Abstract
Exposure of cells to hydrogen peroxide or platelet-derived growth factor (PDGF) induced Akt phosphorylation and oxidation of phosphatase and tensin homolog (PTEN). The Cys124 and Cys71 residues of PTEN were critical for the formation of a disulfide bond and the intermediate glutathionylation in the process of reduction of the disulfide bond. To determine which specific tyrosine residues of the PDGF beta receptor (PDGFβR) is involved in PDGF-induced PTEN oxidation and Akt phosphorylation, we investigated a kinase activity-deficient mutant and PDGFβR mutants where the tyrosine residues in the binding site for phosphoinositide 3-kinase (PI3K), GTPase-activating protein of Ras, Src homology 2 domain containing protein-tyrosine phosphatase-2, and phospholipase C-1 were replaced by Phe. Both PTEN oxidation and Akt phosphorylation did not occur in response to PDGF in the kinase-deficient mutant and in the PDGFβR mutant with a mutation in the PI3K binding site (Tyr740 and Tyr751). Thus, the kinase activity and the constituent Tyr740 and Tyr751 residues of PDGFβR in the cells stimulated with PDGF are responsible for the oxidation of PTEN and the Akt phosphorylation.
Introduction
Hydrogen peroxide (H2O2) plays an important role in the propagation of receptor signaling and growth factor signal transduction in cells.Citation1 Stimulation of cells with growth factors such as epidermal growth factor (EGF) or platelet-derived growth factor (PDGF) induces the generation of H2O2. Inhibition of H2O2 production or scavenging of H2O2 results in the attenuation of mitogenic signaling triggered by growth factors.Citation2,Citation3 The levels of intracellular H2O2 are regulated by superoxide-generating NADPH oxidase and scavenging enzymes such as superoxide dismutase, catalase, and peroxiredoxins.Citation4,Citation5
Stimulation of cells with EGF or PDGF induces the transient activation of phosphoinsitide 3-kinase (PI3K) which catalyzes synthesis of phosphatidylinositol (3,4,5)-triphosphatate (PtdIns (3,4,5)P3).Citation6 Phosphatase and tensin homolog (PTEN) negatively regulates PI3K through dephosphorylation of PtdIns(3,4,5)P3 at the third position of the inositol headgroup.Citation7 PtdIns(3,4,5)P3-induced activation of downstream Akt promotes cell survival and suppresses apoptosis.Citation8 PI3K is required for the generation of H2O2 in non-phagocytic cells when stimulated by growth factorsCitation9 and in phagocytic cells stimulated by chemoattractants,Citation10 suggesting that PtdIns(3,4,5)P3 is necessary for the generation of H2O2 as a second messenger.
PI3K in non-phagocytic cells is activated by recruitment of the p110 catalytic subunit to the phosphorylated growth factor receptor via mediation of the p85 regulatory subunit.Citation6,Citation11 p110, the catalytic subunit of PI3K interacts with Ras.Citation12 PI3K in phagocytic cells is up-regulated by binding Ras as well as Gβ.Citation13 Activated PI3K increases the level of intracellular H2O2 and inhibits the activity of phosphatase.Citation11,Citation14 The activity of PI3K and level of PtdIns(3,4,5)P3 increase in cells exposed to H2O2.Citation14 The PI3K downstream effector, Akt, can also be activated by exogeneous H2O2 in a variety of cell types.Citation15,Citation16
Despite this, however, the mechanism by which the activity of PTEN is regulated remains unclear. Growth factor stimulation induces accumulation of PtdIns(3,4,5)P3 and activation of Akt via a PI3K-dependent pathway.Citation17 In addition, PDGF induces phosphorylation of PDGFR which leads to the activation of NADPH oxidase that catalyzes the production of H2O2. In contrast, PTEN is oxidized and inactivated by H2O2 through the formation of a disulfide bond between the active site Cys124 and a nearby Cys71 residue in NIH 3T3 cells.Citation18,Citation19 In this report, we identified the specific tyrosine residues of PDGF receptor (PDGFR) that are involved in the redox regulation of PTEN in the cells to elucidate the close correlation of activation of PDGFR with the onset of the reversible oxidation of PTEN in the processes of signal transduction via second messengers PtdIns(3,4,5)P3 and H2O2.
