Abstract
The present study was undertaken to evaluate the effect of the aqueous extract of Podophyllum hexandrum against free radical-mediated damage and also explore its anticancer activity. The extract exhibited significant activity in scavenging 1, 1-diphenyl-2-picryl-hydrazyl radicals, •OH radical-mediated DNA damage, and lipid peroxide production in rat liver microsomes. The extract was also tested for its reducing abilities. The activity of liver marker enzymes and antioxidant defense enzymes in rat liver homogenate was assessed in control and carbon tetrachloride (CCl4)-treated animals. It was observed that CCl4-induced changes viz., increases in the activities of aspartate aminotransferase, alanine aminotransferase, and lactate dehydrogenase, a decrease in reduced glutathione as well as decreases in the activities of superoxide dismutase, catalase, glutathione peroxidase, glutathione reductase, and glutathione-S-transferase. All these parameters showed reversal when pretreated with aqueous extract of P. hexandrum. Podophylotoxin and etoposide are the two known anticancer agents derived from P. hexandrum and interestingly the aqueous extract of P. hexandrum showed a typical DNA ladder formation in HL-60 cells confirming its role as an inducer of apoptosis. The results obtained suggest that the plant extract exhibits inhibition of and free radical production and lipid peroxidation, increase in antioxidant enzyme activities, revealing its antioxidant properties, and is also able to show potent anticancer activity as depicted by its ability to cause fragmentation of DNA.
Introduction
Antioxidant research is an important area in the field of medicine and food industry. Recent research with important bioactive compounds in many plant and food materials has received significant attention. The oxidation induced by reactive oxygen species (ROS) has been reported to result in cell membrane disintegration, membrane protein damage, and DNA mutation, which is implicated in many diseases, such as cancer, liver injury, and cardiovascular disease.Citation1 Although the body possesses defense mechanisms, which arrest the damaging properties of ROS,Citation2 continuous exposure to chemicals and contaminants may lead to an increase in the amount of free radicals in the body beyond its capacity to control them and cause irreversible oxidative damage.Citation3 Therefore, antioxidants with free radical scavenging activities may have greater relevance in the prevention of diseases in which oxidants or free radicals are implicated.Citation4 In this respect, polyphenolic compounds, like flavonoids and phenolic acids, commonly found in plants have been reported to have multiple biological effects, including anticancer and antioxidant activities.Citation5,Citation6 It is generally assumed that frequent consumption of plant-derived phytochemicals from vegetables, fruits, tea, and herbs may contribute to shift the balance towards an adequate antioxidant status.Citation7 Plants have played considerable contribution towards the development of anticancer drugs. Over 60% of currently used anticancer agents are derived in one way or another from natural sources, including plants. Thus, interest in natural antioxidants and anticancer agents, especially of plant origin, has greatly increased in recent years.Citation8
Liver is the major site responsible for the metabolism of xenobiotics. Carbon tetrachloride (CCl4) has been widely used as an agent to induce acute liver injury damage,Citation9 as it is reductively dehalogenated by the cytochrome P450 enzyme producing highly reactive free radical trichloromethyl radical.Citation10 The main objective of the present investigation was to evaluate the effects of an aqueous extract of Podophyllum hexandrum against free radical-mediated damages under in vitro and in vivo conditions and to monitor its effect on the genomic DNA of human promyelocytic leukemia cells to invoke its role as an anticancer agent. In vitro assays like 1, 1-diphenyl-2-picryl-hydrazyl (DPPH) radical scavenging activity, •OH-mediated DNA damage, reducing power, and lipid peroxidation in rat liver microsomes were carried out. Liver toxicity was induced by administrating a single dose of CCl4 into experimental adult male albino rats and changes in biochemical and antioxidants markers in the presence or absence of plant extract was evaluated.
P. hexandrum, an erect flabrous, succulent herb, 35–60 cm high with perennial rhizome bearing numerous roots, is found in the inner range of Himalayas from Kashmir to Sikkim at an altitude of 3000–4200 m. P. hexandrum belongs to family Berberidaceae and in Kashmir valley, it is locally known as Banwangon. The dried rhizomes and roots of this plant form the source of medicinal activity. Podophyllin, a resin is commonly used as a purgative agent. Podophyllin is considered a cholagogue, purgative, emetic, and a tonic. The rhizome powder is used as laxative or to get rid of intestinal worms and also used as poultice to treat warts and tumorous growths on the skin.
