Abstract
Introduction
Leptin has lipid peroxidation properties in healthy individuals. Here we aimed to study the correlation between serum-oxidized low-density lipoprotein (ox-LDL) and leptin levels in patients with type 2 diabetes. We also studied the effect of metformin therapy on the correlation between serum ox-LDL and leptin levels in patients with newly diagnosed diabetes.
Methods
We performed a cross-sectional study on two groups of patients with type 2 diabetes stratified according to (1) patients with newly diagnosed diabetes and (2) patients with long-standing diabetes plus healthy controls. Patients with newly diagnosed diabetes were followed for 3 months after the initiation of metformin therapy.
Results
Patients with type 2 diabetes had a higher serum ox-LDL, ox-LDL/LDL ratio, waist circumference, fasting blood sugars (FBSs), hemoglobin A1C (HbA1C), triglyceride, homeostatic model assessment of insulin resistance (HOMA-IR) and a lower serum leptin levels than controls. Serum ox-LDL, ox-LDL/LDL ratio (0.08 (0.08–0.12) vs. 0.06 (0.05–0.08), P < 0.001) and HOMA-IR (3.26 ± 0.23 vs. 2.93 ± 0.32; P < 0.01) were decreased when serum leptin levels (15.9 ± 1.6 vs. 21.4 ± 2.5, P < 0.01) were increased after 3 months of metformin therapy. This remained significant after multiple adjustments for age, body mass index, FBS, HbA1c, and HOMA-IR. Leptin was significantly correlated with ox-LDL/LDL ratio in controls (r = 0.78, P < 0.01), and in patients with newly diagnosed diabetes (r = 0.4, P < 0.05), after metformin therapy. There were not any correlation between leptin and ox-LDL/LDL ratio in patients with long-standing diabetes and patients with newly diagnosed diabetes before treatment.
Discussion
Metformin restores the positive correlation between serum ox-LDL and leptin levels in patients with type 2 diabetes.
Introduction
Oxidative stress is a disturbance in the pro-oxidant, anti-oxidant balance in favor of the former, which leads to the potential damage.Citation1 Indicators of oxidative stress include measures of damaged DNA, protein oxidation, and lipid per-oxidation products.Citation1 Oxidized low-density lipoprotein (LDL) is a particle derived from the circulating LDL that may have peroxide and other degeneration products.Citation2 The concept that oxidative stress and oxidation of LDL might play an important role in the atherosclerosis and diabetes complications was first described over 15 years ago.Citation3 This idea formed from a simple observation that in vitro incubation of macrophages with oxidized LDL (ox-LDL), and not with native LDL led to cholesterol ester accumulation.Citation4 Since then many studies established the role of ox-LDL in the pathogenesis of diabetes complications including albuminuria and coronary heart disease.Citation5,Citation6
Leptin, an adipocyte-derived cytokine, primarily was known to involve in the regulation of food intake and energy expenditure.Citation7 Leptin modulate the proliferation of monocyte/macrophages and the production of oxidative stress.Citation8 Wistar rats injected with leptin had a higher plasma lipid hydroperoxide, malondialdehyde, isoprostane, and protein carbonyl content than non-treated controls.Citation9 They also suggested that leptin has a proatherogenic effect.Citation9 Studies have shown a positive correlation between serum ox-LDL and leptin levels in psoriasis and healthy individuals.Citation10,Citation11 Porreca et al. showed a significant correlation between circulating ox-LDL and leptin levels in postmenopausal women without coronary heart disease risk factors. They suggested that leptin had a stronger effect on oxidative modification of LDL than of C reactive protein (CRP). This was because the association of ox-LDL with CRP disappeared in a multivariate model, and the change in ox-LDL concentrations, from weight loss, was predicted only by changes in leptin.Citation12
Hence, we hypothesized that in chronic inflammation, like type 2 diabetes, there would be a positive correlation between serum ox-LDL and leptin levels. Studies have shown that metformin therapy improves the antioxidant status, enzymatic activity, and inflammatory parameters in type 2 diabetic patients.Citation13,Citation14 The correlation between serum ox-LDL and leptin would be altered after metformin therapy. The purposes of the present study were
| 1. | Comparing the correlation between serum leptin and ox-LDL levels in patients with newly diagnosed diabetes, patients with long-standing type 2 diabetes, and healthy controls. | ||||
| 2. | Studying the effect of 3 months metformin therapy on the ox-LDL and leptin correlation in the group of newly diagnosed diabetes. | ||||
Methods
We performed a cross-sectional study on the established groups of patients with type 2 diabetes defined as (1) patients with long-standing diabetes for more than 5 years; and (2) patients with newly diagnosed diabetes within recent 6 months who were not on any glucose lowering treatment other than by dietary means alone; and (3) healthy controls. Patient recruitment was from the diabetes clinic of Vali-Asr Hospital, affiliated with Tehran University of Medical Sciences. Controls were healthy volunteers from the patients' concomitants or hospital staffs. Healthy controls were selected from those without any known disease including type 2 diabetes, hyperlipidemia, ischemic heart disease, and malignancy. Patients and controls were matched according to age, sex, and body mass index (BMI).
