Abstract
Objectives
Ionizing radiation induces severe oxidative stress in the body resulting an imbalance in prooxidant and antioxidant status in the cell. The aim of the present study is to investigate the protective effect of polysaccharide protein complex (PPC-Pr) isolated from the mushroom Phellinus rimosus against the oxidative stress induced by gamma radiation.
Methodology
PPC-Pr complex was isolated from the aqueous extracts of P. rimosus. The complex was administered to Swiss albino mice at a concentration of 5 and 10 mg/kg body weight intraperitoneally for 5 days consecutively and exposed to 4 Gy of gamma irradiation. Animals were sacrificed 1 day after irradiation and the antioxidant parameters such as glutathione, glutathione peroxidase, superoxide dismutase, catalase, glutathione reductase as well as lipid peroxidation were evaluated in both liver and brain tissues to evaluate oxidative stress. Amifostine, a standard radioprotective agent, was used as a positive control. In vitro DNA damage was assessed using the comet assay. Survival studies were also carried out to determine the protective role of PPC-Pr against radiation-induced delayed oxidative stress.
Results
PPC-Pr treatment enhanced the declined levels of antioxidants and comet parameters to a significant level, indicating its antioxidant as well as DNA protecting potential. Significant increase in the survival rate of animals was also observed in irradiated animals treated with PPC-Pr complex. The results were comparable to the standard drug amifostine.
Discussion
The results indicate profound effects of PPC-Pr against radiation-induced oxidative stress. The findings suggest potential therapeutic use of PPC-Pr in radiotherapy.
Introduction
Cancer treatment by radiation causes general weakening of body's antioxidant status. Reduced antioxidant status is due to increased generation of harmful reactive oxygen species (ROS) by radiolysis of water or by direct attack of ionizing radiation on the genetic material. Biological consequences of ionizing radiation involve generation of various transient ROS, such as superoxide (O2•−), hydrogen peroxide (H2O2), and hydroxyl radical (OH•), which cause tissue injury.Citation1–Citation4 The DNA damage inflicted by these free radicals is mainly strand break.Citation5 In addition to this, ionizing radiation can generate wide spectrum of molecular damages including DNA base alterations, DNA–DNA, and DNA protein cross links, single and double strand breaks. Further, radiotherapy causes general weakening of body's immune system resulting in immunosuppression that can significantly increase patients risk for infection. These facts warrant the concept of boosting an individual's immune system. This goal is validated by current clinical practice, called immunotherapy, which consists of administering immune-modulating drugs in combination with conventional radiotherapy to strengthen patient's tolerance to treatment.
The practice of using medicinal mushrooms to treat a variety of diseases is found in many countries. They are traditionally used in China and Japan for medicinal purposes.Citation6 Phellinus species are mostly tropical mushrooms and 18 species are known from Kerala State, India. Phellinus rimosus is a parasitic host-specific polypore mushroom often found growing on jackfruit tree (Atrocarpus heterophyllatus) trunks.Citation7 Our earlier investigations showed that ethyl acetate and methanol extracts of P. rimosus possessed antioxidant, antitumor, hepatoprotective, and radioprotective activities.Citation8–Citation10 Ethyl acetate extract of P. rimosus showed significant radiation-induced lipid peroxidation inhibiting activity.Citation11
Mushroom metabolites have diverse biological functions. Among them, polysaccharides play a major role due to its potent immunomodulating property.Citation12 Polysaccharides isolated from another species Phellinus linteus had been reported to possess significant antioxidant and immunostimulatory activity.Citation13,Citation14 Our recent investigations have demonstrated profound antioxidant, antiinflammatory, antiarthritic, as well as radioprotective activities of a polysaccharide protein complex (PPC-Pr) isolated from the aqueous extract of P. rimosus.Citation15–Citation17 The aim of our present study is to investigate the protective effect of PPC-Pr complex against radiation-induced DNA damage, tissue oxidative stress, as well as survival of the organism.
