Abstract
When mosses are exposed to increased quantities of ultraviolet (UV) radiation, they produce more secondary metabolites. Antarctica moss Sanionia uncinata (Hedw.) Loeske has presented high carotenoid contents in response to an increase in UVB radiation. This moss has been recommended as a potential source of antioxidants. In the present work, the protective and enhancing effects of aqueous (AE) and hydroalcoholic (HE) extracts of S. uncinata on the cleavage of supercoiled DNA were evaluated through topological modifications, quantified by densitometry after agarose gel electrophoresis. Total phenolic contents reached 5.89 mg/g. Our data demonstrated that the extract does not induce DNA cleavage. Furthermore, both extracts showed antioxidant activity that protected the DNA against cleavage induced by (i) O2•−, 89% (AE) and 94% (HE) (P < 0.05), and (ii) .OH, 17% (AE) and 18% (HE). However, the extracts intensified cleavage induced by Fenton-like reactions: (i) Cu2+/H2O2, 94% (AE) and 100% (HE) (P < 0.05), and (ii) SnCl2, 62% (AE) and 56% (HE). DNA damages seem to follow different ways: (i) in the presence of Fenton-like reactions could be via reactive oxygen species generation and (ii) with HE/Cu2+ could have also been triggered by other mechanisms.
Introduction
Research on Antarctic mosses has provided an interesting perspective on the influence of the increase in ultraviolet (UV) B levels (280–315 nm) caused in recent decades by ozone depletion.Citation1 When they are exposed to greater solar UV radiation, mosses produce more of certain secondary metabolites, mainly flavonoidsCitation2 and carotenoids,Citation3 which can be understood as antioxidants that protect them against UV radiation.Citation4
Antarctica moss Sanionia uncinata (Hedw.) Loeske has presented high contents of the carotenoids violaxanthin, and nexanthin in the presence of higher levels of natural UVB radiation.Citation5 Moreover, no significant increase in cyclobutane pyrimidine dimers has been detected in the S. uncinata genome exposed to natural UVB radiation in Antarctica. This tolerance suggests its acclimatization well to environmental stresses via DNA repair or an increase in the synthesis of UV chromophores.Citation6
Correlations between phenolic content and responses to in vitro antioxidant activity have been found using a hydroalcoholic extract of the moss collected from King George Island, Antarctica. Based on this, it has been suggested that S. uncinata could be an important natural source of antioxidants for applications in medicine and cosmetics.Citation7
Antioxidants protect plant cells from damage induced by free radicals and reactive oxygen species (ROS). ROS accumulation causes damage to the DNA, proteins, lipids, and various molecules in the cells and can lead to cell death.Citation8 DNA oxidative damage is particularly implied in mutagenesis and carcinogenesis processes.Citation9 In fact, UV radiation can induce the damage of DNA chainsCitation10 via a reduction in molecular oxygen and singlet oxygen-mediated photo-oxidation, leading to the formation of ROS, which are directly responsible for DNA damage.Citation11
In the present study, using agarose gel electrophoresis, we investigated DNA topological modifications to evaluate whether aqueous (AE) and hydroalcoholic (HE) extracts from S. uncinata acted as protectors against ROS or as inductors of cleavage in DNA plasmid. As biological systems involve higher complexity of interactions, the present investigation focused in vitro assays to aim primary screening on effects of the extracts following basic mechanisms of ROS generations.
