Abstract
Objectives
Oxidative stress (OS) is defined as an imbalance in the production of reactive oxygen species and the capacity of antioxidant defenses. The objective of this work was to investigate OS and antioxidant capacity in pregnant women.
Methods
Parameters of the oxidative status and antioxidant capacity in serum and whole blood were evaluated in thirty-nine women with normal pregnancy.
Results
The assessment of antioxidants indicated an increase in superoxide dismutase and catalase activities (P < 0.05 and P < 0.01) and a decrease in ascorbic acid levels and the total content of sulfhydryl (P < 0.05 and P < 0.001). Additionally, when the pro-oxidant system was investigated we found an increase (P < 0.01) in malondialdehyde and no significant change (P > 0.05) in protein carbonylation.
Discussion
This study demonstrates that there is a change in the pro-oxidant and antioxidant defenses associated with body and circulation changes that are inherent to the pregnancy process.
Introduction
The principal causes of oxidative stress (OS) are reactive oxygen species (ROS), which may be broadly defined as derivatives of molecular oxygen.Citation1 Small amounts of ROS, including hydroxyl radicals (•OH), superoxide anions (O2−), and hydrogen peroxide (H2O2), are constantly generated in aerobic organisms in response to both external and internal stimuli.Citation2 They are very transient due to their high chemical reactivity, which also leads to lipid peroxidation, oxidation of some enzymes, and massive protein oxidation and degradation.Citation3
ROS attack the phospholipids of cell membranes and react with polyunsaturated fatty acids to form lipid peroxides, resulting in cellular injury, and they have been proposed to be a promoter of lipid peroxidation and the endothelial cell dysfunction that is commonly associated with disorders of pregnancy.Citation4 The prevention of lipid peroxidation is an essential process in all aerobic organisms as products from this process can cause DNA damage. To complicate the situation further, an increase in lipid peroxidation and decreased antioxidant protection frequently occurs. As a result, epoxides may spontaneously react with nucleophilic centers in the cell and covalently bind to DNA, RNA, and protein.Citation5
Low levels of ROS are indispensable in many biochemical processes, including intracellular messaging to control cell differentiation, cell progression, cell growth, apoptosis,Citation6 immunity, and defense against microorganisms.Citation7 In contrast, high doses and/or inadequate removal of ROS result in OS, which may cause severe metabolic malfunctions and damage to biological macromolecules.Citation8
The removal of these ROS is performed by antioxidant enzymes, such as catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase, glutathione transferase, glutathione reductase, and antioxidant molecules, such as glutathione, that work in concert within the cell to metabolize these noxious forms of oxygen into innocuous substances.Citation9 We can define OS as an imbalance between the production of ROS and reactive nitrogen species and the ability of antioxidant defenses to scavenge them. It can arise from increased production of ROS and/or a decrease in antioxidant capacity. Among the ROS, there are several free radicals that can induce a great deal of cellular damage by acting on proteins and lipids.