Materials and methods
Chemicals
Glutathione (GSH), 5,5′dithio-dinitro bisbenzoic acid (DTNB), nitroblue tetrazolium, thiobarbituric acid, riboflavin, and sodium azide were obtained from SRL, Mumbai, India and H2O2 from Merck India Ltd, Mumbai, India. Amifostine (Cytofos) was purchased from Sun pharmaceutical India Ltd, Gujarat, India. All other chemicals and reagents used were of analytical reagent grade.
Animals
Male Swiss albino mice, 8–10-week old and weighing 20–25 g, were purchased from Small Animal Breeding Station of Kerala Agriculture University, Mannuthy, Thrissur and were kept under environmentally controlled conditions (12 hours of light–dark cycle, 26–28°C temperature, and relative humidity of 60–70%) with free access to standard food (Sai Durga Feeds, Bangalore, India) and water ad libitum. All the animals were acclimatized for 1 week before starting the experiment. The experiments were conducted with the approval of Institutional Animal Ethics Committee following the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals, Govt. of India.
Irradiation
The animals were treated with a single dose of radiation of 400 rads (4 Gy). The source of radiation was a 60Co-Theratron Phoenix Teletherapy Unit (Atomic Energy Ltd, Ottawa, Canada) at Amala Cancer Hospital, Thrissur, Kerala, India. The animals were kept immobilized in a specially designed, well-ventilated cage without any anesthesia and exposed to whole-body radiation at a rate of 1.41 Gy/minute. The radiation field size was 25 × 25 cm2 and at a distance of 80 cm from the source.
Isolation of PPC-Pr
Fruiting bodies of P. rimosus were collected from the outskirts of Thrissur, Kerala, India. The voucher specimen was deposited in the Herbarium of Centre for Advanced Studies in Botany, University of Madras, Chennai, India (HERB MUBL 3171). PPC-Pr was isolated from the aqueous extract of P. rimosus by the method described by Meera et al.Citation15–Citation17
Estimation of in vitro DNA damage
Alkaline single-cell gel electrophoresis was performed using the method by SinghCitation18 with minor modifications. Quantification of DNA strand breaks of the stored images was done by the imaging software CASP, by which the percentage DNA in tail, tail length, tail moment, and olive tail moment could be obtained directly.Citation19 The tail length in the comet indicated the extent of damage, because the smaller molecule will move faster on the agarose gel. The tail moment was a commonly accepted unit of DNA damage that normalizes the difference in the nucleus studied. For olive tail moment, distance of center of gravity of DNA is considered instead of usual tail length.Citation20,Citation21
Assay of antioxidant activity
The animals were divided into five groups. First group was given distilled water for five consecutive days and served as normal. The second group was exposed to a single dose of whole-body gamma rays (4 Gy) and served as a control. Third group was injected with the standard drug amifostine (300 mg/kg body weight (bwt), intraperitoneally (i.p.)) 1 hour before whole-body irradiation (4 Gy) and was kept as a positive control. The fourth and fifth groups were injected with PPC-Pr (5 and 10 mg/kg bwt, i.p., respectively) for 5 days consecutively and irradiated with 4 Gy, 1 hour after the last dose of PPC-Pr administration. Animals were sacrificed 1 day after irradiation by cervical decapitation. Liver and brain tissues were quickly excised, washed with saline (0.89%), and blotted with a piece of filter paper. Liver and brain homogenate (10%) was prepared in phosphate buffer (50 mM/l, pH 7.0) and centrifuged at 4°C and 1200 rpm for 30 minutes. The supernatant was used for the analysis. Blood was collected by heart puncture and serum was separated.