Materials and methods
Plant material collection and extraction
The rhizome of P. hexandrum was collected from higher reaches of Aharbal, Shopian, J&K, India, in the months of May and June 2009, identified by the Centre of Plant Taxonomy, Department of Botany, University of Kashmir. A reference specimen has been retained in the herbarium of the Department of Botany at the University of Kashmir under reference number KASH-bot/Ku/PH-702-SAG.
The plant material (rhizome) was dried in shade at 30 ± 2°C. The dried rhizome material was ground into a powder using mortar and pestle and passed through a sieve of 0.3 mm mesh size. The powder obtained was extracted with water using a Soxhlet extractor (60–80°C). The extract was then concentrated with the help of rotary evaporator under reduced pressure and the solid extract was stored in refrigerator for further use.
Animals
Adult male albino rats of Wistar strain weighing 200–250 g used throughout this study were purchased from the Indian Institute of Integrative Medicine Jammu (IIIM-CSIR). The animals had access to food and water ad libitum. The animals were maintained in a controlled environment under standard conditions of temperature and humidity with an alternating 12-hour light-and-dark cycle. Other conditions of maintenance were maintained in accordance with the guidelines prescribed by the National Institute of Nutrition, Indian Council of Medical Research. The study was approved by the Ethical Committee of the University of Kashmir.
Experimental methods
DPPH radical scavenging activity
The DPPH assay was performed by using the method of Braca et al.Citation11 Various concentrations of plant extract (100–800 µg/ml) were added to 1 ml of the 0.004% methanol solution of DPPH, and the mixture was vortexed vigorously. The tubes were then incubated at room temperature for 30 minutes in dark, and the absorbance was measured at 517 nm. Lower absorbance of the reaction mixture indicates higher free radical scavenging activity. Alpha tocopherol was taken as a known free radical scavenger. Percentage scavenging activity was calculated by using the formula:where A0 was the absorbance of the control and A1 was absorbance in the presence of P. hexandrum extract/known antioxidant.
Assessment of hydroxyl radical scavenging property
Hydroxyl radical, generated from the Fe3+-ascorbate-H2O2 (Fenton reaction), was evaluated by degradation of deoxyribose that produced thiobarbituric acid reactive species (TBARS).Citation12 The reaction mixture contained 25 mM deoxyribose, 10 mM ferric chloride, 100 mM ascorbic acid and 2.8 mM H2O2 in 10 mM KH2PO4 (pH 7.4), and various concentrations of P. hexandrum rhizome aqueous extract. The reaction mixture was incubated at 37°C for 1 hour. Then 1 ml of 1% thiobarbituric acid and 1 ml of 3% trichloroacetic acid were added and mixture heated at 100°C for 20 minutes. The TBARS was measured spectrophotometrically by taking absorbance at 532 nm. The results were expressed as percentage inhibition of deoxyribose oxidation, as determined by the following formula:where A was the TBARS produced by Fenton reaction treated alone, and B was the TBARS produced in the presence of P. hexandrum extract/known antioxidant.
Reducing power
The reducing power of P. hexandrum rhizome extract was evaluated according to Oyaizu.Citation13 Different concentrations of the plant extract were mixed with 2.5 ml of 0.2 M phosphate buffer (pH 6.6), and 2.5 ml of 1% potassium hexacyanoferrate II. The mixture was incubated at 50°C for 20 minutes, and after an addition of 2.5 ml of 10% tricarboxylic acid, was centrifuged at 3000 rpm for 10 minutes. The upper layer of the solution (2.5 ml) was mixed with distilled water (2.5 ml) and FeCl3 (0.5 ml, 0.1%), and the absorbance was measured at 700 nm. Butylated hydroxyl toluene (BHT) was taken as the known standard. The percentage reduction of the sample as compared with BHT was calculated by using formula:where AC is absorbance of the standard at maximum concentration tested and AS is absorbance of the sample.