Many studies have shown that there is increased OS during pregnancy, and oxidative damage is increased in pregnancies complicated by disorders such as pre-eclampsia.Citation10 Currently, there is only scattered information regarding baseline levels of OS and antioxidant status in women with uncomplicated pregnancies.Citation11 The cause of increased maternal OS during pregnancy is unknown; nevertheless, accumulating evidence suggests that the placenta plays an important role in the production of OS.Citation12,Citation13
Recent evidence suggests that OS might have an influence during the entire reproductive span of women's life and even thereafter. It might play a significant role during pregnancy, normal parturition, and initiation of preterm labor. Oocyte maturation, ovarian steroidogenesis, ovulation, implantation, formation blastocyst, luteolysis, and luteal maintenance in pregnancy have been shown to be influenced by OS. Pregnancy is characterized by dynamic changes in multiple body systems resulting in increased basal oxygen consumption.Citation9
Studies have also reported the idea that even normal pregnancy (NP) may induce a state of mild OS and lead to peroxidation of lipids, namely arachidonic acid, because plasma F2-isoprostane levels were found to be higher in severe pre-eclampsia, mild pre-eclampsia, normotensive pregnancy, and non-pregnancy. Pregnant women showed higher levels of isoprostanes, which is important because the free radical-mediated arachidonic acid products might have a significant role in the maintenance of pregnancy.Citation14
One of the major F2-isoprostanes, 8-iso-prostaglandin F2α (8-iso-PGF2α), is increased in several syndromes associated with oxidant injury, and estimation of isoprostanes is now regarded as a biomarker for in vivo measurement of OS. In relation to antioxidants, the lipid-adjusted alpha and gamma tocopherol levels showed a significant decrease in mid- to late-stage pregnancy compared to non-pregnancy.Citation15
Because OS can play both physiological and pathological roles in the progression and outcome of pregnancy, we attempted to characterize the profiles of antioxidant response of normal pregnant women at the Federal University of Santa Maria Hospital, Brazil, by determining enzymic antioxidants (SOD, CAT), non-enzymic antioxidants (ascorbic acid and total sulfhydryl content) as well as lipid peroxidation, and protein oxidation profile in the third trimester of pregnancy.
Materials and methods
Patients and controls
Thirty-nine women from the Federal University of Santa Maria Hospital (UFSM, Santa Maria, RS, Brazil) were included in the study. None of these women had a history of other relevant disease states. Women with unfavorable outcome of pregnancy were excluded from this investigation. Each woman was carefully selected by a clinical evaluation, and participants were separated into two patient groups. The control group (CG) consisted of total n = 9 non-pregnant, healthy female volunteers who did not present any disease and had not been submitted to any pharmacological therapy during the previous month. The second group, designated NP, consisted of 30 pregnant women with a singlet pregnancy, normal diastolic and systolic blood pressure (mmHg), and ages ranging from 27 to 45 years. A detailed description of the groups is given in .
Table 1. Clinical characteristics of the study population
None of the subjects in the study were smokers or were using antioxidant supplementation. All women of the NP group were being treated with ferrous sulfate and were in the third-gestational trimester (). Women in the CG were not using drugs of any kind. The Human Ethics Committee of UFSM approved the project under number 86-2006, and subjects gave informed consent.
Collection of blood samples
Eighteen milliliters of peripheral blood was collected by venous puncture from the ante-cubital region. Blood was collected into three vacutainer plastic tubes containing anticoagulant sodium citrate 3.2%, EDTA (K3E), and an additional tube with serum clot activator without anticoagulant for the separation of blood fractions.
Hematological parameters
Tests were run to determine the erythrocyte count, hemoglobin, and hematocrit of both control individuals and patients using an ABX Micros 60 automatic cell counter (ABX Horiba, Kyoto, Japan) Measurements were taken using the impedance and photometry method and provided 18 reading parameters.
Determination of lipid peroxidation
We used the protocol previously described by Jentzsch that uses the formation of thiobarbituric acid reactive substances (TBARS) during an acid-heating reaction to determine an index of lipid peroxidation.Citation16 Briefly, 200 µl of serum samples was mixed with 1.5 ml of 0.2 M orthophosphoric acid, 250 µl of 0.1 M of thiobarbituric acid, and 550 µl of distilled water. The samples were then heated in a boiling water bath for 45 minutes. TBARS were determined by the absorbance at 532 nm and were expressed as malondialdehyde (MDA) equivalents (nmol MDA/ml of serum).
Determination of the total sulfhydryl content
Total-plasma sulfhydryl groups were determined as described by Ellman.Citation17 In short, an aliquot of plasma was reacted with 250 µM DTNB (5,5′-dithiobis-(2-nitrobenzoic acid) in a final volume of 2 ml of potassium phosphate at 500 mM, and the absorbance was read at 412 nm. A standard curve with cysteine (0.01–0.5 µmol SH/ml) was constructed to calculate the total sulfhydryl concentration in the samples.