Antioxidant enzyme assays were carried out in tissue homogenate as well as in serum, using a double beam spectrophotometer (Systronics India Ltd, Hyderabad, India.). Reduced glutathione content (GSH) was assayed by the method of Moron et al.Citation22 based on the reaction with DTNB to produce a yellow-colored complex. Glutathione peroxidase (GPx) was assayed by the method of Hafeman et al.Citation23 based on the decrease in GSH content after incubating the sample in the presence of H2O2 and NaN3. Activity of enzyme superoxide dismutase (SOD) was assayed from the ability of the sample to scavenge superoxide anion generated from the photo illumination of riboflavin according to the method of Mc Cord and Fridovich.Citation24 The activity of CAT in tissue was estimated by the method of AebiCitation25 and calculated using the molar extinction coefficient of H2O2 (43.6 M−1 Cm−1) and expressed in millimoles of H2O2 decomposed/minute/mg protein (U/mg protein). The level of lipid peroxidation was measured as TBARS (thiobarbituric acid reactive substances) and is expressed in terms of malondialdehyde equivalents formed using 1′1′3′3′-tetramethoxypropane as standard.Citation26 Activity of GR in serum was assayed using the method of RackerCitation27 where the amount of reduced form of NADP consumed during the conversion of oxidized glutathione to GSH was measured. Protein content in tissue and serum was determined using Folin's phenol reagentCitation28 and compared to bovine serum albumin standard.
Survival studies
The effect of administration of PPC-Pr on survival of irradiated mice was investigated. The mice were divided into five groups having 10 animals each.
(i) Normal 0 Gy.
(ii) Irradiated control 9 Gy.
(iii) Amifostine (300 mg/kg bwt, i.p.) administered 1 hour before irradiation of 9 Gy.
(iv) PPC-Pr (5 mg/kg bwt, i.p.) administered daily for five consecutive days and irradiated with 9 Gy, 1 hour after the last dose.
(v) PPC-Pr (10 mg/kg bwt, i.p.) administered daily for five consecutive days and irradiated with 9 Gy, 1 hour after the last dose.
Statistical analysis
All experimental data were expressed as mean ± SD. The data were analyzed by one-way analysis of variance followed by Bonferroni's multiple comparison test (using the Graph Pad Instat Software Package). A P value <0.05 was considered as significant.
Results
DNA protective effects of PPC-Pr
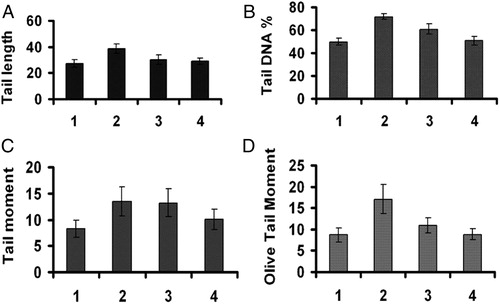
Gamma radiation (4 Gy) resulted in an increase in the comet parameters (such as tail length, %DNA in tail, tail moment, and olive tail moment) of blood cells as a result of damage to cellular DNA. When cells were exposed to gamma radiation (4 Gy), tail length was increased from 27.30 ± 2.73 to 38.56 ± 3.54, %DNA in tail was increased from 49.77 ± 3.06 to 71.88 ± 2.53, tail moment was increased from 8.23 ± 1.64 to 13.49 ± 2.70, and olive tail moment was increased from 8.70 ± 1.74 to 17.08 ± 3.42 in blood cells. However, PPC-Pr at a concentration of 100 µg brought down tail length, %DNA in tail, tail moment, and olive tail moment to 28.96 ± 2.5, 50.80 ± 3.8, 10.08 ± 2.01, and 8.88 ± 1.27, respectively, at 4 Gy of irradiation. Thus administration of PPC-Pr inhibited the increase of these parameters significantly indicating DNA protective effects of PPC-Pr ().
In vivo antioxidant assay
Significant decrease (P < 0.05) in antioxidant enzymes such as GSH, GPx, SOD, CAT, GR were observed in the irradiated control group 24 hours after irradiation when compared to normal ( and and ). It was also observed that PPC-Pr treatment (10 mg/kg bwt) significantly elevated (P < 0.001) antioxidant enzymes CAT and GSH when compared to control. PPC-Pr at both the concentrations (5 and 10 mg/kg bwt) as well as amifostine effectively improved (P < 0.05) antioxidant enzymes activity such as SOD, GPx, and GR in irradiated animals. Further, PPC-Pr isolated from P. rimosus exhibited dose-dependent increase in the activity of antioxidant enzymes consequent to the radiation-induced oxidative stress.