Materials and methods
Chemicals, bacterial strains, and plasmid DNA
Stannous chloride anhydrous (SnCl2, CAS 7772-099-78), l-histidine (CAS 7048-02-4), pyrocatechol (CAS 120-80-9), ethylenediaminetetraacetic acid (EDTA, CAS 60-00-4), and sodium azide (NaN3, CAS 26628-22-8) were purchased from Sigma Co (St Louis, MO, USA). Analytical-grade dimethyl sulfoxide (DMSO) and 30% H2O2 were purchased from Merck (Darmstadt, Germany). Pyrogallol (99%, CP95 Pharm grade) was obtained from Heco Trading (Hamburg, Germany). All other reagents were analytical grade. Highly purified E-pure water with a resistivity of 18.1 MΩ-cm (Barnstead/Thermolyne, Dubuque, IA, USA) was used throughout the preparations and assays. Plasmid pUC18 (2686 bp, double strand) was isolated from Escherichia coli DH5alpha strain using the standard alkaline lysis procedure.Citation12
Sampling site at King George Island
Samples of S. uncinata were collected from King George Island, in the South Shetland Islands, Antarctic Peninsula, in February 2004. Throughout the year, the monthly mean air temperature at the island ranges from +2 to −10°C. On King George Island, there are ice-free areas in the summer (December to late March), when soil and air temperatures typically remain above freezing. During this period, mosses growing on rocky terrains are exposed to direct UV radiation. From Autumn to Spring, the mosses are covered by a snow/ice layer that varies from a few centimeters to ∼2 m in depth. At sub-Antarctic sites such as King George Island, UV radiation levels reaching the surface are ∼4 times higher in the summer (in terms of maximum daily UV index) than in the winter or autumn, leading to a high gradient in seasonal UV irradiation. Samples were collected in the vicinity of the Brazilian Comandante Ferraz Antarctic Station (62°05′S, 58°24′W) using aseptic procedures, stored in plastic bags and kept frozen until being processed in laboratory conditions.
Preparation and characterization of the extracts
The S. uncinata specimen was identified by Dr Denise Pinheiro da Costa of the Botanical Garden Research Institute of Rio de Janeiro (Rio de Janeiro, Brazil). Moss material (9 g) was washed four-fold with 200 ml water for 15 minutes under magnetic agitation for the removal of visible debris and sediments. The vegetal material was extracted by boiling for 15 minutes consecutively with 300 ml water and 300 ml 70% v/v ethanol. The extracts were filtered through Whatman no. 1 filter paper, centrifuged at 3900 × g for 20 minutes at 4°C, and then the supernatant was sterilized by membrane filtration (nylon, 0.22 µm pore size). The ethanol was then evaporated under 300 mTorr pressures in an SC-110 SpeedVac® (Savant Instruments, Holbrooks, NY, USA). The extracts were freeze-dried and further stored in a desiccator in the dark at −20°C until use. The yields from wet moss were 1.88 and 0.73% (w/w), respectively, for AE and HE. The total phenolic content (mg pyrocatechol equivalent per g of dried extract) was determined in triplicate by the Folin–Ciocalteu method as described previously,Citation13 using a standard curve (with five pyrocatechol concentration levels) at 760 nm in a Shimadzu UV-160A spectrophotometer.
DNA treatment
Plasmid DNA (pUC18, 394 µg/ml) was incubated in a tris-acetate-EDTA buffer (TAE), pH 7.8, with increasing concentrations of AE and HE (20, 500, and 900 µg/ml) for 60 minutes at 37°C under aerobic conditions to evaluate its genotoxic potential. SnCl2 at 200 µg/ml was used as a positive control of DNA cleavage using the same incubation conditions as previously described.Citation14 Reaction mixtures (20 µl) containing S. uncinata extracts (fixed at 900 µg/ml) and plasmid DNA in TAE were incubated at 37°C under aerobic conditions as described below.
The protective activity of the extracts was evaluated, including ROS inducers that had been diluted in TAE. The experimental design was adapted from previous studies of DNA cleavage caused by ROS.Citation15–Citation18 Thus, pyrogallol (250 µM), H2O2 (2 mM), Fe2+/H2O2 (200 mM, 50 mM, Fenton reaction), and Cu2+/H2O2 (1.25 mM, 0.125 mM, Fenton-like reaction) were included individually in the reaction medium (20 µl) and this was incubated for 60 minutes (except for H2O2, 30 minutes). DMSO 5% was added as an inhibition control since this compound is a scavenger of O2•− and OH.Citation9
The extracts were also incubated in the presence of SnCl2 (200 µg/ml) for 60 minutes. Well-known antioxidants or ROS scavengers were included following previously reported proceduresCitation20 butylated hydroxytoluene (BHT, 20 mM), sodium benzoate (benz, 2 M), l-histidine (50 mM), DMSO (5%), and NaN3 (100 mM).
