/6 donors died at 12–15 days after irradiation, with a median of 13 days. Significantly, sensitized BALB/c mice receiving BMCs of BALB/c donors remained alive 28 days after irradiation. By log-rank analysis there was no significant difference between the irradiated control group and the C57BL/6 donor group (P > 0.05), but there was a significant difference between the C57BL/6 donor group and the BALB/c donor group (P < 0.0001).
Figure 2. Survival analysis after irradiation with or without bone marrow transplantation. The irradiation control group was treated with lethal irradiation of 800 cGy, but without bone marrow transplantation. The sensitized BALB/c mice were transplanted with 1 × 107 BMCs derived from C57BL/6 or BALB/c mice after the irradiation, respectively. Each group contained 10 recipients, and the survival events were monitored daily. Values of log-rank P were determined using the Kaplan–Meier method comparing survival curves performed with SPSS 16.0.
In addition, hematopoietic recovery of recipients was detected post-transplantation. As shown in , the white blood cells and BMCs of recipients in C57BL/6 donor group deceased with time post-transplantation, while those in BALB/c donor group increased with time post-transplantation. Moreover, no CFU-GM colonies could be found for BMCs in the C57BL/6 donor group, but the numbers of CFU-GM colonies in the BALB/c donor group increased with time post-transplantation. All these results showed that the transplantation of BMCs from fully identical donors could rescue irradiated sensitized recipients.
Table 1. Hematopoietic recovery of recipients post-transplantation (x¯ ± s, n = 5)
Discussion
Our data showed that high levels of antibody were present in the serum of primed mice, indicating that a murine model of sensitization was established by repeated transfusion of allogeneic splenocytes. In our present study, sensitized BALB/c mice were used as transplanted recipients, while C57BL/6 mice and BALB/c mice were used as fully disparate and identical donors, respectively. In tracking the cells we found that the CFSE+ donor cells decreased with time in recipients of C57BL/6 donor group while the CFSE+ donor cells increased with time in recipients of BALB/c donor group. As an indicator of engraftment all the sensitized recipients transplanted with BMCs derived from C57BL/6 donors died after lethal irradiation. In contrast, all the recipients transplanted with BMCs derived from fully identical donors survived long term and recovered rapidly. Furthermore, by histological section assays, the recipients of C57BL/6 donor group were shown to die of bone marrow failure, while the recipients of BALB/c donor group recovered rapidly (data not shown). Taken together, our experiments showed that only the BMCs derived from fully identical donors succeeded in homing and engraftment in sensitized recipients.
These results agree with clinical findings. In our center, 14 patients with thalassemia major underwent transplantation with cells from unrelated donors with one or two HLA allelic mismatches. Only two patients had successful engraftment, while the others developed primary graft failure. In contrast, during the same period, six patients with thalassemia major underwent transplantation from HLA-identical related donors, and five patients had successful engraftment.Citation20 Clinical results suggest that HLA-matched related donor transplantation is the preferred option for sensitized recipients.Citation21–Citation23 The mechanism of marrow graft rejection in sensitized recipients has not been clearly defined. Our previous data have shown that the antibodies in sensitized serum were capable of inducing high level of cell and complement-mediated cytotoxicity to donor graft.Citation9 Other research groups also found that the antibody-dependent cellular cytotoxicity pathway played an important role in sensitized recipients; in addition to humoral immunity, memory T cells from sensitized recipients could also be responsible for BMCs rejection.Citation24–Citation26
In conclusion, BMCs derived from fully disparate donors were completely rejected in the sensitized recipients, while the BMCs derived from fully identical donors underwent successful homing and engraftment. Our results suggest that it is crucial to have HLA identical donors for sensitized recipients during hematopoietic stem cell transplantation.
Acknowledgements
This work was supported in part by National Natural Science Foundation of China (81100370).
References
- Schrier SL, Angelucci E. New strategies in the treatment of the thalassemias. Annu Rev Med. 2005;56:157–71.
- Kremastinos DT, Farmakis D, Aessopos A, Hahalis G, Hamodraka E, Tsiapras D, et al. Beta-thalassemia cardiomyopathy: history, present considerations, and future perspectives. Circ Heart Fail. 2010;3:451–8.
- Noetzli LJ, Coates TD, Wood JC. Pancreatic iron loading in chronically transfused sickle cell disease is lower than in thalassaemia major. Br J Haematol. 2011;152:229–33.
- Marsh JC, Ball SE, Cavenagh J, Darbyshire P, Dokal I, Gordon-Smith EC, et al. Guidelines for the diagnosis and management of aplastic anaemia. Br J Haematol. 2009;147:43–70.
- Marsh J, Socie G, Tichelli A, Schrezenmeier H, Hochsmann B, Risitano AM, et al. Should irradiated blood products be given routinely to all patients with aplastic anaemia undergoing immunosuppressive therapy with antithymocyte globulin (ATG)? A survey from the European Group for Blood and Marrow Transplantation Severe Aplastic Anaemia Working Party. Br J Haematol. 2010;150:377–9.