On the basis of the results of an exploratory analysis, we designed a follow-up study of patients with newly diagnosed diabetes before and after 3 months of metformin treatment. The patients commenced on treatment immediately following diagnosis. All these patients had poorer glycemic control, sufficient to merit an oral hypoglycemic agent. Metformin was the first-line choice of glucose lowering therapy in these patients. Treatment was started with metformin monotherapy and the patients had a 3-month period of therapy with three phases of initiation, titration, and maintenance. The average dose of metformin was 1000–1500 mg per day. Diabetes was diagnosed according to the criteria of the American Diabetes Association.Citation15 Groups of patients and controls were matched for age, sex, and BMI. Exclusion criteria were smoking, pregnancy, creatinine >1.5 mg/dl or GFR < 70 cc/minute, glomerulonephritis, congestive heart failure, use of antioxidant, and hospital admission in recent 6 months. None of the participants had overt diabetes complications. None of the participants were on hormone replacement therapy. Demographic and anthropometric data including age, sex, duration of diabetes, height, weight in light clothing, and blood pressure in sitting position were recorded. Blood pressure was re-measured twice after 5 minutes and averaged. The BMI (kg/m2) was calculated according to the Quetelet formula. The research was carried out according to the principles of the Declaration of Helsinki. All the patients received and signed written informed consent. The local ethics review committee of Tehran University of Medical Science approved the study protocol.
Blood samples
Blood samples were collected after 12 hours of fasting, centrifuged, and kept at −70°C until analysis. Serum creatinine, fasting blood sugar (FBS), total cholesterol, triglycerides (TGs), high-density lipoprotein-cholesterol (HDL-C), LDL-cholesterol (LDL-C), and HbA1c were measured for all participants. Glucose measurements (intra-assay coefficient of variants (CV) 2.1%, inter-assay CV 2.6%) were carried out using the glucose oxidase method. Ceratinine was measured using calibrated Jaffe method (Parsazmoon, Karaj, Iran). Cholesterol, HDL-C, LDL-C, and TG were determined using direct enzymatic methods. Serum samples underwent measurement of ox-LDL-beta2GPI using a commercially available sandwich enzyme-linked immunometric assays (ELISA, Cayman, Ann Arbor, MI, USA). The intra- and inter-assay coefficient of variation for the assay ranged between 6.4 and 3.4%. Insulin was measured by radioimmunoassay, using an antibody with no cross reaction against pro-insulin and C-peptide (Immunotech, Prague, Czech Republic). The intra- and inter-assay coefficients of variation were lower than 4.3 and 3.4, respectively. The homeostatic model assessment of insulin resistance (HOMA-IR) was calculated according to; fasting insulin (μU/ml) × fasting plasma glucose (mg/dl)/405, as described by Matthews et al.Citation16 Serum leptin concentration was determined by an enzyme-linked immunosorbent assay (DRG Instruments GmbH, Germany) with an intra-assay coefficient of variation of 5.9–6.9% and an inter-assay coefficient of variation of 8.6–11.5%.
Statistical analysis
The statistical package SPSS 17 for windows (Chicago, IL, USA), was used for analysis. Kolmogorov–Smirnov test was employed to test the normality of the variables in each group. Variables distributed normally are presented as mean and standard error of mean (SEM). Variables with skewed distribution are presented as median (interquantile range). One-way analysis of variance followed by Student's t-test and Kruskal–Wallis followed by Mann–Whitney U test were employed for the comparison of the normally distributed and skewed distributed variables between the studied groups, respectively.