Figure 2. Effect of administration of PPC-Pr on tissue GSH levels of irradiated mice. Values are mean ± SD, n = 6, ***P < 0.001, *P < 0.05 compared to irradiated control (Bonferroni test); a, denotes P < 0.001 compared to normal (Bonferroni test).
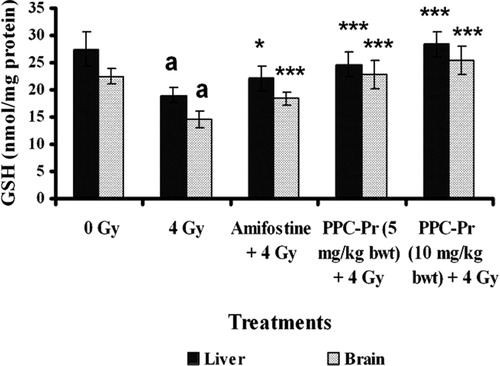
Figure 3. Effect of administration of PPC-Pr on GR activity in the serum of irradiated mice. Values are mean ± SD, n = 6, ***P < 0.001 compared to irradiated control; a, denotes P < 0.001 compared to normal (Bonferroni test).
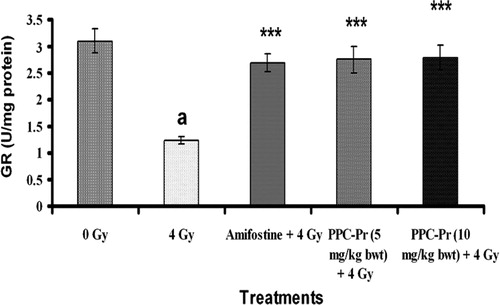
Table 1. Effect of administration of PPC-Pr on antioxidant enzyme activity in liver and brain tissues of irradiated mice (4 Gy)
In vivo lipid peroxidation
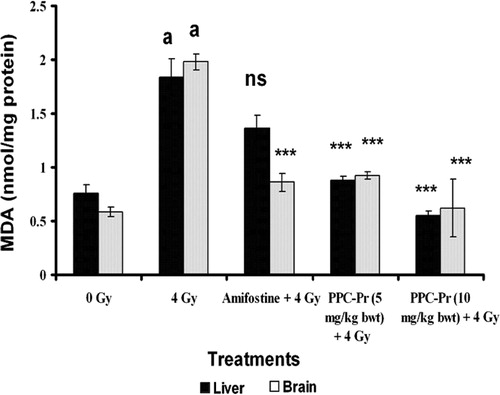
Tissue TBARS level was increased significantly (P < 0.001) in the irradiated control mice when compared to normal. There was 3.2 and 1.9-fold increase in TBARS level in brain and liver homogenate, respectively. PPC-Pr at both the concentrations (5 and 10 mg/kg bwt) offered significant improvement in the inhibition of TBARS level. Further, dose-dependent decrease in TBARS level was observed in both the treated group when compared to irradiated control ().
Survival studies
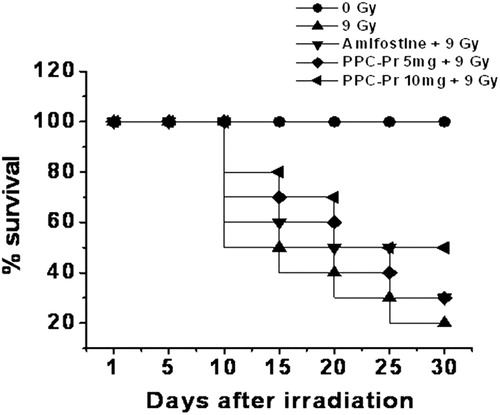
Animals were observed up to 30 post-irradiation days. The survival rate in the irradiated control group was found to be 30% on 15th post-irradiation day, while PPC-Pr at 5 and 10 mg/kg bwt offered 50 and 60% survival. The survival rate of animals after 30th post-irradiation day was found to be 20% in the untreated control group. PPC-Pr at 10 mg/kg bwt offered 40% survival on 30th post-irradiation day ().