Assay of protein carbonyls
The concentration of protein carbonyls was measured by reaction with 2,4-dinitrophenyl hydrazine (DNPH) following the method of Levine et al.Citation18 Assays were performed in duplicate for both the DNPH-treated samples and blanks. A serum aliquot containing approximately 5 mg of protein was diluted in 20 mM HEPES buffer at pH 7.2 and then placed in four tubes. A half-volume of 10% trichloroacetic acid (TCA) was added to precipitate the protein. Tubes were centrifuged at 3700 rpm for 5 minutes in a tabletop centrifuge, and the supernatant was discarded. Two tubes then received 250 µl of 10 mM DNPH in 2 M HCl, and the same volume of 2 M HCl was added to the other two tubes. The pellets were resuspended using a pipette tip and vigorous vortexing. The reaction was left in the dark at room temperature for 30 minutes and vortexed after 15 minutes. The protein was precipitated by addition of 250 µl of 10% TCA followed by centrifugation for 5 minutes at 3700 rpm. After discarding the supernatant, the pelleted protein was washed two times with 1.0 ml of 1:1 ethyl acetate and ethanol at room temperature. Pellets were broken up with a pipette tip and vortexing and then centrifuged at 3700 rpm for 5 minutes before discarding the supernatant. After the third wash, the pellets were incubated at 37°C for 10 minutes in 1.5 ml of 80 mM phosphate-buffered saline buffer at pH 8.0 with 2% sodium dodecyl sulfate, and 0.5% EDTA, and the sample was then analyzed at 370 nm. The results are expressed as nmol of protein carbonyl per mg of protein.
SOD and CAT activities
Citrated whole blood was used to determine SOD and CAT activities. SOD activity was assayed by measuring the inhibition of 1 mM adrenaline auto-oxidation by absorbance at 480 nm using a glycine buffer (50 mM, pH 10.2) as described by Bannister and Calabrese.Citation19 CAT activity was determined with the method of Aebi by measuring the rate of catalysis of 30 mM H2O2 at 240 nm in 50 mM potassium phosphate buffer at pH 7.0.Citation20
Ascorbic acid (vitamin C) quantification
Vitamin C analysis was performed using the method described by Lloyd et al.Citation21 with the following modifications. The serum samples were de-proteinized with 15% TCA, using 400 µl of sample and 800 µl of 15% TCA. The sample was then mixed by vortexing for 15 seconds and centrifuged at 1800 × g for 15 minutes. Then 400 µl of supernatant was transferred to another tube, and 120 µl of 2,4-dinitrophenyl hydrazine-thiourea-CuSo4, DTC (a solution of 5 ml of 2.4 dinitrophenylhydrazine at 0.1 mol/l in 4.5 MH2SO4 with 0.25 ml of 0.66 mol/l thiourea and 0.25 ml of 0.027 mol/l cupric sulfate) was added. Samples were vortex mixed for 10 seconds, closed with filmed paper, and incubated at 60°C for 60 minutes. After incubation, samples were transferred to an ice bath for 10 minutes and 600 µl of 12 M H2SO4 was added. Samples were vortex mixed for 10 seconds and analyzed at 520 nm. All samples were measured in duplicate. Calibration curves were prepared with L(+)-ascorbic acid (Vetec Quimica Fina Ltda, Rio de Janeiro, Brazil) following the same procedure as used with the samples and were used to determine the concentration of ascorbic acid in the samples.
Protein determination
Protein was determined with the Coomassie blue method according to BradfordCitation22 with bovine serum albumin as the standard.
Statistical analysis
Data were analyzed by parametric unpaired t-test with a level of significance of 5%. All results were statistically analyzed and were found to follow a normal distribution when analyzed by the Kolmogorov-Smirnov (KS) normality test. Values of P < 0.05 were regarded as statistically significant. Correlation was evaluated by Pearson's test, and P < 0.05 was considered significant.
Results
Hematological parameters
A significant difference between the two groups of women was evident with regard to hematological parameters measured in peripheral blood. The erythrocyte count, hemoglobin, and hematocrit all were significantly decreased in the NP group compared with the CG ().