All experiments were performed in triplicate.
DNA electrophoresis
Agarose gel (1.5%) electrophoresis was performed using standard procedures as previously describedCitation12 and as used elsewhereCitation14,Citation15,Citation21–Citation23 in order to separate different structural conformations found after DNA incubation: form I (supercoiled DNA); form II (relaxed circular) resulting from a single-strand break; and form III (linear) resulting from double-strand breaks. As such, aliquots (15 µl) from the incubation mixture were mixed with a loading buffer (0.25% xylene cyanol FF; 0.25% bromophenol blue; 30% glycerol in water), added to a horizontal gel electrophoresis chamber in TAE buffer, pH 8.0, and then electrophoresed at 60 V for 90 minutes. The final volume was 20 µl. After electrophoresis, the distinct DNA bands were stained with 0.5 µg/ml ethidium bromide, visualized under UV light using a UV transilluminator (UVP, Cambridge, UK), and then digitally photographed (Kodak Digital ID, EDAS 120, Rochester, NY, USA). The stained bands were scanned by densitometry using the ImageJ 1.34a software (National Institute of Health; Bethesda, MD, USA) and expressed as an intensity ratio ranging between form II and total (I + II + III). Differences among the densitometry data were statistically evaluated using z test of proportions.
Results and discussion
Phenolic content estimation
The phenolic content of AE and HE was 1.04 ± 0.27 and 5.89 ± 2.05 mg/g, respectively. The statistically higher content (P < 0.05) in the HE was probably due to the intense presence of derivatives with few or no combined glycoside groups. Natural phenolic compounds can contain glycosides in their chemical structures, and glycosilation usually leads to high solubility in water and low solubility in low-polarity and non-polar solvents.Citation24 The results found in HE were two times lower as those values reported elsewhereCitation7 because the data in the literature are based on freeze-dried moss material.
Effects on DNA topology
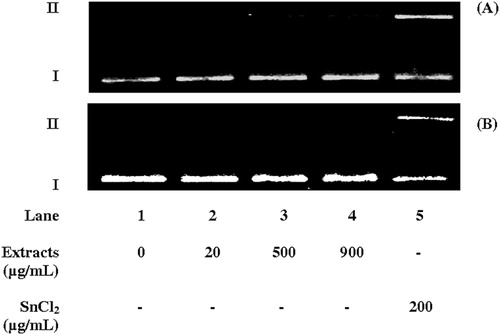
shows the structural conformation of pUC18 DNA after incubation with AE and HE at three concentrations. Lane 1 shows the results from incubation without the extracts, representing the negative control. SnCl2 was used as a positive control (lane 5) since it is a genotoxic agent that induces DNA damages via Fenton-like reactions.Citation14,Citation21–Citation23 No induction of DNA cleavage by either extract was detected (lane 2–4), meaning that molecular genotoxicity cannot be attributed to them.
Figure 1. Agarose gel electrophoresis of plasmid DNA (pUC18, 394 µg/ml) incubated for 1 hour at 37oC with aqueous (A) or hydroalcoholic (B) extracts of S. uncinata (lanes 2–4). Lane 1 represents the negative control. Stannous chloride at 200 µg/ml was used as a positive control (lane 5). The direction of electrophoresis is top to bottom. Supercoiled (I) and relaxed circular (II) plasmid DNA forms are indicated. The gels are representative of experiments with similar results.