Paired sample t-test and Wilcoxon signed-rank test were employed to compare the normally distributed and skewed distributed variables in patients with newly diagnosed diabetes, before and after treatment. General linear models were employed to study the changes in serum ox-LDL, ox-LDL/LDL ratio, and leptin levels, before and after metformin therapy, controlling for age, BMI, FBS, HbA1C, and HOMA-IR. Pearson correlation was employed to study the correlation between serum ox-LDL, ox-LDL/LDL ratio, leptin, HOMA-IR, and BMI levels in the group of patients with type 2 diabetes and controls.
Results
Primary characteristics of the groups of patients and controls are presented in . The drop-out rate due to side effects following commencement of metformin therapy was (0%) in patients with newly diagnosed diabetes. The frequency of insulin therapy was (9/45; 20%) in patients with long-standing diabetes. Forty-six percent (21/45) of the patients with long-standing diabetes were on statin therapy. Leptin was significantly correlated with ox-LDL/LDL ratio in controls (r = 0.78, P < 0.01), and patients with newly diagnosed diabetes after 3 months of metformin therapy (r = 0.4, P < 0.05), when serum leptin levels were not correlated with ox-LDL/LDL ratio (r = −0.09, r = −0.02) in patients with long-standing diabetes and patients with newly diagnosed diabetes before treatment ().
Figure 1. Presenting correlation coefficient between serum leptin and ox-LDL/LDL ratio in A: controls, B: patients with long-standing diabetes, C: patients with newly diagnosed diabetes before treatment, and D: patients with newly diagnosed diabetes after treatment.
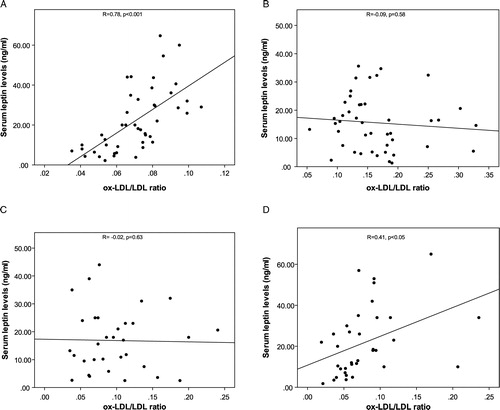
Table 1. Comparing demographic and biochemical variables among patients with long-standing diabetes, patients with newly diagnosed diabetes and healthy controls
Patients with type 2 diabetes had a higher serum ox-LDL, ox-LDL/LDL ratio, waist circumference, FBS, HbA1C, TG, and HOMA-IR levels than controls (). Likewise, ox-LDL, ox-LDL/LDL ratio, HbA1C, and HOMA-IR were higher in patients with long-standing diabetes than newly diagnosed patients (). Participants in the control group had a higher serum leptin and HDL-C levels compared to patients with type 2 diabetes ().
There was a significant decrease in serum ox-LDL (10(9.7–14.1) vs. 6(5.6–7.8); P < 0.001), ox-LDL/LDL (0.082 (0.080–0.12) vs. 0.06 (0.05–0.08); P < 0.001) (), HOMA-IR (3.26 ± 0.23 vs. 2.93 ± 0.32; P < 0.001), cholesterol (206.77 ± 6.161 vs. 182.97 ± 5.57, P < 0.01), and LDL-C (123.96 ± 6.03 vs. 102.171 ± 4.19; P < 0.01) in patients with newly diagnosed diabetes after treatment (). Serum leptin levels increased after metformin therapy in patients with newly diagnosed diabetes (15.9 ± 1.6 vs. 21.4 ± 2.5; P < 0.01) (). The observed change in serum ox-LDL (11.3 (9.6–13.4) vs. 6.6 (6.3–7.5)), P < 0.001), ox-LDL/LDL ratio (0.10(0.079–0.12) vs. 0.064 (0.053–0.074), P < 0.001), and leptin levels (15.2 ± 1.4 vs. 20.7 ± 2.7, P < 0.05) remained significant after multiple adjustments for age, BMI, FBS, HbA1c, and HOMA-IR using general linear models.
Figure 2. Serum A: leptin and B: ox-LDL/LDL ratio in patients with newly diagnosed diabetes, before and after 3 months of metformin therapy. The bars represent mean and the handles represent SEM.