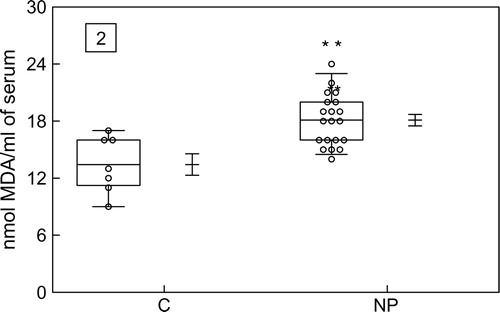
Determination of lipid peroxidation
To verify the oxidative damage in membrane lipids, MDA content was measured, and it was found to be significantly enhanced in the NP group compared with the CG as shown in .
Determination of total sulfhydryl content
A significant difference in levels of the total sulfhydryl content of pregnant women was found. The plasma total sulfhydryl content was decreased in the NP group (0.36 ± 0.02 µmol/ml, SEM) with P < 0.001 when compared with the CG (0.67 ± 0.04, SEM) ().
Table 2. Values of antioxidant substances in blood from NP and controls
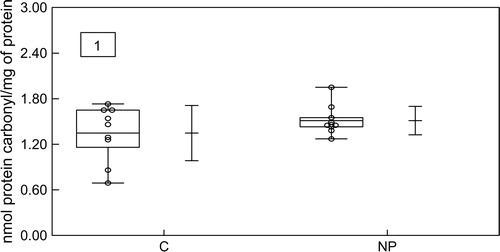
Determination of carbonylation of serum proteins
To assess the oxidative damage to proteins, the carbonyl groups were evaluated. It was found that proteins in the NP group had a larger carbonyl content than in the CG, but this difference was not statistically significant (P > 0.05) ().
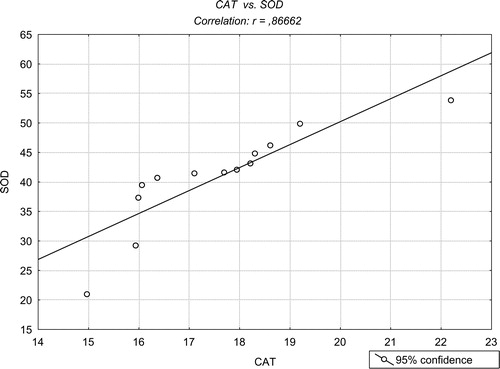
SOD and CAT activities
The levels of SOD and CAT activity are presented in . SOD activity was increased in the NP group (43.5 ± 1.47 U/mg protein, SEM) with P < 0.05 when compared with the CG (38.0 ± 2.09, SEM). The CAT activity was also increased in the NP group (17.15 ± 0.33 U/mg protein, SEM) with P < 0.01 when compared with the CG (13.75 ± 1.43, SEM) (). SOD and CAT activities demonstrated a positive correlation as illustrated in (P < 0.05).
Plasma vitamin C quantification
This exogenous antioxidant showed a statistically significant difference between groups. The plasma vitamin C was decreased in the NP group (2.77 ± 0.25 µmol/l, SEM) with P < 0.05 when compared with the CG (4.25 ± 0.75, SEM) ().
Discussion
Our results showed that the amounts of MDA, a pro-oxidant, were increased in women during the third trimester of gestation. In parallel with this change, there was an increase in the amounts of the antioxidants SOD and CAT during the same period. As previously reported,Citation9,Citation12,Citation13 these changes might be related to the process of pregnancy itself and the placental circulation that plays an important role in OS during this period.