Protective effects against induction of ROS generation
In view of the above results, the possible protective effects of the extracts at 900 µg/ml against the induction of ROS were investigated using four experimental designs performed independently. The electrophoresis analysis of the DNA topology after incubation is illustrated in , in which lanes 1 and 2 are the negative and DNA damage controls, respectively. The relative signal intensities of form II obtained by densitometry analysis are presented in .
Figure 2. Agarose gel electrophoresis of plasmid DNA (pUC18, 394 μg/ml) incubated for 1 hour at 37oC with aqueous and hydroalcoholic extracts of S. uncinata at 900 µg/ml in the presence and absence of ROS generation systems: (A) Pyrogalol 250 µM for O2•−, (B) Fe2+/H2O2 (200 µM, 50 mM) for •OH, via Fenton reaction, (C) Cu2+/H2O2 (200 µM, 50 mM) for •OH and O2−•, via Fenton-like reaction, (D) SnCl2 (200 µg/ml) for •OH and O2−•, via in situ Fenton-like reaction. ROS inhibitors: DMSO 5%, except for SnCl2 in which EDTA 25 mM was used. The direction of electrophoresis is top to bottom. Supercoiled (I), relaxed circular (II), and linear (III) forms are indicated. The gels are representative of experiments with similar results.
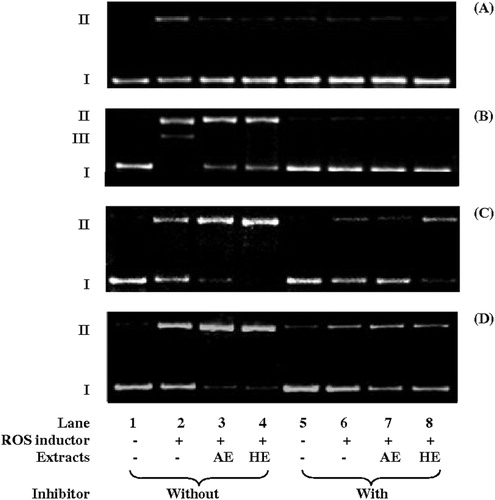
A shows the agarose gel electrophoresis of plasmid DNA after incubation with pyrogallol, an inducer of topological changes in DNA from form I to II via O2•− generation.Citation15,Citation25 In , the action of both extracts in inhibiting pyrogallol-induced conformational changes (lane 2) (P < 0.05) can be observed, since AE and HE inhibited 89 and 94% (lanes 3 and 4, respectively) of DNA cleavage in form II (lane 2). This inhibition effect was statistically identical to the negative control.
Table 1. Intensity (%, mean relative to total band density and standard deviation) of form II DNA (plasmid pUC18) treated with the aqueous (AE) and hydroalcoholic (HE) extracts of S. uncinata in the absence (–) or presence (+) of ROS generation systems and without (wo) or with (w) ROS inhibitors
Hydrogen peroxide is known to be the second ROS formed by the reduction of O2. The induction of single- and double-strand breaks of plasmid DNA by 50–400 µM H2O2 has been demonstrated.Citation16,Citation26 However, in our experiments 50 µM to 2 mM H2O2 did not induce pUC18 DNA cleavage under the same conditions in the absence or presence of either extract (data not shown) after 30 minutes of incubation. Since OH generation by H2O2 in the presence of appropriate inducers has been considered an appropriate model for ROS generation,Citation19 these results suggest that neither extract contained compounds that induce .OH generation.
The effects of AE and HE were investigated in the situations where DNA cleavage was induced by the Fenton reaction (B). In this case, the reduction of H2O2 was observed, particularly by Fe2+, producing •OH.Citation17 Extensive DNA cleavage in forms II and III induced by the Fenton reaction can be observed in lane 2 (P < 0.01). The inhibition control was effective (lanes 5–8), showing that ROS were responsible for DNA cleavage in the assay. AE and HE protected the plasmid DNA (lanes 3 and 4) against cleavage induced by the Fenton reaction, with the intensity of the form II band decreasing by up to 17% for AE and by up to 18% for HE (). Form III generation was also inhibited. No statistically significant differences between the effects of the extracts (P > 0.05) were found, regardless of the presence of an inhibitor (comparing lanes 3 and 4, and 7 and 8).