Dosage and treatment
Rats were divided into six groups each containing seven rats. The plant extract was administered at oral doses of 20, 30, and 50 mg/kg-day. The extract was suspended in normal saline such that the final volume of extract at each dose was 1 ml which was fed to rats by gavage.
Group I – Received olive oil vehicle only at 5 ml/kg body weight for 15 days.
Group II – Received CCl4 in corn oil vehicle only.
Group III – Were administered with vitamin E (50 mg/kg body weight) 15 days.
Group IV – Received 20 mg/kg-day extract orally for 15 days.
Group V – Received 30 mg/kg-day extract orally for 15 days.
Group VI – Received 50 mg/kg-day orally for 15 days.
On the 13th day, animals from Groups II–VI were injected intraperitoneally (i.p.) with CCl4 in olive oil vehicle at a dosage of 1 ml/kg body weight. The rats were sacrificed 48 hours after CCl4 administration, the livers were collected and the post-mitochondrial supernatant (PMS) of the tissue was then prepared.
Blood collection for estimation of aspartate aminotransferase, alanine aminotransferase, and lactate dehydrogenase
Before sacrificing the experimental animals, blood was collected from retro-orbital plexus without the use of anticoagulant. The blood was allowed to stand for 10 minutes before being centrifuged at 5000 g for 10 minutes to obtain serum for analysis of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and serum lactate dehydrogenase (LDH).
Serum ALT and AST
ALT and AST were estimated following the method of Reitman and Frankel.Citation14
Serum LDH
LDH was assayed according to the method of King and Van.Citation15
Preparation of PMS
Liver tissue was washed in ice-cold 1.15% KCl and homogenized in a homogenizing buffer (50 mM Tris-HCl, 1.15% KCl pH 7.4) using a Teflon homogenizer. The homogenate was centrifuged at 9000 g for 20 minutes to remove debris. The supernatant was further centrifuged at 15 000 g for 20 minutes at 4°C to get PMS, subsequently used for various biochemical assays.
Protein estimation
Protein concentration was estimated by the method of Lowry et al.Citation16
Estimation of lipid peroxidation
Lipid peroxidation in liver tissue homogenate was estimated by the formation of thiobarbituric acid reactive species (TBARS) by the method of Nichans and Samuelson.Citation17
Glutathione peroxidase activity
Glutathione peroxidase (GPx) activity was assayed using the method of Sharma et al.Citation18 Oxidation of NADPH was recorded spectrophotometrically at 340 nm and the enzyme activity was calculated as nmoles of NADPH oxidized/minute/mg of protein, using extinction coefficient of 6.22 × 103/M/cm.
Glutathione reductase activity
Glutathione reductase (GR) was assayed by the method of Sharma et al.Citation18 The enzyme activity measured as absorbance at 340 nm was calculated as nmoles of NADPH oxidized/minute/mg of protein using extinction coefficient of 6.22 × 103/M/cm.
Glutathione-S-transferase activity
Glutathione-S-transferase (GST) was assayed using the method of Haque et al.Citation19 The change in absorbance was recorded at 340 nm and the enzyme activity was calculated as nmoles of CDNB conjugates formed/min/mg protein using extinction coefficient of 9.6 × 103/M/cm.
Catalase activity
Catalase (CAT) was assayed by the method of Claiborne.Citation20 Change in absorbance was recorded at 240 nm. CAT activity was calculated in terms of nmoles of H2O2 consumed/minute/mg of protein.
Superoxide dismutase activity
Superoxide dismutase (SOD) activity was estimated by the method of Beauchamp and Fridovich.Citation21 Units of SOD activity were expressed as the amount of enzyme required to inhibit the reduction of NBT (Nitroblue tetrazolium) by 50%.