The human placenta is classified as hemochorial, and the establishment of maternal placental circulation is influenced by trophoblastic invasion. Extravillous trophoblastic invasion transforms the low-caliber, high-resistance spiral arteries into high caliber with low resistance.Citation23 This remodeling of the placental vascular bed causes invasion of the cytotrophoblast cells on the maternal spiral arteries that feed the intervillous space, which involves the endothelium and muscular tunica media. The arteries lose their smooth muscle and become sinusoids that lack any contractibility, which results in an oxidative burst.Citation24
The maternal–placental interface is formed by syncytiotrophoblasts (STBs). These cells are the likely origin of the factors that disturb the maternal endothelium. Significant quantities of syncytiotrophoblast microparticles (STBMs), a product of the normal turnover and renewal of the syncytial surface by apoptosis, circulate in maternal blood during pregnancy.Citation25 The STBMs may lead to activation of maternal neutrophils and subsequent production of ROS mediated by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase.Citation26
The NADPH oxidase is an important enzyme that generates superoxide radicals localized in the placenta syncytial microvillous membrane.Citation27,Citation28 This enzyme may play a role in placental lipid peroxidation by generating increased amounts of the superoxide radicals, which could explain the increased activity of the enzyme SOD observed in this work. We propose that the STBMs may be contributing to the formation of ROS during pregnancy when they are released into the maternal–placental interface.
Other researchers have reported that pregnancy itself may produce OS as a result of increased metabolic activity in the placental mitochondria and a reduced scavenging power of antioxidants. Increased levels of plasma thiols and placental lipid peroxides in pregnant women have been reported.Citation29,Citation30 In another study, researchers showed that PGF2α levels were significantly higher during the NP period than during non-pregnancy. The researchers suggested that an increased level of PGF2α might be necessary in the NP process and parturition.Citation31
Other reports have demonstrated that plasma levels of lipid hydroperoxides and/or TBARS, CAT, and SOD increase with advancing gestational age.Citation9,Citation32 Another cross-sectional study has shown that MDA levels are significantly increased in NP compared to non-pregnancy.Citation33 In addition, pregnancy was associated with successively increasing levels of 8-iso-PGF2α with advancing gestational age, but lipid-adjusted alpha and gamma-tocopherol levels decreased in the same period.Citation15
This is important because substantial increases in OS have been hypothesized to lead to acute pregnancy complicationsCitation34 or spontaneous abortion.Citation35 Additionally, under pro-thrombotic conditions related to both thrombophilia and pregnancy, hemostatic responses, and microthrombi are generated, which could lead to ischemic conditions and mechanical stress in blood vessels and the consequent generation of ROS.Citation36,Citation37
Still, there are factors that are associated with endothelium injury and coagulation resulting from inflammation, which increase the activity of leukocytes and result in oxidative bursts. Thus, a cascade of events initiated by OS leads to a series of responses, such as vasoconstriction, membrane oxidation, or further pro-coagulation, each of which includes further generation of ROS.Citation38
Our results are consistent with other reports in the literatureCitation9,Citation32,Citation33 that suggest that the increase in SOD activity should be followed by increased CAT activity. The increase in SOD activity is an important antioxidant defense system that involves the catalysis of the superoxide anion (O2−) by SOD and the formation of peroxide (H2O2), and oxygen. The peroxide, in turn, is converted to water by CAT.
Another cause for the increase of pro-oxidants in NP that is reported here could be the iron supplementation these women were undergoing. We could hypothesize that iron supplementation is responsible for stress. Iron supplementation during pregnancy is currently a routine practice for most pregnancy care centers, particularly in developing countries. Reports indicate that iron is a free radical generator.Citation39 Indeed, the chemical properties of Fe render it a potential hazard within the organism, and ferrous iron (Fe2+) in small, non-protein shielded chelates can catalyze the production of oxygen radicals or ROS, which in turn can lead to peroxidation and radical chain reaction with molecular damage.Citation40
Iron is also involved in the Fenton reaction, generating a hydroxyl radical (•OH) that is particularly toxic. In fact, it is one of the most reactive forms of the free radicals. Thus, iron is significantly involved in toxic cellular injuryCitation41 and is probably responsible for a portion of the formation of ROS in pregnant women.
Together, these results and the knowledge of the importance of the balance between the generation of oxygen species and antioxidant systems in our body, both in healthy individuals and those with pathology, suggest that more studies should be conducted. The objective of these future endeavors should be to determine other important enzymes, and their possible mechanisms of action, that could be involved in OS in uncomplicated gestational processes.