Interaction with Fenton-like reactions
Fenton-like reactions have a significant role in the induction of oxidative DNA damageCitation27 following the generation of ROS via the reduction of H2O2 by metal ions, except in ferrous salts.Citation28,Citation29 As such, the effect of both extracts on DNA cleavage induced by Fenton-like reactions was also investigated (C) using the Cu2+/H2O2 system. In contrast with the protective effect of both extracts against Fenton reaction-induced cleavage, both AE and HE induced a statistically significant (P < 0.01) increase in DNA cleavage induced by a Fenton-like reaction (lanes 3 and 4). The maximum increase observed was 94% for AE and 100% for HE (P < 0.05) (). DMSO, which was used as an inhibition control, decreased damage by Fenton-like reactions, even in the presence of AE (lanes 6 and 7). However, this inhibition was lower in the presence of HE/Fenton-like reactions (comparing lanes 6 and 7 with 8).
Antioxidant activity of natural phenolic compounds it is well known. However, prooxidant effects of these compounds have been also observed with the participation of transition metal ions and dissolved oxygen. The resulting rise to oxidative damage has also been detected.Citation30 The antioxidant and prooxidant activities of these substances are generally concentration dependents.Citation31–Citation33 Some natural phenolic compounds associated with Cu2+ promote extensive in vitro DNA cleavage under aerobic conditions.Citation15,Citation25 Even so, no topological modification was observed when plasmid DNA was treated for 1 hour with this ion in the presence of AE and HE (data not shown), excluding any possibility of a reaction between Cu2+ and the extracts causing DNA cleavage, such as observed above in the Fenton-like reactions.
The distinct effect of HE in the presence of Fenton-like reactions can be associated on the higher amounts of phenolic ingredients. Furthermore, the antioxidant and the copper-initiated prooxidant activities of phenolic compounds depend on the number and position of –OH substituents in its backbone structure, apart from the presence of glycosides, and the overall degree of conjugation are important in determining activity.Citation32,Citation33
Fenton-like reaction with hydrogen peroxide formed in situ
D shows the conformational forms observed for plasmid DNA treated with both extracts in the presence of SnCl2. In this system, H2O2 is generated in situ after the reduction of molecular oxygen (in aerobic conditions) by the metal ion.Citation21 Moreover, Sn2+ also presents direct mutagenic effects via the oxidation of DNA resulting in 8-oxoguanine.Citation22
The SnCl2-induced DNA cleavage (lanes 3 and 4, in ) may have occurred via Fenton-like reactions (P < 0.01), since this effect was also previously observed in the Cu2+/H2O2 system assay (C). Both extracts increased the damage to the plasmid DNA: AE by 62% and HE by 56%. EDTA, a metal chelating compound that inhibits DNA damage by SnCl2, was included as a control (lanes 5–8). EDTA also inhibited the DNA cleavage induced by SnCl2 in the presence of each extract. Thus, we suggest that the increased activity of the extracts was associated with the presence of the non-complexed metal ion.
The effects of ROS scavengers (DMSO for O•−2 and •OH; NaN3 and histidine for singlet oxygenCitation19) and antioxidants (BHT and sodium benzoateCitation34) on the induction of DNA cleavage by the AE and HE were also investigated. The electrophoresis analysis is illustrated in and the densitometry results are shown in . DNA cleavage fell sharply and significantly (P < 0.01) in the presence of ROS scavengers and antioxidants. These results indicate that the increased SnCl2-induced DNA cleavage induced by the extracts was caused via ROS generation, but without generation of specific ROS.