- Friedman DF, Lukas MB, Jawad A, Larson PJ, Ohene-Frempong K, Manno CS. Alloimmunization to platelets in heavily transfused patients with sickle cell disease. Blood. 1996;88:3216–22.
- Lo SC, Chang JS, Lin SW, Lin DT. Platelet alloimmunization after long-term red cell transfusion in transfusion-dependent thalassemia patients. Transfusion. 2005;45:761–5.
- Eikmans M, Waanders MM, Roelen DL, van Miert PP, Anholts JD, de Fijter HW, et al. Differential effect of pretransplant blood transfusions on immune effector and regulatory compartments in HLA-sensitized and nonsensitized recipients. Transplantation. 2010;90:1192–9.
- Fang JP, Xu LH, Yang XG, Wu YF, Weng WJ, Xu HG. Panel reactive antibody in thalassemic serum inhibits proliferation and differentiation of cord blood CD34+ cells in vitro. Pediatr Hematol Oncol. 2009;26:338–44.
- Ciurea SO, de Lima M, Cano P, Korbling M, Giralt S, Shpall EJ, et al. High risk of graft failure in patients with anti-HLA antibodies undergoing haploidentical stem-cell transplantation. Transplantation. 2009;88:1019–24.
- Kataoka K, Yamamoto G, Nannya Y, Yoshimi A, Okada S, Asai T, et al. Successful engraftment following HLA-mismatched cord blood transplantation for patients with anti-HLA Abs. Bone Marrow Transplant. 2008;42:129–30.
- Khoury R, Abboud MR. Stem-cell transplantation in children and adults with sickle cell disease: an update. Expert Rev Hematol. 2011;4:343–51.
- Gaziev D, Galimberti M, Lucarelli G, Polchi P, Giardini C, Angelucci E, et al. Bone marrow transplantation from alternative donors for thalassemia: HLA-phenotypically identical relative and HLA-nonidentical sibling or parent transplants. Bone Marrow Transplant. 2000;25:815–21.
- Li CG, Li CF, Li Q, Li M. Thalassemia incidence and treatment in China with special reference to Shenzhen City and Guangdong province. Hemoglobin. 2009;33:296–303.
- Petrányi GG, Réti M, Harsányi V, Szabó J. Immunologic consequences of blood transfusion and their clinical manifestations. Int Arch Allergy Immunol. 1997;114:303–15.
- Andreu G, Perrot JY, Pirenne F, Boccaccio C. The effect of ultraviolet B light on antigen-presenting cells: implications for transfusion-induced sensitization. Semin Hematol. 1992;29:122–31.
- Dooner M, Cerny J, Colvin G, Demers D, Pimentel J, Greer D, et al. Homing and conversion of murine hematopoietic stem cells to lung. Blood Cells Mol Dis. 2004;32:47–51.
- Roda E, Coccini T, Acerbi D, Castoldi AF, Manzo L. Comparative in vitro and ex-vivo myelotoxicity of aflatoxins B1 and M1 on haematopoietic progenitors (BFU-E, CFU-E, and CFU-GM): species-related susceptibility. Toxicol In Vitro. 2010;24:217–23.
- Lock RB, Liem N, Farnsworth ML, Milross CG, Xue C, Tajbakhsh M, et al. The nonobese diabetic/severe combined immunodeficient (NOD/SCID) mouse model of childhood acute lymphoblastic leukemia reveals intrinsic differences in biologic characteristics at diagnosis and relapse. Blood. 2002;99:4100–8.
- Fang J, Huang S, Chen C, Zhou D, Li CK, Li Y, et al. Umbilical cord blood transplantation in Chinese children with beta-thalassemia. J Pediatr Hematol Oncol. 2004;26:185–9.
- Lucarelli G, Gaziev J. Advances in the allogeneic transplantation for thalassemia. Blood Rev. 2008;22:53–63.
- Fang JP, Xu LH. Hematopoietic stem cell transplantation for children with thalassemia major in China. Pediatr Blood Cancer. 2010;55:1062–5.
- Smiers FJ, Krishnamurti L, Lucarelli G. Hematopoietic stem cell transplantation for hemoglobinopathies: current practice and emerging trends. Pediatr Clin North Am. 2010;57:181–205.
- Nagata S, Okano S, Yonemitsu Y, Nakagawa K, Tomita Y, Yoshikai Y, et al. Critical roles of memory T cells and antidonor immunoglobulin in rejection of allogeneic bone marrow cells in sensitized recipient mice. Transplantation. 2006;82:689–98.
- Xu H, Chilton PM, Tanner MK, Huang Y, Schanie CL, Dy-Liacco M, et al. Humoral immunity is the dominant barrier for allogeneic bone marrow engraftment in sensitized recipients. Blood. 2006;108:3611–9.
- Taylor PA, Ehrhardt MJ, Roforth MM, Swedin JM, Panoskaltsis-Mortari A, Serody JS, et al. Preformed antibody, not primed T cells, is the initial and major barrier to bone marrow engraftment in allosensitized recipients. Blood. 2007;109:1307–15.