Materials and methods
Materials
Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), and calf serum (CS) were purchased from Hyclone (Thermo Scientific, UT, USA). PDGF-BB and rabbit polyclonal antibodies to PTEN were obtained from Upstate Biotechnology. A monoclonal antibody to PTEN was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Rabbit polyclonal antibodies to phospho-Akt (Ser-473) were obtained from Cell Signaling Technology (Cell signaling, MA, USA). Mouse monoclonal antibody to glutathione (GSH) was obtained from ViroGen (Watertown, MA, USA). Dithiothreitol (DTT) and N-ethylmaleimide (NEM) were purchased from Sigma-Aldrich (St Louis, MO, USA). H2O2 (30%) was obtained from Fluka (St Louis, MO, USA).
Cell culture and transfection
HepG2 human hepatocelluar carcinoma cells expressing various PDGF beta receptor (PDGFβR) mutantsCitation20 were provided by Dr Y. S. Bae.Citation9 NIH 3T3 mouse embryonic fibroblast cells and HepG2 cells were grown at 37°C under 5% carbon dioxide in DMEM supplemented with 10% CS or 10% FBS, respectively. NIH 3T3 cells were incubated for 20 hours in DMEM alone before stimulation with 50 ng/ml PDGF. HepG2 cells were incubated for 16 hours in DMEM alone before stimulation with 25 ng/ml PDGF. cDNAs encoding wild-type PTEN, the Cys71/Ser (C71S) mutant, and the Cys124/Ser (C124S) mutant tagged at their NH2 termini with FLAG were subcloned into pIRES plasmids (Clontech, CA, USA), and the resulting vectors were introduced into NIH 3T3 cells by transfection with the use of Fugene HD (Roche, Indianapolis, IN, USA).
Analysis of reduced and oxidized forms of PTEN by immunoblotting
Reduced and oxidized forms of PTEN were identified by non-reducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and subsequent immunoblot analysis (IB) as described previously.Citation19 Briefly, the cells were lysed in 20 mM Tris-HCl (pH 7.5) buffer containing 150 mM NaCl, 5% glycerol, 0.1% NP-40, 1 µM phenylmethylsulfonyl fluoride, and 10 mM NEM. The lysates were boiled and the constituent proteins were resolved by SDS-PAGE under non-reducing conditions, and the separated proteins were transferred onto nitrocellulose membranes, which were then probed with antibodies against PTEN.
Expression, purification, and in vitro assay of recombinant PTEN
Human PTEN cDNA encoding the wild-type PTEN was cloned into the pQE30 vector (Qiagen, Germantown, MD, USA) for expression of the protein with a histidine tag at the NH2 terminus. The histidine-tagged wild-type protein was expressed in Escherichia coli according to a standard procedure, and was purified by nickel nitrilotriacetic acid resin (Qiagen). Purified PTEN was incubated with 7 mM GSH for 60 minutes. Samples were then subjected to IB with either antibodies against PTEN or GSH under non-reducing condition.
Results
Redox regulation of PTEN by H2O2 and GSH
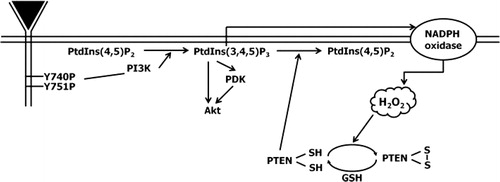
The increased electrophoretic mobility shift of PTEN has been successfully used to demonstrate the amount of the oxidized PTEN formed on exposure to H2O2.Citation19 Appropriately, non-reducing SDS-PAGE and immunoblot analyses were used to examine whether PTEN was oxidized in NIH 3T3 cells when exposed to 0.3 mM H2O2. The level of oxidized PTEN reached a maximum at 10 minutes and then gradually decreased (A). This result was consistent with the H2O2-mediated oxidation of PTEN and subsequent reactivation by cellular reducing compounds.Citation19
Figure 1. Reversible oxidation and reduction of PTEN by H2O2 and GSH. (A) NIH 3T3 cells were incubated with 0.3 mM H2O2 for the indicated times, after which cell lysates were alkylated with NEM and immunoblotted with antibodies against PTEN. (B) Recombinant PTEN was incubated with 7 mM GSH for the indicated times. Samples were then alkylated with NEM and subjected to non-reducing SDS-PAGE followed by immunoblotting with antibodies against PTEN (B, left) and GSH (B, right) to analyze the redox state of PTEN. (C) Cells overexpressing wild-type and PTEN mutants were incubated with 1 mM H2O2 for the indicated times. Immunoprecipitates were prepared from NIH 3T3 cell lysates with polyclonal antibodies against PTEN and subjected to immunoblot analysis by using a monoclonal anti-FLAG-M2 antibody.