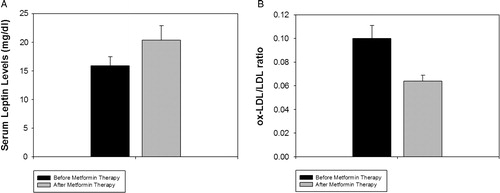
Table 2. Characteristics of patients with newly diagnosed diabetes before and after 3 months of metformin therapy
Discussion
Our findings clearly demonstrated that there were no significant correlation between ox-LDL, ox-LDL/LDL ratio, and leptin levels in patients with diabetes, when serum leptin and ox-LDL/LDL ratio were significantly correlated in controls. However, 3 months metformin therapy reestablished the positive correlation between serum leptin and ox-LDL levels in patients with newly diagnosed diabetes. Patients with type 2 diabetes had a higher serum ox-LDL, ox-LDL/LDL ratio, and a lower serum leptin level than controls. Serum ox-LDL, ox-LDL/LDL ratio, and HOMA-IR were reduced when serum leptin levels were increased after 3 months of metformin therapy. The changes in serum ox-LDL, ox-LDL/LDL ratio, and leptin levels after metformin therapy were independent of the improvement in HOMA-IR and glycemic index.
Traditionally, leptin has been regarded as a link between fat mass and food intake.Citation17 Later on, studies showed that in humans, there are many instances where leptin dissociate from the strict role of communicating nutritional status; like decreased by testosterone and increased by estrogen.Citation18 Leptin increases hepatic lipid peroxidation and lipolysis in skeletal muscle and adipocytes.Citation19,Citation20 Leptin increases ox-LDL phagocytosis in the monocytes of the patients.Citation21 Moreover, it is increased in the situation of chronic inflammation, related to white blood cell count.Citation18 This may be an explanation for the positive correlation between serum leptin and ox-LDL in normal subjects.Citation10,Citation11 However, we did not find any positive correlation between serum leptin and ox-LDL levels in patients with long-standing diabetes and patients with newly diagnosed diabetes before treatment. Consistent with our findings, Stringer et al.Citation22 did not report any correlation between serum leptin and ox-LDL levels in patients with type 2 diabetes. Kassi et al.Citation23 did not report any positive correlation between serum leptin and ox-LDL levels in postmenopausal women with impaired glucose tolerance test. Likewise Demirel et al.Citation24 did not find any correlation between leptin and ox-LDL, when leptin was significantly correlated with very LDL, lipoprotein A, and TG in patients with poly-cystic ovary (PCO) syndrome.
Why the correlation between serum leptin and ox-LDL levels fades out in diabetes. In 1998, Lord et al.Citation25 first reported the role of leptin as a modulator of T lymphocytes. Serum leptin levels were significantly elevated in patients with multiple myeloma and chronic lymphatic leukemia.Citation26 Earlier studies on leptin-deficient mouse have shown marked reduced number of lymphocytes with impaired humoral response.Citation27,Citation28 Lipoatrophy in HIV-infected patients is assumed to be a result of reduced leptin in these patients.Citation29 Paz-Filho et al.Citation30 showed that immune defects are not an obligatory feature of congenital leptin deficiency and even in the absence of significant immune defects, leptin replacement therapy enhanced T-cell responsiveness. Obese leptin-deficient ob/ob mice exhibit a low-grade chronic inflammation together with a low muscle mass.Citation31 Sainz et al.Citation32 showed that leptin administration down-regulates the increased expression levels of genes related to oxidative stress and inflammation in the skeletal muscle of ob/ob mice. Interestingly, it is shown that leptin modulates innate and adaptive immune cell recruitment after cigarette smoke exposure in ob/ob mice.Citation33
So leptin has a dimorphic action on the markers of oxidative stress Citation19–Citation21,Citation32,Citation33 as both leptin deficiency and excessive leptin administration induce oxidative stress. It could be hypothesized that the positive correlation between serum leptin and oxidative stress is observed only in the stable situations, when they both induce the other one, and hence we observe a linear relationship. In type 2 diabetes the inflammatory markers are produced via different pathways and biochemical cascades. We also found a lower serum leptin levels in patients with type 2 diabetes, so the positive linear relationship, which is normally observed between leptin and ox-LDL, would be confounded by other inflammatory cytokines. Interestingly, 3 months metformin therapy could rebuild the correlation between serum leptin and ox-LDL levels in patients with newly diagnosed diabetes.