Discussion
Ionizing radiation has become an integral part of modern medicine. It is used for diagnostic as well as therapeutic purposes. Low linear energy transfer radiations act by generating ROS. However, it causes severe oxidative stress and causes excessive accumulation of reactive oxidant species such as superoxide (O2•−) and hydroxyl radical (OH•) resulting in increased lipid peroxidation in irradiated animals. Thus radiation exposure induces delayed oxidative stress that ultimately determines survival and death. The supplement of non-enzymatic free radical scavengers, such as naturally occurring polysaccharides that stimulate antioxidant defense for the restoration of redox balance can be exploited for radioprotection processes.
Polysaccharides protect animals against radiation through the elevation of endogenous production of antioxidants, which may reduce the oxidative damage via induction of mitochondrial enzymes and other scavenging proteins.Citation29,Citation30 It had been reported that the association of polysaccharides with proteins potentiate free radical scavenging activity.Citation31 PPC-Pr complex was found to contain 52% carbohydrate and 28% protein by phytochemical analysis.Citation17 This complex contains only d-glucose as a carbohydrate moiety as evident from high-performance liquid chromatography analysis of acid hydrolyzed PPC-Pr complex (unpublished observation). In the present study, it had been observed that the activities of SOD, catalase, and GPx were stimulated in the irradiated mice with PPC-Pr treatment. Further, survival studies revealed that pre irradiation administration of PPC-Pr complex at 5 and 10 mg/kg bwt rendered 30 and 40% survival, respectively, against 9 Gy whole-body irradiation on 30th post-irradiation day. Irradiated control mice suffered 80% mortality within 30 days. Therefore, survival of animals by PPC-Pr treatment reflects its wholesome effect of scavenging of radiation-induced free radicals by the enhanced antioxidant status in the treated group. Further, polysaccharides isolated from medicinal mushrooms act as immunopotentiator and enhance immune status in the body by a variety of mechanisms including production of immune mediators like cytokines.Citation14 Hence, they are excellent agents against radiation-induced immunosuppression. Polysaccharides, especially beta-glucan, are considered to be responsible for their biological activity.
In vitro DNA damage was assessed by alkaline comet assay. It is a sensitive method by which DNA strand breaks at a single-cell level can be monitored. It has been widely used to study the genotoxicity and DNA lesions such as single strand breaks, double strand breaks, alkali labile sites, DNA protein cross links, DNA repair, and apoptosis induced by environmental and chemical agents.Citation32 The alkaline single-cell electrophoresis analysis of irradiated peripheral blood leukocytes increased comet parameters indicating radiation-induced damage like formation of alkali labile sites, DNA single strand, and double strand breaks. The decrease in comet parameters after PPC-Pr treatment indicates radioprotecting property of PPC-Pr complex. The PPC-Pr complex through the free radical scavenging property protected the genomic DNA from the ROS-induced strand breaks.
In our present study, amifostine was used as a positive control to evaluate radioprotective activity of polysaccharide. Amifostine is a Food and Drug Administration approved radioprotector used clinically and is considered as ‘gold standard’ for radioprotection. It is considered as a prototype pharmacologic radioprotector that functions via free radical scavenging mechanism. The side effects associated with amifostine like hypotension, nausea, vomiting, etc. limits its clinical usefulness.Citation33 Polysaccharides are biological response modifiers and cause no harm and additional stress on the body. Different polysaccharide preparations such as protein-bound polysaccharide isolated from Coriolus versicolor referred to as PSK (polysaccharide K) and PSP (polysaccharide P) have been used as chemotherapeutic agents in the treatment of cancer in Asia for over 30 years.Citation34,Citation35 P. linteus polysaccharides had been reported to increase the production of immune mediators such as interleukin-1 in mice.Citation36
However, when using an antioxidant or a radioprotective compound, its inherent toxicity should be evaluated. For this purpose, the toxicity of PPC-Pr was evaluated using male Swiss albino mice. Acute toxicity studies showed that PPC-Pr produced no mortality up to 100 mg/kg bwt (i.p.). Further, sub-acute toxicity studies with PPC-Pr showed no hematological toxicity. Blood parameters, liver function and kidney function tests, as well as histopathology were also showed no significant change in the treated groups (unpublished).