Our previous data showed that oxidized PTEN was reduced by GSH in vitro.Citation21 At present, recombinant PTEN was reduced by treatment with 7 mM GSH for 60 minutes (B, left). Glutathionylated PTEN was also detected by immunoblotting using GSH antibody (B, right). To examine the effects of mutation of Cys71 and Cys124 residues on the formation of a disulfide bond and glutathionylation in cells, NIH 3T3 cells were transfected with cDNA encoding wild-type, C71S mutant, and C124S mutant forms of human PTEN tagged with FLAG. The cells were incubated for the indicated times with H2O2, and were then immunoprecipitated with antibodies against PTEN. immunoblotting of the immunoprecipitates with antibody to FLAG M2 revealed that wild-type PTEN formed a disulfide bond and glutathionylated in cells, but the mutants were not (C). These results indicated that intact Cys124 and Cys71 residues of PTEN are required for the formation of a disulfide bond when oxidized by H2O2. The results also suggest that either the Cys124 or Cys71 residue of the oxidized PTEN is intermediately glutathionylated in the process of reduction of the disulfide bond by GSH.
Effect of PDGFβR mutations on PDGF-induced PTEN oxidation
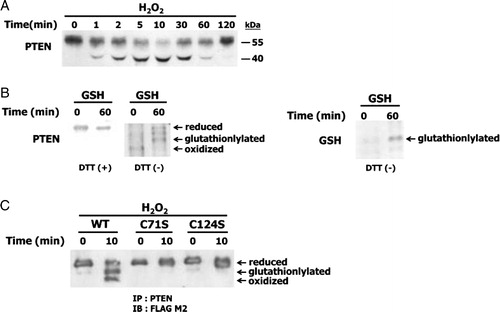
Peptide growth factors such as PDGF and EGF induce the transient production of H2O2 in cells which is required for downstream signaling.Citation2,Citation3 To determine the effect of specific amino acid residues on PDGF-induced PTEN oxidation, various PDGFβR mutants were constructed (B). It has been previously shown that the binding of PDGF to its receptors results in dimerization and autophosphorylation of tyrosine residues, which then initiate downstream signaling, including PI3K, GTPase-activating protein of Ras (GAP), Src homology 2 domain containing protein-tyrosine phosphatase (SHP-2), and phospholipase C-1 (PLC-1).Citation9,Citation22 We examined whether PTEN was oxidized in HepG2 cells expressing wild-type PDGFβR, a kinase-deficient PDGFβR, F740/751, and Y741/751 PDGFβR mutants using an established non-reducing SDS-PAGE mobility shift assay and immunoblot analysis. PDGF failed to induce H2O2 production in HepG2 cells expressing either the kinase-deficient PDGFβR mutant in which Lys634 was replaced by an Arg, or a PDGFβR mutant in which the Tyr740 and Tyr751 residues required for binding PI3K were replaced by Phe (F740/751).Citation9 As expected, PTEN was not oxidized in the kinase-deficient and F740/751 mutants (A). However, PDGF-induced H2O2 production in cells expressing a wild-type PDGFβR or a mutant form designated as Y740/751 where the tyrosine residues, Tyr771 (required for binding of GAP), Tyr1009 (required for SHP-2 binding), and Tyr1021 (required for PLC-1 binding) were all replaced by Phe, led to PTEN oxidation (A).
Figure 2. Effect of PDGF beta receptor mutations on PDGF-induced oxidation of PTEN. (A) HepG2 cells were transfected with the vectors encoding wild-type PDGFβR, a kinase-deficient PDGFβR (kinase (−)), a PDGFβR mutant in which the Tyr740 and Tyr751 residues were replaced with Phe (F740/751 mutant), and a PDGFβR mutant in which the Tyr771, Tyr1009, and Tyr1021 residues were replaced with Phe (Y740/751). All cells were then stimulated with 25 ng/ml PDGF for the indicated times. The oxidation of PTEN in the cellular extracts was analyzed as described in . (B) Schematic representation of wild-type or PDGFβ mutant receptors. The tyrosine, phenylalanine, lysine, and arginine residues are represented by Y, F, K, and R, respectively, at the indicated amino acid positions. PI3 K, GAP, SHP-2, and PLC are proteins that are associated with the tyrosine residues of PDGFR.