The finding of a lower serum leptin level in diabetic patients confirms those from previous studies.Citation34–Citation36 Consistent with our findings, Sivitz et al.Citation37 showed that plasma leptin levels are reduced in untreated type 2 diabetes probably as a consequence of reduced insulin secretion. A low serum leptin levels is a predictor of early mortality in patients on chronic hemodialysis.Citation38 Serum leptin levels are negatively correlated with serum C-reactive protein levels, in patients with chronic kidney disease.Citation39 It is also suggested that serum leptin levels increases after glycemic improvement.Citation40,Citation41 However, our findings are in contrast with some studies which have reported a higher serum leptin levels in patients with type 2 diabetes.Citation42,Citation43 Morin-Papune et al.Citation44 showed that metformin therapy reduces serum leptin levels in women with PCO after 2 months of therapy. However, serum leptin levels were increased after 4–6 months of metformin therapy in their study. We matched the patients and controls according to age, sex, and BMI, which is not usually performed in other studies. Furthermore serum leptin levels were significantly increased after 3 months of metformin therapy. It seems that the role of diabetes on serum leptin levels is not completely understood.
Consistent with our findings, studies have shown the role of metformin in reducing oxidative and nitrosative stress in patients with type 2 diabetes.Citation14,Citation45 Hyperglycemia is the leading cause of oxidative stress, inflammation, and vascular complications in patients with type 2 diabetes.Citation46 Hyperglycemia cause insulin resistance in skeletal muscle and damage the endothelial cells. In vitro and animal studies have shown a positive correlation between serum ox-LDL and insulin levels.Citation47,Citation48 The production of circulating ox-LDL is accelerated by insulin resistance in healthy individuals and diabetic patients.Citation14,Citation49 We showed that 3 months metformin therapy rebuild the positive correlation between serum leptin and ox-LDL levels.
Studies have shown the positive role of metformin on the defense mechanisms against reactive oxygen species and other reactive compounds.Citation50,Citation51 Matsumoto et al.Citation52 suggested that hypoglycemic action of metformin does not play an important role on the vascular protection in rats with type 2 diabetes, when its antioxidative effect plays a significant role. Moreover, metformin provides protection against vascular endothelial dysfunction induced by LDL, probably with protection against oxidative stress in rats.Citation53 Metformin is shown to improve oxidative stress and preserve antioxidant function by increasing superoxide dismutase activity in patients with newly diagnosed diabetes.Citation54 We concluded that metformin improves serum leptin and ox-LDL levels, as two inflammatory markers, through its antioxidative properties.Citation55 Based on the currently available evidence the effects of metformin may also be related to the decreasing glucose levels in the patients. It has been shown that thiazolidinediones have beneficial effects on lipid oxidation and peroxidation properties,Citation56 which is comparable to those of metformin. However, our findings, using general linear models demonstrated that the beneficial role of metformin in reducing serum ox-LDL levels is independent from its glucose-lowering effects.
The effects of metformin could also be related to the fact that it activates AMP-activated protein kinase (AMPK).Citation57 AMPK is an important inhibitor of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB).Citation58 NF-κB is a protein complex that controls the transcription of DNA. NF-κB is found in almost all animal cell types and is involved in the cellular responses to stimuli such as stress, cytokines, free radicals, and ox-LDL.Citation59 All these events are inhibited by the activation of AMPK, which also enhances insulin action in muscle.Citation57 This may explain the effect of metformin in reducing serum ox-LDL levels.
At the end, the effect of metformin can in some way be related to the immune system.Citation60 Oxidation of LDL is carried out by macrophages and monocytes.Citation61 It is shown that the production of ROS by circulating monocytes in the hyperlipidemic subjects contributes to the systemic oxidative stress.Citation61 On the other hand it is shown that metformin attenuate the inflammatory responses, at least in part, by suppressing the production of TNF-alpha in human monocytes.Citation60 The beneficial role of metformin in reducing ox-LDL, increasing leptin levels, and restoring serum leptin and ox-LDL correlation may be someway related to its effect on the immune system.
We believe that different outcomes in different studies may be the result of differences in the inclusion criteria and differences in baseline characteristics of the patients. Future prospective studies which measure serum leptin levels with other inflammatory markers including high-sensitivity CRP, interleukin 6, and TNF-alpha level may elucidate the role of leptin as a negative or positive acute phase reactant in patients with type 2 diabetes. The principal limitation of the present study is its short-term follow-up duration. Furthermore we did not have placebo group, as it is considered unethical not to commence patients on treatment immediately following diabetes diagnosis. However, we took advantage of a relatively large sample size and close similarity between the groups in most of the potentially confounding clinical and laboratory variables. In conclusion, we showed a higher serum ox-LDL and a lower serum leptin level in patients with type 2 diabetes. Metformin modulated serum ox-LDL and leptin levels, independent from its glucose-lowering effect.