Figure 3. Agarose gel electrophoresis of plasmid DNA (pUC18, 394 µg/ml) incubated for 1 hour at 37oC with aqueous (A) and hydroalcoholic (B) extracts of S. uncinata at 900 µg/ml in the presence of SnCl2 (200 µg/ml) with ROS scavengers or antioxidants: l-histidine 50 mM (his), DMSO 5%, NaN3 100 mM, BHT 20 mM, and sodium benzoate 2 M (benz). The direction of electrophoresis is from top to bottom. Supercoiled (I) and relaxed circular (II) forms are indicated. The gels are representative of experiments with similar results.
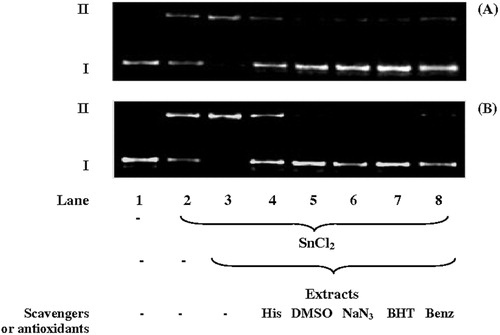
Table 2. Intensity (%, mean relative to total band density and standard deviation) of form II DNA (plasmid pUC18) treated with the aqueous (AE) and hydroalcoholic (HE) extracts of S. uncinata in the absence (wo) or presence (w) of SnCl2 and without (–) or with (+) ROS scavengers and antioxidants
Conclusion
The aqueous and hydroalcoholic extracts of the moss S. uncinata induced neither plasmid DNA cleavage nor ROS generation in the presence of H2O2. Furthermore, our results also showed that the potential antioxidant activity of extracts of S. uncinata, which was previously shown elsewhere using conventional chemical antioxidant assays,Citation7 effectively protected the plasmid DNA against cleavage induced by O2•− (by pyrogallol) and .OH (interanging with Fenton reaction); and that this effect is likely due to the presence of antioxidant phenolic compounds. On the other hand, both extracts intensified the DNA cleavage induced by Fenton-like reactions. EDTA, ROS scavengers, and antioxidants inhibited the enhancing effect of both extracts on Fenton-like reactions, which take place via in situ ROS generation. Nevertheless, DMSO did not inhibit the HE-induced plasmid DNA damage in the Cu2+/H2O2 system, suggesting that the HE increased the induction of DNA cleavage by Fenton-like reactions, and that this could have been via mechanisms other than ROS generation. For instance, the main sequences of events in this type of DNA damage starts with Cu2+ reducing to Cu+, which rapidly binds to DNA with high affinity.Citation35 The combination of this complex with H2O2 primarily produces DNA modifications,Citation36–Citation37 such as 8-oxoguanine, and can be an important source of mutagenesis, carcinogenesis, and the aging process.Citation38–Citation40
It has been suggested that the moss S. uncinata has potentially promising qualities for photoprotection and medical and cosmetic applications. Our results showed that the AE and HE of this moss had protective effects against plasmid DNA cleavage by ROS. However, potential risks in the direct use of these extracts were also identified, in view of the various possible combinations involving metal ions in formulations.
Acknowledgments
The authors wish to thank Dr Denise Pinheiro da Costa from the Botanical Garden Research Institute of Rio de Janeiro (Brazil) for the identification of the moss. This work was supported by CNPq (Brasília, Brazil) and Faperj (Rio de Janeiro, Brazil). We also thank PROANTAR (CNPq556971/2009–4) and INCT-Criosfera for allowing sampling in Antarctica.
References
- Robinson SA, Wasley J, Tobin AK. Living on the edge – plants and global change in continental and maritime Antarctica. Glob Change Biol 2003;9:1681–717.
- Markham KR, Franke A, Given DR, Brownsey P. Historical Antarctic ozone level trends from herbarium specimen flavonoids. Bull Liaison –- Groupe Polyphenols 1990;15:230–5.