Effect of mutations in the PDGFβR on PDGF-induced Akt phosphorylation
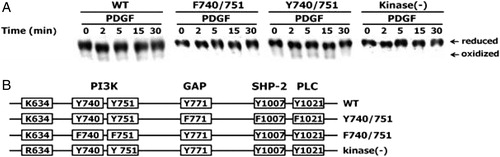
H2O2 generated in response to EGF reversibly inactivates protein-tyrosine phosphatase 1B in cells.Citation23 The PtdIns(3,4,5)P3 levels in NIH 3T3 cells stimulated with PDGF increase when the H2O2 concentration is increased by the expression of Nox1 which is a homolog of the gp91phox catalytic subunit of phagocytic NADPH oxidase.Citation24 These data suggest that the generation of H2O2 in response to growth factors modulates the PI3K/Akt pathway by increasing the level of PtdIns(3,4,5)P3. To confirm this suggestion, we determined the level of Akt phosphorylation induced by stimulation with H2O2 or PDGF (A). NIH3T3 cells were incubated with 0.5 mM H2O2 or PDGF (50 ng/ml) for the indicated times and were then subjected to IB with antibody to phospho-Akt. After stimulation, Akt phosphorylation reached a maximum level at 10 minutes.
Figure 3. Effect of PDGF beta receptor mutations on PDGF-induced Akt phosphorylation. (A) NIH 3T3 cells were treated with 50 ng/ml PDGF or 0.5 mM H2O2 for the indicated times. (B) HepG2 cells expressing wild-type or various PDGFβR mutants were incubated with 25 ng/ml PDGF for the indicated times. Cellular protein extracts were subjected to SDS-PAGE followed by IB with antibodies against phospho-Akt and Akt.
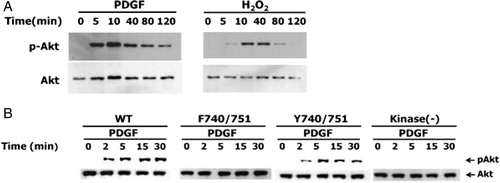
We investigated the PDGF-induced Akt phosphorylation in HpeG2 cells expressing wild-type, kinase-deficient, F740/751, and Y740/751 PDGFβR mutants. PDGF stimulation of HepG2 cells expressing wild-type PDGFβR or the Y740/751 PDGFβR mutants resulted in the phosphorylation of Akt. In contrast, stimulation of HepG2 cells expressing kinase-deficient or F740/751 mutants did not induce the apparent phosphorylation of Akt (B). These results indicate that the kinase activity and PI3K binding of PDGFβR are associated with Akt phosphorylation in response to PDGF.
Discussion
Binding of certain growth factors to their receptors activates PI3K, which then phosphorylates PtdIns(4,5)P2 to produce PtdIns(3,4,5)P3. PtdIns(3,4,5)P3 is in turn dephosphorylated to PtdIns(4,5)P2 by PTEN or to PtdIns(3,4)P2 by 5-phosphatases such as SHIP.Citation6 Thus, intracellular levels of PtdIns(3,4,5)P3 are determined by the opposing activities of PI3K and PTEN. Elevated intracellular levels of H2O2 achieved by expression of a Prx II dominant-negative mutant or Nox1 also increase the level of intracellular PtdIns(3,4,5)P3.Citation18 This result suggests that increases in the level of PtdIns(3,4,5)P3 are due to enhanced PI3K activity and/or inactivation of PTEN by H2O2.
Exogenous H2O2 activates receptor-type protein tyrosine kinases such as PDGFR and EGF receptor, which subsequently recruits and activates PI3K, resulting in accumulation of PtdIns(3,4,5)P3 and activation of downstream Akt.Citation14 Therefore, the increase in intracellular H2O2 is expected to enhance PI3K activity. The PtdIns(3,4,5)P3 product of PI3K is required for the generation of H2O2 in nonphagocytic cells, and the small GTP-binding protein Rac1 regulates signaling between PtdIns(3,4,5)P3 and NADPH oxidase.Citation9 In the case of EGFR, it was shown that generation of H2O2 in A431 cells stimulated with EGF requires the kinase activity of EGFR.Citation3 These observations suggest that a positive feedback loop in which PI3K acting as both an upstream regulator and a downstream effector in signaling via the growth factor receptor, can be modulated by the redox regulation of PTEN.