Acknowledgments
This study is not financially supported by any institution.
Conflicts of interest: The authors have not declared any conflicts of interest.
References
- Sies H. Oxidative stress: from basic research to clinical application. Am J Med 1991;91:31S–8S.
- Parthasarathy S, Raghavamenon A, Garelnabi MO, Santanam N. Oxidized low-density lipoprotein. Methods Mol Biol 2010;610:403–17.
- Schwartz CJ, Valente AJ, Sprague EA, Kelley JL, Cayatte AJ, Rozek MM. Pathogenesis of the atherosclerotic lesion. Implications for diabetes mellitus. Diabetes Care 1992;15:1156–67.
- Quinn MT, Parthasarathy S, Fong LG, Steinberg D. Oxidatively modified low density lipoproteins: a potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proc Natl Acad Sci USA 1987;84:2995–8.
- Holvoet P, Lee DH, Steffes M, Gross M, Jacobs DR Association between circulating oxidized low-density lipoprotein and incidence of the metabolic syndrome. Jama 2008;299:2287–93.
- Nakhjavani M, Esteghamati A, Asgarani F, Khalilzadeh O, Nikzamir A, Safari R. Association of oxidized low-density lipoprotein and transforming growth factor-beta in type 2 diabetic patients: a cross-sectional study. Transl Res 2009;153:86–90. Epub 2008 Dec 9.
- Dardeno TA, Chou SH, Moon HS, Chamberland JP, Fiorenza CG, Mantzoros CS. Leptin in human physiology and therapeutics. Front Neuroendocrinol 2010;31:377–93. Epub 2010 Jun 17.
- Lam QL, Lu L. Role of leptin in immunity. Cell Mol Immunol 2007;4:1–13.
- Beltowski J, Wojcicka G, Jamroz A. Leptin decreases plasma paraoxonase 1 (PON1) activity and induces oxidative stress: the possible novel mechanism for proatherogenic effect of chronic hyperleptinemia. Atherosclerosis 2003;170:21–9.
- Kaur S, Zilmer K, Leping V, Zilmer M. The levels of adiponectin and leptin and their relation to other markers of cardiovascular risk in patients with psoriasis. J Eur Acad Dermatol Venereol 2011;1111:1468–3083.
- Konstantinidis D, Paletas K, Koliakos G, Kaloyianni M. Signaling components involved in leptin-induced amplification of the atherosclerosis-related properties of human monocytes. J Vasc Res 2009;46:199–208. Epub 2008 Oct 9.
- Porreca E, Di Febbo C, Moretta V, Angelini A, Guglielmi MD, Di Nisio M, et al. Circulating leptin is associated with oxidized LDL in postmenopausal women. Atherosclerosis 2004;175:139–43.
- Njajou OT, Kanaya AM, Holvoet P, Connelly S, Strotmeyer ES, Harris TB, et al. Association between oxidized LDL, obesity and type 2 diabetes in a population-based cohort, the Health, Aging and Body Composition Study. Diabetes Metab Res Rev 2009;25:733–9.
- Chakraborty A, Chowdhury S, Bhattacharyya M. Effect of metformin on oxidative stress, nitrosative stress and inflammatory biomarkers in type 2 diabetes patients. Diabetes Res Clin Pract 2011;93:56–62.
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2009;32 (Suppl 1):S62–7.
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9.
- Stofkova A. Leptin and adiponectin: from energy and metabolic dysbalance to inflammation and autoimmunity. Endocr Regul 2009;43:157–68.
- Fernandez-Riejos P, Najib S, Santos-Alvarez J, Martin-Romero C, Perez-Perez A, Gonzalez-Yanes C, et al. Role of leptin in the activation of immune cells. Mediators Inflamm 2010;23. DOI: 10.1155/2010/568343.
- Cheung CC, Clifton DK, Steiner RA. Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology 1997;138:4489–92.
- Long YC, Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest 2006;116:1776–83.
- Sarigianni M, Bekiari E, Tsapas A, Kaloyianni M, Koliakos G, Paletas K. Effect of leptin and insulin resistance on properties of human monocytes in lean and obese healthy participants. Angiology 2010;61:768–74. Epub 2010 May 12.
- Stringer DM, Sellers EA, Burr LL, Taylor CG. Altered plasma adipokines and markers of oxidative stress suggest increased risk of cardiovascular disease in first Nation youth with obesity or type 2 diabetes mellitus. Pediatr Diabetes 2009;10:269–77. Epub 2008 Oct 19.