Conclusion
This study demonstrated that PPC-Pr complex isolated from mushroom P. rimosus at a concentration of 5 and 10 mg/kg bwt significantly protected against radiation-induced suppression of antioxidant status in both liver and brain tissues. PPC-Pr complex also protected against in vitro DNA damage as demonstrated by comet assay. Increased survival rate of radiation-exposed animals after PPC-Pr treatment further confirms radioprotective effect of PPC-Pr complex.
Acknowledgement
The authors sincerely thank Dr T.A. Ajith, Associate Professor, Amala Institute of Medical Sciences, Thrissur, Kerala, India for his valuable suggestions.
References
- Levinson W. Toxic effects of X-irradiated medium on chick embryo cells. Exp Cell Res 1966;43:398–413.
- Walburg HE. Radiation-induced life-shortening and premature aging. In: , Lett JT, Adler DH, (eds.) Advances in radiation biology. New York: Academic; 1975. p. 145–79.
- Singh A, Singh H, Henderson J. Radioprotection of mice with ascorbic acid, desferal and mercaptoethylamine. In: , Simic MG, Taylor KA, Ward JF, Von Sonntag C, (eds.) Oxygen radicals in biology and medicine. New York: Plenum: 1989. p. 587–90.
- Agrawal A, Chandra D, Kale RK. Radiation induced oxidative stress: II studies in liver as a distant organ of tumor bearing mice. Mol Cell Biochem 2001;224:9–17.
- Sonntag CV. The chemical basis of radiation biology. London: Francis & Taylor; 1987. p. 36.
- Jong SC, Birmingham JM. Edible mushrooms in biotechnology. Proceedings of Asian Mycology Symposium. Seoul: Seoul National University; 1992. p. 899–900.
- Leelavathy KM, Ganesh PN. Polypores of Kerala. India: Daya Publishing House; 2000. p. 50–60.
- Ajith TA, Janardhanan KK. Antioxidant and anti-inflammatory activities of methanolic extract of Phellinus rimosus (Berk) Pilat. Ind J Exp Biol 2001;39:1166–9.
- Ajith TA, Janardhanan KK. Antioxidant and antihepatotoxic activities of Phellinus rimosus (Berk) Pilat. J Ethanopharmacol 2002;81:387–91.
- Ajith TA, Janardhanan KK. Cytotoxic and antitumor activities of a polypore macro fungus, Phellinus rimosus (Berk) Pilat. J Ethnopharmcol 2003;84:157–62.
- Lakshmi B, Tilak JC, Adhikari S, Devasagayam TPA, Janardhanan KK. Inhibition of lipid peroxidations induced by γ-radiation and AAPH in rat liver and brain mitochondria by mushrooms. Curr Sci 2005;88:484–8.
- Gao Y, Zhou S, Jiang W, Huang M, Dai X. Effect of ganopoly (a Ganoderma lucidum polysaccharide extract) on the immune functions in advanced – stage cancer patients. Immunol Invest 2003;32:201–15.
- Samchai S, Seephonkai P, Sangdee A, Puntumchai A, Klinhom U. Antioxidant, cytotoxic and antimalarial activities from crude extracts of mushroom Phellinus linteus. J Biol Sci 2009;9:778–83.
- Gi-Su-Oh , Myeong-Su Lee, Hyun-OK Pae, Joseph Kwon, Sang-Soo Lee, Jong-Gil Jeong. Effects of oral administration of P. linteus on production of Th-1 and Th-2 type cytokines in mice. Immunopharmcol Immunotoxicol 2006;28:281–93.