H2O2 may positively regulate intracellular levels of PtdIns(3,4,5)P3 via oxidation of critical cysteine residues in PTEN, resulting in decreased activity of phospholipid phosphatase. PtdIns(3,4,5)P3 levels are expected to be reduced by PTEN. Our data showing the dependence of the PtdIns(3,4,5)P3 level on intracellular H2O2 concentration suggest that phospholipid phosphatases are regulated by their redox properties. The redox-active cysteine in the active site of phospholipid phosphatases provides a means by which these enzymes can be regulated in vivo by H2O2 generated in response to agonists.
PTEN has a sequence motif in its active site, C(X)3R. We previously showed that PTEN is reversibly oxidized and inactivated in cells by exogeneous H2O2 through the formation of a disulfide bond between Cys71 and Cys124 residues in the active site.Citation19 At present, PTEN is reversibly oxidized and inactivated by H2O2 produced in the cells by PDGFR stimulation. Akt phosphorylation and PTEN oxidation observed in PDGF-stimulated HepG2 cells were lower than that observed in cells treated with exogeneous H2O2. It is likely that H2O2 is produced and accumulated only locally at sites near the activated PDGFRs as a result of rapid elimination by various intracellular antioxidants such as peroxiredoxns and GSH. Therefore, the concentration of H2O2 is sufficiently high enough to induce and maintain PTEN oxidation only at these sites. GSH is involved in the reduction of disulfide bonds during oxidative stress and the events of PDGF signal pathway, which may influence the activity of PTEN.Citation25,Citation26 In addition, PTEN may be rapidly and transiently released from the plasma membrane sites generating H2O2 in response to PDGFR stimulation, as previously observed in Dictyostelium cells stimulated with cAMP.Citation27 Thus, only a subset of PTEN molecules is likely to undergo oxidation in stimulated cells. Despite a small extent of oxidation, H2O2-dependent oxidation of PTEN might be necessary for the local accumulation of PtdIns(3,4,5)P3 and propagation of signaling cascades. This notion is consistent with the previous observations that H2O2 generation and accumulation are required for the downstream actions of PDGF receptor activation, such as chemotaxis, DNA synthesis, and cell cycle progression that are mediated by PI3K.Citation1
A schematic of the proposed mechanism by which the redox state of PTEN is regulated through PDGF-induced H2O2 is shown in . Our results suggest that H2O2 generated by NADPH oxidase following activation of PDGFR acts as an intracellular messenger and leads to the accumulation of PtdIns(3,4,5)P by inactivating PTEN. The oxidized PTEN is in turn reduced to activated form by GSH. Receptor-mediated H2O2 production in cells might serve additional functions by increasing the level of PtdIns(3,4,5)P3. For example, PtdIns(3,4,5)P3 activates small GTPases such as Rac and Rho, which increase PI3K activity, thereby creating a positive feedback loop.Citation28 In addition, activated Rac is essential for the activation of NADPH oxidase, which is responsible for the production of H2O2.Citation9 Thus, the results suggest that a reversible oxidation and reduction of PTEN by H2O2 and by GSH creates another positive feedback loop for the propagation of intracellular signaling via PtdIns(3,4,5)P3 and H2O2.
Acknowledgments
We thank Y S Bae for the generous donation of HepG2 cells expressing PDGF receptor mutants. The present study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011-0003869), Republic of Korea. S-R Lee was partly supported by the Research Fund of Chonnam National University in 2007. IK, YK, and S-JH were supported by the BK21 Research Fellowship from the Ministry of Education, Science and Technology, Republic of Korea. I.K. and S.-J.H. have contributed equally to this work.
References
- Finkel T. Oxygen radicals and signaling. Curr Opin Cell Biol 1998;10:248–53.
- Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 1995;27:296–9.
- Bae YS, Kang SW, Seo MS, Baines IC, Tekle E, Chock PB, et al. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J Biol Chem 1997;272:217–21.
- Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu S, et al. Cell transformation by the superoxide-generating oxidase Mox1. Nature 1999;401:79–82.
- Rhee SG, Kang SW, Netto LE, Seo MS, Stadtman ER. A family of novel peroxidases, peroxiredoxins. Biofactors 1999;10:207–9.