- Kassi E, Dalamaga M, Hroussalas G, Kazanis K, Merantzi G, Zachari A, et al. Adipocyte factors, high-sensitive C-reactive protein levels and lipoxidative stress products in overweight postmenopausal women with normal and impaired OGTT. Maturitas 2010;67:72–7. Epub 2010 Jun 8.
- Demirel F, Bideci A, Cinaz P, Camurdan MO, Biberoglu G, Yesilkaya E, et al. Serum leptin, oxidized low density lipoprotein and plasma asymmetric dimethylarginine levels and their relationship with dyslipidaemia in adolescent girls with polycystic ovary syndrome. Clin Endocrinol (Oxf) 2007;67:129–34. Epub 2007 Apr 25.
- Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 1998;394:897–901.
- Pamuk GE, Demir M, Harmandar F, Yesil Y, Turgut B, Vural O. Leptin and resistin levels in serum of patients with hematologic malignancies: correlation with clinical characteristics. Exp Oncol 2006;28:241–4.
- Mancuso P, Gottschalk A, Phare SM, Peters-Golden M, Lukacs NW, Huffnagle GB. Leptin-deficient mice exhibit impaired host defense in gram-negative pneumonia. J Immunol 2002;168:4018–24.
- Gruver AL, Sempowski GD. Cytokines, leptin, and stress-induced thymic atrophy. J Leukoc Biol 2008;84:915–23. Epub 2008 May 21.
- Papaevangelou V, Papassotiriou I, Vounatsou M, Chrousos G, Theodoridou M. Changes in leptin serum levels in HIV-infected children receiving highly active antiretroviral therapy. Scand J Clin Lab Invest 2007;67:291–6.
- Paz-Filho GJ, Delibasi T, Erol HK, Wong ML, Licinio J. Cellular immunity before and after leptin replacement therapy. J Pediatr Endocrinol Metab 2009;22:1069–74.
- Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 1995;269:540–3.
- Sainz N, Rodriguez A, Catalan V, Becerril S, Ramirez B, Gomez-Ambrosi J, et al. Leptin administration downregulates the increased expression levels of genes related to oxidative stress and inflammation in the skeletal muscle of ob/ob mice. Mediators Inflamm 2010;30. DOI: 10.1155/2010/784343.
- Vernooy JH, Bracke KR, Drummen NE, Pauwels NS, Zabeau L, van Suylen RJ, et al. Leptin modulates innate and adaptive immune cell recruitment after cigarette smoke exposure in mice. J Immunol 2010;184:7169–77. Epub 2010 May 19.
- Clement K, Lahlou N, Ruiz J, Hager J, Bougneres P, Basdevant A, et al. Association of poorly controlled diabetes with low serum leptin in morbid obesity. Int J Obes Relat Metab Disord 1997;21:556–61.
- Passaro A, Calzoni F, Zamboni PF, Manservigi D, Alberti L, Dalla Nora E, et al. Role of diabetes in influencing leptin concentration in elderly overweight patients. Eur J Endocrinol 2001;145:173–9.
- Marita AR, Sarkar JA, Rane S. Type 2 diabetes in non-obese Indian subjects is associated with reduced leptin levels: study from Mumbai, Western India. Mol Cell Biochem 2005;275:143–51.
- Sivitz WI, Wayson SM, Bayless ML, Larson LF, Sinkey C, Bar RS, et al. Leptin and body fat in type 2 diabetes and monodrug therapy. J Clin Endocrinol Metab 2003;88:1543–53.
- Scholze A, Rattensperger D, Zidek W, Tepel M. Low serum leptin predicts mortality in patients with chronic kidney disease stage 5. Obesity (Silver Spring) 2007;15:1617–22.
- Don BR, Rosales LM, Levine NW, Mitch W, Kaysen GA. Leptin is a negative acute phase protein in chronic hemodialysis patients. Kidney Int 2001;59:1114–20.
- Ozata M, Gungor D, Turan M, Ozisik G, Bingol N, Ozgurtas T, et al. Improved glycemic control increases fasting plasma acylation-stimulating protein and decreases leptin concentrations in type II diabetic subjects. J Clin Endocrinol Metab 2001;86:3659–64.
- Nar A, Gedik O. The effect of metformin on leptin in obese patients with type 2 diabetes mellitus and nonalcoholic fatty liver disease. Acta Diabetol 2009;46:113–8. Epub 2008 Oct 7.