DNA agarose gel electrophoresis for evaluating DNA fragmentation
HL-60 cells (2 × 106 cells/well/2 ml) were grown in six-well plates and treated with indicated concentrations of plant extract. After treatments, cells were centrifuged at 1500 rpm for 10 minutes, and washed in phosphate-buffered saline. The pellet was lysed in 250 µl of lysis buffer (100 mM NaCl, 5 mM EDTA, 10 mM Tris-HCl, pH 8.0, 5% Triton X-100) containing (200 µg/ml) proteinase-K and incubated at 50°C for 1 hour followed by 90 minutes incubation with 400 µg/ml DNase-free RNase. The DNA was extracted with 100 µl phenol: chloroform: isoamyl alcohol (25:24:1) and centrifuged. DNA was precipitated from aqueous phase with three volumes of chilled alcohol and 0.3 M sodium acetate at 20°C overnight. The precipitate was centrifuged at 13 000 × g for 10 minutes. The DNA pellet was washed in 80% alcohol, dried, dissolved in 50 µl TE buffer, mixed in loading buffer, and electrophoresed in 1% agarose gel at 80 V for 1.5 hours in TAE buffer.Citation22
DNA nicking assay
DNA nicking assay was performed using supercoiled pBR322 DNA by the method of Lee et al.Citation23 A mixture of 5 µl of plant extract of different concentrations (20–80 mg/ml) and plasmid DNA (0.5 µg) was incubated for 10 minutes at room temperature followed by the addition of 10 µl of Fenton's reagent (30 mM H2O2, 50 mM ascorbic acid, and 20 mM FeCl3). The final volume of the mixture was made up to 20 µl and incubated for 30 minutes at 37°C. DNA was analyzed on 1% agarose gel using ethidium bromide staining.
Statistical analysis
The values are expressed as mean ± standard deviation (SD). The results were evaluated by using the SPSS (version 12.0) and Graphpad Prism 5 softwares and evaluated by one-way analysis of variance followed by Dunnett's test. Statistical significance was considered when value of P was <0.05.
Results
DPPH radical scavenging activity
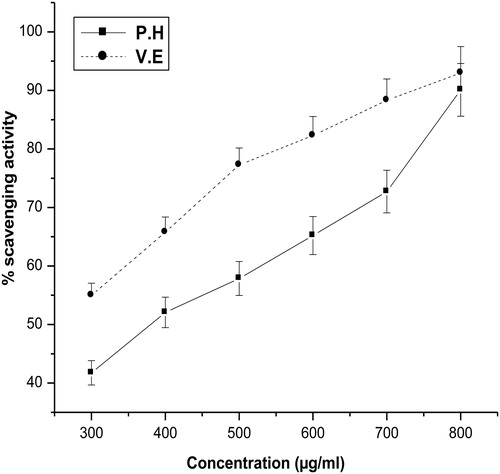
The DPPH radical scavenging activity of aqueous extract of P. hexandrum was performed as mentioned in Materials and methods. DPPH antioxidant assay is based on the ability of DPPH, a stable free radical, to decolorize in the presence of antioxidants. It is accepted that the DPPH free radical scavenging by antioxidants is due to their hydrogen donating ability.Citation24 As shown in , the scavenging of DPPH radicals increased with increasing concentrations of the plant extract. The scavenging effect of the extract on DPPH radical was 90% at a concentration of 800 µg/ml. At the same concentration the scavenging activity of vitamin E (a known antioxidant) was highly comparable.
Hydroxyl radical scavenging activity
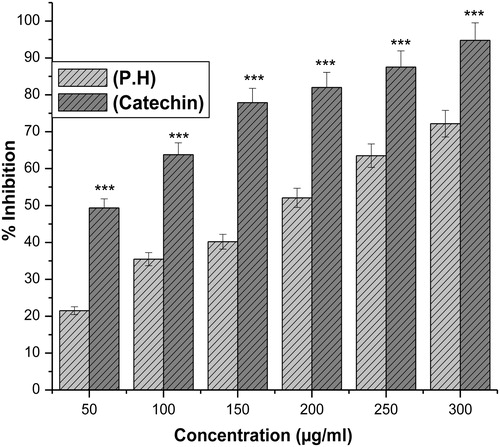
The hydroxyl radical an extremely reactive free radical formed in biological systems, and has been implicated as a highly damaging species in free radical pathology, capable of damaging the biomolecules of living cells. The hydroxyl radical scavenging activity of an aqueous extract of P. hexandrum was investigated using the Fenton reaction in which deoxyribose is degraded into malonaldehyde on exposure to hydroxyl radicals generated by Fenton systems and was detected by its ability to react with thiobarbituric acid to form a pink chromogen.Citation12 The hydroxyl radical scavenging activity of the extract increased in a concentration-dependent manner. The extract showed a dose-dependent scavenging activity which was comparable to that of catechin, a known hydroxyl radical scavenger ().