- Fruman DA, Meyers RE, Cantley LC. Phosphoinositide kinases. Annu Rev Biochem 1998;67:481–507.
- Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem 1998;273:13375–8.
- Franke TF, Kaplan DR, Cantley LC. PI3K: downstream AKT blocks apoptosis. Cell 1997;88:435–7.
- Bae YS, Sung JY, Kim OS, Kim YJ, Hur KC, Kazlauska A, et al. Platelet-derived growth factor-induced H2O2 production requires the activation of phosphatidylinositol 3-kinase. J Biol Chem 2000;275:10527–31.
- Hirsch E, Katanaev VL, Garlanda C, Azzolino O, Pirola L, Silengo L, et al. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science 2000;287:1049–53.
- Auger KR, Serunian LA, Soltoff SP, Libby P, Cantley LC. PDGF-dependent tyrosine phosphorylation stimulates production of novel polyphosphoinositides in intact cells. Cell 1989;57:167–75.
- Rodriguez-Viciana P, Warne PH, Dhand R, Vanhaesebroeck B, Gout I, Fry MJ, et al. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature 1994;370:527–32.
- Stephens L, Smrcka A, Cooke FT, Jackson TR, Sternweis PC, Hawkins PT. A novel phosphoinositide 3 kinase activity in myeloid-derived cells is activated by G protein beta gamma subunits. Cell 1994;77:83–93.
- Wang X, McCullough KD, Franke TF, Holbrook NJ. Epidermal growth factor receptor dependent Akt activation by oxidative stress enhances cell survival. J Biol Chem 2000;275:14624–31.
- Konishi H, Matsuzaki H, Tanaka M, Takimura Y, Kuroda S, Ono Y, et al. Activation of protein kinase B (Akt/RAC-protein kinase) by cellular stress and its association with heat shock protein Hsp27. FEBS Lett 1997;410:493–8.
- Shaw M, Cohen P, Alessi DR. The activation of protein kinase B by H2O2 or heat shock is mediated by phosphoinositide 3-kinase and not by mitogen-activated protein kinase-activated protein kinase-2. Biochem J 1998;336:241–6.
- Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci 1999;96:4240–5.
- Kwon J, Lee SR, Yang KS, Ahn Y, Kim YJ, Stadtman ER, et al. Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc Natl Acad Sci 2004;101:16419–24.
- Lee SR, Yang KS, Kwon J, Lee C, Jeong W, Rhee SG. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem 2002;277:20336–42.
- Valius M, Kazlauskas A. Phospholipase C-gamma 1 and phosphatidylinositol 3 kinase are the downstream mediators of the PDGF receptor's mitogenic signal. Cell 1993;73:321–34.
- Kim Y, Song YB, Kim TY, Kim Y, Han SJ, Ahn Y, et al. Redox regulation of the tumor suppressor PTEN by glutathione. FEBS Lett 2010;584:3550–6.
- Kang SW. Two axes in platelet-derived growth factor signaling: tyrosine phosphorylation and reactive oxygen species. Cell Mol Life Sci 2007;64:533–41.
- Lee SR, Kwon KS, Kim SR, Ghee SG. Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. J Biol Chem 1998;273:15366–72.
- Arnold RS, Shi J, Murad E, Whalen AM, Sun CQ, Polavarapu R, et al. Hydrogen peroxide mediates the cell growth and transformation caused by the mitogenic oxidase Nox1. Proc Natl Acad Sci 2001;98:5550–5.
- Rigacci S, Iantomasi T, Marraccini P, Berti A, Vincenzini MT, Rampono G. Evidence for glutathione involvement in platelet-derived growth-factor-mediated signal transduction. Biochem J 1997;324:791–6.
- Iantomasi T, Favilli F, Catarzi S, Vincenzini MT. GSH role on platelet-derived growth factor receptor tyrosine phosphorylation induced by H2O2. Biochem Bioph Res Commun 2001;280:1279–85.
- Funamoto S, Meili R, Lee S, Parry L, Firtel RA. Spatial and temporal regulation of 3-phosphoinositides by PI 3-kinase and PTEN mediates chemotaxis. Cell 2002;109:611–23.
- Zheng Y, Bagrodia S, Cerione RA. Activation of phosphoinositide 3-kinase activity by Cdc42Hs binding to p85. J Biol Chem 1994;269:18727–30.