- Lovelock CE, Robinson SA. Surface reflectance properties of Antarctic moss and their relationship to plant species, pigment composition and photosynthetic function. Plant Cell Environ 2002;25:1239–50.
- Rozema J, Noordijk AJ, Broekman RA, Van Beem A, Meijkamp BM, De Bakker NVJ, et al. Polyphenolic compounds in pollen and spores of Antarctic plants as indicators of solar UV-B. Plant Ecol 2001;154:11–26.
- Newsham KK, Hodgson DA, Murray WA, Peat HJ, Lewis Smith RI. Response of two Antarctic bryophytes to stratospheric ozone depletion. Glob Change Biol 2002;8:972–83.
- Lud D, Moerdijk T, Van de Poll WH, Buma AGJ, Huiskes AHL. DNA damage and photosynthesis in Antarctic and Arctic Sanionia uncinata (Hedw.) Loeske under ambient and enhanced levels of UV-B radiation. Plant Cell Environ 2002;25:1579–89.
- Bhattarai HD, Paudel BP, Lee HS, Lee YK, Yim JH. Antioxidant activity of Sanionia uncinata, a polar moss species from King Island, Antarctica. Phytother Res 2008;22:1635–9.
- Scandalios JG. The rise of ROS. Trends Biochem Sci 2002;9:483–6.
- Poulsen HE, Prieme H, Loft S. Role of oxidative DNA damage in cancer initiation and promotion. Eur J Cancer Prev 1998;7(1):9–16.
- Friedberg EC. DNA damage and repair. Nature 2003;421:436–40.
- Leccia MT, Richard MJ, Favier A, Beani JC. Zinc protects against ultraviolet A1-induced DNA damage and apoptosis in cultured human fibroblast. Biol Trace Elem Res 1999;69(3):177–90.
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory, 1989.
- Kiralp S, Toppare L. Polyphenol content in selected Turkish wines, an alternative method of detection of phenolics. Process Biochem 2006;41(1):236–9.
- Dantas FJS, Moraes MO, De Mattos JCP, Bezerra RJAC, Carvalho EF, Bernardo-Filho M, et al. Stannous chloride mediates single strand breaks in plasmid DNA through reactive oxygen species formation. Toxicol Lett 1999;110:129–36.
- Mazzei JL, Lapa JS, Felzenszwalb I. The influence of pH on the inhibition of DNA cleavages induced by pyrogallol. Redox Rep 2008;13(5):208–12.
- Choi D, Kim S, Jung MY. Inhibitory activity of berberine on DNA strand cleavage induced by hydrogen peroxide and cytochrome C. Biosci Biotechnol Biochem 2001;65(2):452–5.
- Henle ES, Han Z, Tang N, Rai P, Luo Y, Linn S. Sequence-specific DNA cleavage by Fe2+-mediated Fenton reactions has possible biological implications. J Biol Chem 1999;274(2):962–71.
- Sagripanti J, Kraemer KH. Site-specific oxidative DNA damage at polyguanosines produced by copper plus hydrogen peroxide. J Biol Chem 1989;264:1729–34.
- Lee SF, Liang YC, Lin JK. Inhibition of 1,2,4-benzenetriol generated active oxygen species and induction of phase II enzymes by green tea polyphenols. Chem-Biol Interact 1995;98:283–301.
- Hiramoto K, Yasuhara Y, Sako K, Aoki K, Kikugawa K. Supression of free radical-induced DNA strand breaks by linoleic acid and low density lipoprotein in vitro. Biol Pharm Bull 2003;26(8):1129–34.
- Caldeira-de-Araújo A, Dantas FJS, Moraes MO, Felzenszwalb I, Bernardo-Filho M. Stannous chloride participates in the generation of reactive oxygen species. J Brazil Assoc Adv Sci 1996;48:109–13.