Reducing power of the extract
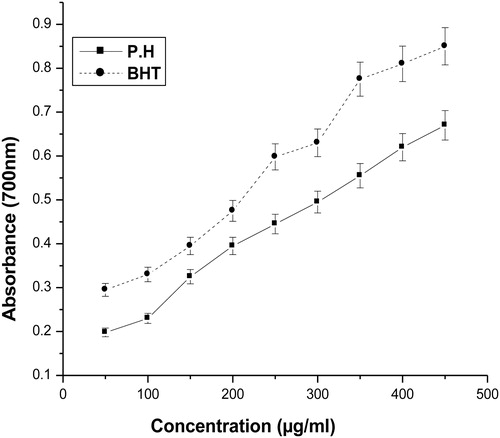
For the measurement of the reductive ability, it has been previously postulated that the Fe3+–Fe2+ transformation occurs in the presence of certain plant extracts.Citation13 Also earlier authorsCitation25 have observed a direct correlation between antioxidant activities and reducing power of some plant extracts. In the present study, it was found that the reducing powers of the plant extract and BHT on Fe3+ were concentration dependent. The reducing power increased with increasing concentration of P. hexandrum aqueous extract (). A comparable effect was seen when BHT was used as a positive control.
Effect of extract on hepatic markers
As evidenced by the significant elevation of the serum AST, ALT, and LDH levels () i.p. administration of CCl4 induced severe hepatocellular damage on the animal models. However, elevations were attenuated in P. hexandrum pretreated rats, in a dose-dependent manner. The plant extract was found to work efficiently when administered at a 50 mg/kg-day dosage, the effect of the extract at this dose being comparable to that when vitamin E was used.
Table 1. Effects of P. hexandrum aqueous extract on biochemical parameters in CCl4-induced hepatotoxicity in albino rats
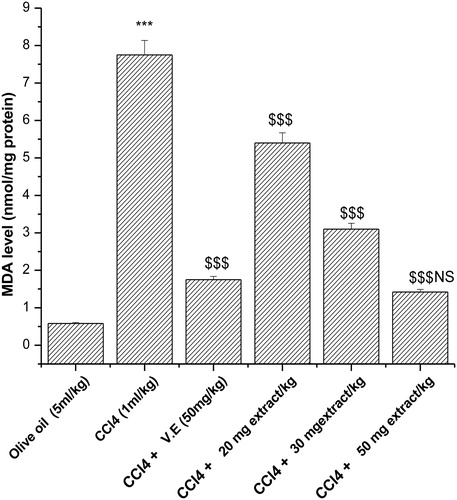
Effect of extract on malondialdehyde levels
Malondialdehyde (MDA) is one of the deleterious lipid peroxidative metabolites. Out of various concentrations of plant extract that were able to decrease the MDA levels following CCl4 administration, it was observed that at a higher concentration, i.e. 50 mg/kg-day P. hexandrum aqueous extract decreased the MDA level to 1.42 nmol/mg protein from 7.75 nmol/mg protein (), which is comparable with that of vitamin E, a known antioxidant, and decreased the MDA level to 1.75 nmol/mg protein from 7.75 nmol/mg protein.
Figure 4. Effect of aqueous extract of P. hexandrum on lipid peroxidation in CCl4-treated rats. V.E stands for vitamin E. Values represent mean ± SD of seven animals in each group. ***P < 0.001 when compared with olive oil only, $$$P < 0.001 when compared with the CCl4 group, NS, non-significant when compared with CCl4 + V.E.
Effect of extract on antioxidant enzyme activities
The activities of SOD in the tissue homogenate, of all experimental rats are shown in . As expected, treatment of the liver homogenate, with CCl4 caused a significant reduction in the SOD activity compared to the normal controls. There was, however, a concentration-dependent restoration of the enzyme activity in the extract-treated animals. Similar results were obtained with the antioxidant vitamin E.