Acknowledgements
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundação de Amparo a Pesquisa do Rio Grande do Sul (FAPERGS) and by the Universidade Federal de Santa Maria (UFSM).
References
- Gupta S, Wen JJ, Garg NJ. Oxidative stress in Chagas` disease. Hindawi Publishing Corporation: Interdiscip Perspect on Infect Dis 2009; volume 2009, 8 pages doi:10.1155/2009/190354.
- Jornot L, Petersen H, Junod AF. Hydrogen peroxide induced DNA damage is independent of nuclear calcium but dependent on redox-active ions. Biochem J 1998;335:85–94.
- Matés JM, Pérez-Gómez C, Núnez de Castro I. Antioxidant enzymes and human diseases. Clin Biochem 1999;32:595–603.
- Hung TH, Skepper JN, Charnock-Jones DS, Burton GJ. Hypoxia-reoxygenation: a potent inducer of apoptotic changes in the human placenta and possible etiological factor in preeclampsia. Circ Res 2002;90:1274–81.
- Matés JM. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology 2000;153:83–104.
- Ghosh J, Myers CE. Inhibition of arachidonate 5-lipoxygenase triggers massive apoptosis in human prostate cancer cells. Proc Natl Acad Sci 1998;95:13182–7.
- Le YJ, Galoforo SS, Berns CM, Chen JC, Davis BH, Sim JE, et al. Glucose deprivation-induced cytotoxicity and alterations in mitogen-activated protein kinase activation are mediated by oxidative stress in multidrug-resistant human breast carcinoma cells. J Biol Chem 1998;273:5294–9.
- Chopra S, Wallace HM. Induction of spermidine/spermine N1-acetyltransferase in human cancer cells in response to increased production of reactive oxygen species. Biochem Pharmacol 1998;55:1119–23.
- Ademuyiwa O, Odusoga OL, Adebawo OO, Ugbaja RN. Endogenous antioxidants defences in plasma and erythrocytes of pregnant women during different trimesters of pregnancy. Acta Obst et Gynec 2007;86:1175–80.
- Hubel CA. Oxidative stress in the pathogenesis of preeclampsia. Proc Soc Exp Biol Med 1999;222:222–35.
- Little RE, Gladen BC. Levels of lipid peroxides in uncomplicated pregnancy: a review of the literature. Reprod Toxicol 1999;13:347–52.
- Burton GJ, Jauniaux E. Placental oxidative stress: from miscarriage to preeclampsia. J Soc Gynecol Investig 2004;11:342–52.
- Hung TH, Burton GJ. Hypoxia and reoxygenation: a possible mechanism for placental oxidative stress in pleeclampsia. Taiwan J Obstet Gynecol 2006;45:189–200.
- McKinney ET, Shouri R, Hunt RS, Ahokas RA, Sibai BM. Plasma urinary and salivary 8-epi-prostagladin F2α levels in normotensive and pre-eclamptic pregnancies. Am J Gynecol 2000;183:874–7.
- Palm M, Axelsson O, Wernroth L, Basu S. F2 – isoprostanes, tocopherols and normal pregnancy. Free Radic Res 2009;43:546–52.
- Jentzsch AM, Bachmann H, Fürst P, Biesalski HK. Improved analysis of malondialdehyde in human body fluids. Free Radic Biol Med 1996;20:251–6.
- Ellman GL. Tissue sulphydryl groups. Arch Biochem Biophys 1959;82:70–7.
- Levine LR, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, et al. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 1990;186:464–78.
- Bannister JV, Calabrese L. Assays for superoxide dismutase. Methods Biochem Anal 1987;32:279–312.
- Aebi H. Catalase in vitro. Methods Enzymol 1984;105:121–6.
- Lloyd B, Sinclair HM, Webster GR. The estimation of ascorbic acid for clinical purposes by the hydrazine method. Biochem J 1945;39:17.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–54.