- De Mattos JCP, Dantas FJS, Bezerra JAC, Bernardo-Filho M, Cabral-Neto JB, Lage C, et al. Damage induced by stannous chloride in plasmid DNA. Toxicol Lett 2000;116:159–63.
- Felzenszwalb I, De Mattos JCP, Bernardo-Filho M, Caldeira-de-Araújo A. Shark cartilage preparation: protection against reactive oxygen species. Food Chem Toxicol 1998;36:1079–84.
- Antolovich M, Prenzler KR, Ryan D. Sample preparation in the determination of phenolic compounds in fruits. Analyst 2000;125:989–1009.
- Hayakawa F, Kimura T, Maeda T. DNA cleavage reaction and linoleic acid peroxidation induced by tea catechins in the presence of cupric ion. Biochim Biophys Acta 1997;1336:123–31.
- Jun T, Bochu W, Liancai Z. Hydrolytic cleavage of DNA by quercetin zinc (II) complex. Bioorg Med Chem Lett 2007;17:1197–99.
- Lloyd DR, Phillips DH. Oxidative DNA damage mediated by copper (II), iron (II) and nickel (II) Fenton reactions: evidence for site specific mechanisms in the formation of double-strand breaks, 8-hydroxydeoxyguanosine and putative intra strand cross-links. Mutat Res 1999;424(1–2):23–36.
- Aruoma OI, Halliwell B, Gajewski E, Dizdaroglu M. Copper ion-dependent damage to the bases in DNA in the presence of hydrogen peroxide. Biochem J 1991;273:601–4.
- Simpson JA, Cheeseman KH, Smith SE, Dean RT. Free-radical generation by copper ions and hydrogen peroxide. J Bacteriol 1988;254:519–23.
- Apak R, Güçlü K, Demirata B, Özyürek M, Çelik SE, Bektasoglu B, et al. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules 2007;12:1496–547.
- Gutteridge JMC. Free radicals and aging. Rev Clin Gerontol 1994;4:279–88.
- Cao G, Sofic E, Prior RL. Antioxidant and prooxidant behavior of flavonoids: structure-activity relationships. Free Radic Biol Med 1997;22:749–60.
- Tripoli E, Guardia ML, Giammanco S, Majo DD, Giammanco M. Citrus flavonoids: molecular structure, biological activity and nutritional properties: a review. Food Chem 2007;104:466–79.
- Omura K. Antioxidant synergism between butylatedhydroxyanisole and butylatedhydroxytoluene. J Am Oil Chem Soc 1995;72:1565–70.
- Goldstein S, Czapski G. Mechanisms of the reactions of some copper complexes in the presence of DNA with O2.−, H2O2 and molecular oxygen. J Am Chem Soc 1986;108:2244–50.
- Drouin R, Rodriguez H, Gebreyes Z, O'Connor TR, Holmquist GP, Akman SA. Cupric ion/ascorbate/hydrogen peroxide-induced DNA damage: DNA-bound copper ion primarily induces base modifications. Free Radical Bio Med 1996;21(3):261–73.
- Lee D, ÓConnor TR, Pfeifer GP. Oxidative DNA damage induced by copper and hydrogen peroxide promotes CG-TT tandem mutations at methylated Cpg dinucleotides in nucleotide excision repair-deficient cells. Nucleic Acids Res 2002;30:3566–73.
- Floyd RA. The role of 8-hydroxyguanine in carcinogenesis. Carcinogenesis 1990;11:1447–50.
- Tchou J, Kasai S, Shibutani MH, Chung J, Laval AP, Grollman SN. 8-Oxoguanine (8-hydroxyguanine) DNA glycosilase and its substrate specificity. Proc Natl Acad Sci USA 1991;88:4690–4.
- Rozalski R, Gackowski D, Roszkwski K, Foksinski M, Olinski R. The level of 8-hydroxyguanine, a possible repair product of oxidative DNA damage, is higher in urine of cancer patients than in control subjects. Cancer Epidemiol Biomar 2002;11:1072–5.