Table 2. Effects of treatment of aqueous extract of P. hexandrum on antioxidant enzymes in CCl4-challenged rats
CAT activities in the liver homogenate of rats for all experimental groups are shown in . The CAT activity in the liver tissue homogenates of CCl4-treated rats was considerably lower than that of normal control rats. In the pretreated group, which got the aqueous extract for 15 days prior to CCl4, CAT activity was found to be significantly higher compared to that of the CCl4-treated group. Vitamin E treatment prior to CCl4 intoxication restored the activity of catalase to a large extent.
GST activity as measured from the liver tissue homogenate of all the experimental rats is shown in . Decreased GST activity was observed in CCl4-treated rats in comparison with that of the normal control group. Pretreatment with the aqueous extract for 15 days prior to CCl4 intoxication enhanced that activity significantly. GST activities in vitamin E and aqueous extract pretreated groups at concentrations of 50 mg/kg body weight were close to each other. Similar results were observed with GPx and GR activities.
DNA fragmentation (a hallmark of apoptosis induction) by methanolic extract of P. hexandrum
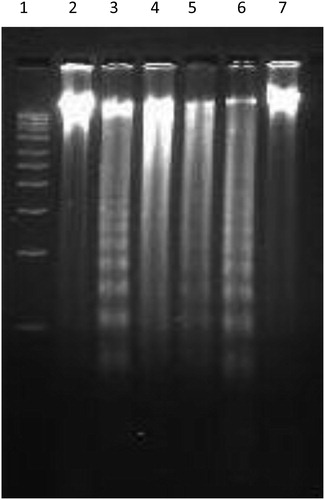
DNA fragmentation is a biochemical hallmark of apoptotic cell death which involves internucleosomal chromatin cleavage by endonucleases in multiples of 180 bp resulting in a typical DNA laddering. To investigate whether the aqueous extract of P. hexandrum had any effect on the DNA, genomic DNA was isolated from HL-60 cells, and treated with different concentrations of plant extract. Electrophoretic analysis of DNA showed that exposure of HL-60 cells with aqueous extract of P. hexandrum at 50 µM resulted in a characteristic fragmentation of DNA. This can be seen as a diffused band interspersed with smear, and is indicative of some post-apoptotic necrosis in HL-60 both at 70 and 100 µg/ml. Camptothecin, used as a positive control, showed fragmentation of DNA in HL-60 cells after treatment of cells with 5 µM Camptothecin for 6 hours ().
Figure 5. Aqueous extract of P. hexandrum induced DNA fragmentation in HL-60 cells. Cells 2 × 106/ml/well were treated with indicated conc. of plant extract for 24 hours. Genomic DNA was isolated and put to electrophoresis as described in the Materials and methods to assess the fragmentation at different concentrations. Lane 1, 1 kb ladder; Lane 2, –ve control (untreated cells); Lane 3, +ve control (camptothecin at 5 µM for 6 hours); Lanes 4–7, extract at 100, 70, 50, 30 µg/ml.
DNA nicking assay
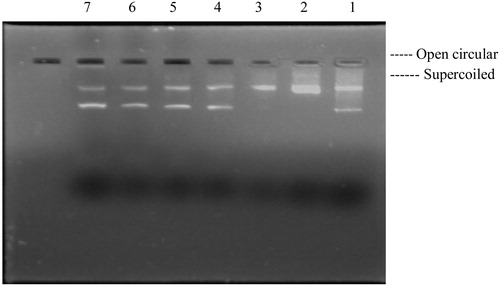
Hydroxyl radicals generated by the Fenton reaction are known to cause oxidatively induced breaks in DNA strands to yield its open circular (Oc DNA) or relaxed forms. The concentration-dependent (20–80 mg/ml) free radical scavenging effect of aqueous extract of P. hexandrum was studied () on plasmid DNA damage. The extract (lanes 4–7) showed significant reduction in the formation of nicked DNA and increased the native form of DNA. pBR322 plasmid showed two bands on agarose gel electrophoresis ( and lane 1) the faster-moving prominent band corresponded to the native supercoiled circular DNA (Sc DNA) and the slower-moving very faint band was the Oc DNA. The plasmid in the presence of H2O2/ferric chloride and ascorbic acid (, lane 2) resulted in the cleavage of Sc DNA to give prominent Oc DNA and a faint linear (Lin) DNA indicating that •OH generated from Fenton reaction produced DNA strand scission.