- Kanaley JA, Fenicchia LM, Miller CS, Ploutz-Synder LL, Weinstock RS, Carhart R, et al. Resting leptin responses to acute and chronic resistance training in type 2 diabetic men and women. Int J Obes Relat Metab Disord 2001;25:1474–80.
- Wasim H, Al-Daghri NM, Chetty R, McTernan PG, Barnett AH, Kumar S. Relationship of serum adiponectin and resistin to glucose intolerance and fat topography in South-Asians. Cardiovasc Diabetol 2006;5:10.
- Morin-Papunen LC, Koivunen RM, Tomas C, Ruokonen A, Martikainen HK. Decreased serum leptin concentrations during metformin therapy in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab 1998;83:2566–8.
- Cusi K, Consoli A, DeFronzo RA. Metabolic effects of metformin on glucose and lactate metabolism in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1996;81:4059–67.
- Wright E, Scism-Bacon JL, Glass LC. Oxidative stress in type 2 diabetes: the role of fasting and postprandial glycaemia. Int J Clin Pract 2006;60:308–14.
- Erguder IB, Karagenc N, Karaca L. Reduced antioxidant potential & sensitivity to oxidation in plasma low density lipoprotein fraction in type 2 diabetes mellitus patients. Indian J Med Res 2006;124:207–10.
- Rifici VA, Schneider SH, Khachadurian AK. Stimulation of low-density lipoprotein oxidation by insulin and insulin like growth factor I. Atherosclerosis 1994;107:99–108.
- Carantoni M, Abbasi F, Warmerdam F, Klebanov M, Wang PW, Chen YD, et al. Relationship between insulin resistance and partially oxidized LDL particles in healthy, nondiabetic volunteers. Arterioscler Thromb Vasc Biol 1998;18:762–7.
- Kanigur-Sultuybek G, Ozdas SB, Curgunlu A, Tezcan V, Onaran I. Does metformin prevent short-term oxidant-induced dna damage? In vitro study on lymphocytes from aged subjects. J Basic Clin Physiol Pharmacol 2007;18:129–40.
- Correia S, Carvalho C, Santos MS, Proenca T, Nunes E, Duarte AI, et al. Metformin protects the brain against the oxidative imbalance promoted by type 2 diabetes. Med Chem 2008;4:358–64.
- Matsumoto T, Noguchi E, Ishida K, Kobayashi T, Yamada N, Kamata K. Metformin normalizes endothelial function by suppressing vasoconstrictor prostanoids in mesenteric arteries from OLETF rats, a model of type 2 diabetes. Am J Physiol Heart Circ Physiol 2008;295:H1165–76. Epub 2008 Jul 18.
- Xie W, Zhang SD, Ou XP, Yang TL. Protective effects of metformin on low-density lipoprotein-induced endothelial dysfunction in rats. Nan Fang Yi Ke Da Xue Xue Bao 2009;29:890–3.
- Formoso G, De Filippis EA, Michetti N, Di Fulvio P, Pandolfi A, Bucciarelli T, et al. Decreased in vivo oxidative stress and decreased platelet activation following metformin treatment in newly diagnosed type 2 diabetic subjects. Diabetes Metab Res Rev 2008;24:231–7.
- Rabbani N, Chittari MV, Bodmer CW, Zehnder D, Ceriello A, Thornalley PJ. Increased glycation and oxidative damage to apolipoprotein B100 of LDL cholesterol in patients with type 2 diabetes and effect of metformin. Diabetes 2010;59:1038–45.
- Iida KT, Kawakami Y, Suzuki M, Shimano H, Toyoshima H, Sone H, et al. Effect of thiazolidinediones and metformin on LDL oxidation and aortic endothelium relaxation in diabetic GK rats. Am J Physiol Endocrinol Metab 2003;284:E1125–30.
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 2001;108:1167–74.
- Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene 2006;25:6680–4.
- Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol 2007;8:49–62.
- Arai M, Uchiba M, Komura H, Mizuochi Y, Harada N, Okajima K. Metformin, an antidiabetic agent, suppresses the production of tumor necrosis factor and tissue factor by inhibiting early growth response factor-1 expression in human monocytes in vitro. J Pharmacol Exp Ther 2010;334:206–13.
- Vasconcelos EM, Degasperi GR, de Oliveira HC, Vercesi AE, de Faria EC, Castilho LN. Reactive oxygen species generation in peripheral blood monocytes and oxidized LDL are increased in hyperlipidemic patients. Clin Biochem 2009;42:1222–7.