- Meera CR, Nitha B, Janardhanan KK, Vishwakarma RA. Anti-inflammatory and free radical scavenging activities of polysaccharide – protein complex isolated from Phellinus rimosus (Berk.) Pilat (Aphyllophoromycetideae). Int J Med Mushroom 2009a;11:365–73.
- Meera CR, Smina TP, Nitha B, Mathew J, Janardhanan KK. Antiarthritic activities of a polysaccharide – protein complex isolated from Phellinus rimosus (Berk.) Pilat (Aphyllophoromycetideae) in Freunds complete adjuvant induced arthritic rats. Int J Med Mushroom 2009b;11:21–8.
- Jini J, Smina TP, Janardhanan KK. Polysaccharide protein complex isolated from mushroom Phellinus rimosus (Berk.) pilat alleviates gamma radiation-induced toxicity in mice. Cancer Biother Radiopharm 2011;26:299–308.
- Singh NP. Microgels for estimation of DNA strand breaks, DNA-protein cross links and apoptosis. Mutat Res 2000;455:111.
- Konca K, Lankoff A, Banasik A, Lisowska H, Kuszewski T, Gozdz S. A cross platform public domain PC image analysis program for the comet assay. Mutat Res 2003;534:15–20.
- Maurya DK, Salvi VP, Nair CKK. Radioprotection of normal tissues in tumour-bearing mice by troxerutin. J Radiat Res 2004;45:221–8.
- Gandhi NM, Nair CKK. Radiation protection by diethyldithiocarbamate: protection of membrane and DNA in vitro and in vivo against γ radiation. J Radiat Res 2004;45:175–80.
- Moron MA, Depicrre JW, Mannervick K. Levels of glutathione, glutathione – S transferase activities in rat liver. Biochem Biophys Acta 1979;582:67–8.
- Hafeman DG, Sundae RA, Houestra WG. Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rat. J Nutr 1974;104:580–7.
- Mc Cord JM, Fridovich I. Superoxide dismutase enzymes function for erythrocuprein (hemocuprein). J Biol Chem 1969;244:6049–56.
- Aebi H. Enzyme activities: oxidoreductases. In: , Bergmeyer HV, (ed.) Methods in enzymatic analysis. New York: Verlag Chemic; 1974. p. 673–84.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxide in animal tissue by thiobarbituric acid reaction. Anal Biochem 1979;95:351–8.
- Racker E. Glutathione reductase (liver and yeast). In: , Sidney PC, Nathan OK, (eds.) Methods in enzymology. New York: Academic Press; 1955. p. 722–5.
- Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with Folin Wu reagent. J Biol Chem 1951;193:265–75.
- Chen L, Dong JX. Study of anti-irradiation effect of polysaccharides. Carcinogenesis, Teratogenesis Mutagenesis. 2004;6:56–70.
- Trommer H, Neubert HHR. Screening for new antioxidative compounds for topical administration using skin lipid model systems. J Pharm Pharm Sci 2005;8:494–506.
- Staub AM. Removal of protein-Sevag method. Meth Carbohydr Chem 1965;5:5–6.
- Chaubey RC, Bhilwade HN, Rajagopalan R, Bannur SV. Gamma ray induced DNA damage in human and mouse leucocytes measured by SCGE-Pro: software developed for automated image analysis and data processing for comet assay. Muat Res 2001;4900:187–97.
- Devita VT. Cancer: principles and practice of oncology. Philadelphia: Lippincott- Raven; 1997. p. 3087–92.
- Kidd PM. The use of mushroom glucans and proteoglycans in cancer treatment. Altern Med Rev 2000;5:4–27.
- Fisher M, Yang LX. Anticancer effects and mechanism of polysaccharide-k (PSK); implications of cancer immunotherapy. Anticancer Res 2002;22:1737–54.
- Kim GY, Roh SI, Park SK. Alleviation of experimental septic shock in mice by acidic polysaccharide isolated from medicinal mushroom Phellinus linteus. Biol Pharm Bull 2003;26:1418–23.