Discussion
In the last few years, there has been a growing interest in the study of involvement of ROS in several pathological situations. ROS include the superoxide radical (O2•–), hydrogen peroxide (H2O2), and hypochlorous acid (HOCl). H2O2 and O2•– can interact in the presence of certain transition metal ions to yield a highly reactive oxidizing species, the hydroxyl radical (•OH).Citation12 Phenolic compounds and flavonoids have been reported to be associated with antioxidative action in biological systems, acting as scavengers of singlet oxygen and free radicals.Citation26 Many naturally occurring triterpenoids exhibiting a good antioxidant and antiinflammatory activity have been isolated from various plants.Citation27 There is a growing interest in the study of natural polyphenolic components, owing to their biological activities viz., bactericidal, fungicidal, antiviral, cytotoxic, analgesic, antiinflammatory, anticancer, and antiallergic.Citation28
In our study, antioxidant effects of aqueous extract of P. hexandrum rhizome were measured through a variety of biological parameters and compared with some of the known antioxidants. Because of the well-known complexity of the phytochemicals, their antioxidant potential could not be evaluated by a single method. Therefore, enzymatic and non-enzymatic methods under in vitro and in vivo conditions were employed to evaluate the total antioxidant potential of plant extracts.
The use of DPPH radical provides an easy, rapid, and convenient method to evaluate the antioxidants and free radical scavenging properties under in vitro conditions.Citation29 The scavenging effect of our test extract on DPPH increased with increasing concentration probably by its hydrogen donating ability. Similar results were reported earlier, showing that the aqueous extract of carob pods reduces DPPH in a concentration-dependent manner.Citation30
Hydroxyl radical is an extremely reactive free radical formed in biological systems and has the capacity to cause DNA strand breakage, which contributes to its carcinogenesis, mutagenesis, and cytotoxicity.Citation31 The extract was examined for its ability to scavenge •OH radicals and it was seen that that the extract has the potential to scavenge free radicals generated by the Fenton reaction, which increased in a concentration-dependent manner but the effect was comparatively lesser than the scavenging activity of the positive control – catechin ().
The reducing properties were generally associated with the presence of reductones,Citation32 which have been shown to exert antioxidant action by donating the hydrogen atom generated by breaking of the free radical chain.Citation31 Reductones are also reported to react with certain precursors of peroxide, thus preventing peroxide formation. The reductive influence of an aqueous extract of P. hexandrum was found to be gradually increasing with the increasing concentration of the extract ().
The assessment of the antioxidant potential of an aqueous extract P. hexandrum was also extended to in vivo situations. CCl4, one of the most commonly used hepatotoxins in the experimental study of liver diseases, was used as an oxidant. The hepatotoxic effects of CCl4 are largely thought to be due to its active metabolite, trichloromethyl radical (•CCl3).Citation33 Lipid peroxidation due to (•CCl3) radical will initiate various pathological changes such as depression of protein synthesis,Citation34 elevation of serum marker enzymes such as AST, ALT, and LDH,Citation35 depletion of glutathione content, CAT, SOD, GPx, GR, and GST activity,Citation36 which further increase in lipid peroxidation. The aqueous extract at the dose of 20, 30, and 50 mg/kg, significantly decreased the levels of AST, ALT, and LDH in CCl4-treated rats indicating the maintenance of functional integrity of hepatic cell membrane (). MDA levels in tissue were found to be significantly elevated in CCl4-treated rats. This toxic effect is the consequence of CCl4 activation by cytochrome P450 to trimethyl radical (•CCl3) which readily reacts with oxygen to form trichloromethyl peroxyl radicals (CCl3O2•–).Citation37 The reduction in lipid peroxidation after treatment with the P. hexandrum extract may be attributed to the antioxidant activity of the plant through its scavenging of •CCl3 free radical. Similar results have been reported earlier where decrease in LPO (Lipid Peroxidation) in CCl4-treated rats has been attributed to the antioxidant activity of Chamomile recutita by scavenging the •CCl3 radical.Citation38 During the course of this present study, we also found a significant decrease in hepatic SOD, CAT, GPx, GR, and GST levels following CCl4 exposure. Aqueous extract of P. hexandrum treatment significantly increased the above-mentioned levels which clearly indicate the effect of extract in quenching the reactive intermediates and radical species generated during oxidative stress. These observations suggest that the administration of extract has significantly neutralized the toxic effects of CCl4 and probably helped in the regeneration of hepatocytes.