- Gupta S, Agarwal A, Sharma RK. The role of placental oxidative stress and lipid peroxidation in preeclampsia. Obstet Gynecol Surv 2005;60:807–16.
- Chamy MV, Lepe J, Catalán A, Retamal D, Escobar JA, Madrid EM. Oxidative stress is closely related to clinical severity of pre-eclampsia. Biol Res 2006;39:229–36.
- Huppertz B, Frank HG, Kingdon JC, Reister F, Kaufmann P. Villous cytotrophoblast regulation of the syncytial apoptotic cascade in the human placenta. Histochem Cell Biol 1998;110:495–508.
- Lee VM, Quinn PA, Jennings SC, Ng LL. Neutrophil activation and production of reactive oxygen species in preeclampsia. J Hypertens 2003;21:395–402.
- Matsubara S, Sato I. Enzyme histochemically detectable NAD(P)H oxidase in human placental trophoblasts:normal, preeclamptic, and fetal growth restriction-complicated pregnancy. Histochem Cell Biol 2001;116:1–7.
- Raijmakers MT, Peters WH, Steegers EA, Poston L. NAD(P)H oxidase associated superoxide production in human placenta from normotensive and pre-eclamptic women. Placenta 2004;25 (suppl A):S85–89.
- Wisdon SJ, Wilson R, McKillop JH, Walker JJ. Antioxidant systems in normal pregnancy and in pregnancy-induced hypertension. Am J Obstet Gynecol 1991;165:1701–74.
- Myatt L, Cui X. Oxidative stress in the placenta. Histochem Cell Biol 2004;122:369–82.
- Ishihara O, Hayashi M, Osawa H, Kobayashi K, Takedas S, Vessby B. Isoprostanes, prostaglandins and tocopherols in pre-eclampsia, normal pregnancy and non-pregnancy. Free Radic Res 2004;38:913–8.
- Toescu V, Nuttall SL, Martin U, Kendall MJ, Dunne F. Oxidative stress and normal pregnancy. Clin Endocrinol 2002;57:609–13.
- Morris JM, Gopaul NK, Endressen MJ, Knight M, Linton EA, Dhir S, Anggard EE, et al. Circulating markers of oxidative stress are raised in normal pregnancy and pre-eclampsia. Br J Obstet Gynaecol 1998;105:1195–9.
- Wang Y, Sharma KR, Falcone T, Goldberg J, Agarwal A. Importance of reactive oxigen species in the peritoneal fluid of women with endometriosis or idiopathic infertility. Fertil Steril 1997;68:826–30.
- Vural P, Akgul C, Yildirim A, Canbaz M. Antioxidant defence in recurrent abortion. Clin Chim Acta 2000;295:169–77.
- Crimi E, Ignarro LJ, Napoli C. Microcirculation and oxidative stress. Free Radic Res 2007;41:1364–75.
- Laude I, Rongieres-Bertrand C, Boyer-Neumann C, Wolf M, Mairovitz V, Hugel B, et al. Circulating procoagulant microparticles in women with unexplained pregnancy loss: a new insight. Thromb Haemost 2001;85:18–21.
- Krotz F, Sohn HY, Pohl U. Reactive oxygen species: players in the platelet game. Arterioscler Thromb Vasc Biol 2004;24:1988–96.
- Haliwell B, Gutteridge JMC. Free radicals in biology and medicine. 3rd ed. England: Oxford University Press; 1998.
- Simsek M, Naziroglu M, Simsek H, Cay M, Aksakal M, Kumru S. Blood plasma levels of lipoperoxide, glutathione peroxidase, beta-carotene, vitamins A and E in women with habitual abortions. Cell Biochem Funct 1998;16:227–31.
- Hubel CA, Kozlov AV, Kagan VE, Evans RW, Davidge ST, McLaughin MK, et al. Decreased transferrin and increased transferrin saturation in sera of women with pre-eclampsia: implications for oxidative stress. Am J Obstet Gynecol 1996;175:692–700.