In order to further establish the biological significance of the antioxidant potential of an aqueous extract of P. hexandrum, its protective effect was assessed on DNA, which is one of the major targets of free radicals. Untreated plasmid DNA showed a major band corresponding to the supercoiled form. When pBR322 plasmid was dissolved in the nicking reaction mixture, within 30 minutes an increase in the formation of single-stranded nicked DNA, i.e. open circular form was observed. However, when the aqueous extract of P. hexandrum was added to the nicking reaction mixture, the formation of open circular form reduced. A dose-dependent increase in the supercoiled form, and almost complete loss of the hydroxyl radical was observed. So, the polyphenolic compounds present in the P. hexandrum aqueous extract, which was confirmed by total phenolic content, might be responsible for inhibiting DNA nicking.
In order to substantiate our findings about its role as an anticancer agent, the DNA fragmentation assay was performed on HL-60 cells. It is already reported that some of the derivatives of P. hexandrum like etoposide kill cancerous cells leaving the normal cells unaffected. In the present study, we observed that the aqueous extract of P. hexandrum did not stop the fragmentation of DNA in HL-60 cells at 50 µg/ml, but could stop it at concentrations of 70 and100 µg/ml. In the present study we observed that the aqueous extract of P. hexandrum caused fragmentation of DNA at 50 μg/ml followed by formation of smear at higher concentration.
Conclusion
The results of the study indicate that an aqueous extract of P. hexandrum possesses anticancer activity in vitro and antioxidant activity under both in vitro and in vivo conditions. In the present study, the results of P. hexandrum aqueous extract with the various in vitro antioxidant investigations proved that the plant possess reducing activity, has hydrogen-donating ability as well as effectiveness as scavengers of hydroxyl radical. Current study also demonstrates that aqueous extract could reduce CCl4-induced toxicity, particularly hepatotoxicity, by inhibiting lipid peroxidation, suppressing ALT, AST, LDH activities, and increasing antioxidant enzyme activity. Therefore, aqueous extract of P. hexandrum is hepatoprotective, and that effect might be correlated with its antioxidant and free radical scavenger activities. The present study also shows that aqueous extract of P. hexandrum rhizome possesses in vitro cytotoxicity effect on different human leukaemia cell line.
References
- Liao KL, Yin M. Individual and combined antioxidant effects of seven phenolic agents in human erythrocyte membrane ghosts and phosphatidylcholine liposome systems: importance of the partition coefficient. J Agric Food Chem 2000;48:2266–70.
- Halliwell B, Aeschbach R, Loliger J, Aruoma OI. The characterization of antioxidants. Food Chem Toxicol 1995;33:601–17.
- Tseng TH, Kao ES, Chu CY, Chou FP, Linwu HW, Wang CJ. Protective effects of dried flower extracts of Hibiscus sabdariffa L. against oxidative stress in rat primary hepatocytes. Food Chem Toxicol 1997;35:1159–64.
- Soares JR, Dinis TC, Cunha AP, Almeida LP. Antioxidant activities of some extracts of thymus zygis. Free Radic Res 1997;26:469–78.
- Gil MI, Ferreres FT, Barberan FA. Effect of postharvest storage and processing on the antioxidant constituents (flavonoids and vitamin C) of fresh-cut spinach. J Agric and Food Chem 1999;4:2213–17.
- Kahkonen MP, Hopia AI, Vuorela HJ, Rauha JP, Pihlaja KK, Heinonen TS. Antioxidant Activity of Plant Extracts Containing Phenolic Compounds. J Agric Food Chem 1999;4:3954–62.
- Halliwell B. Oxidative stress, nutrition and health. Experimental strategies for optimization of nutritional antioxidant intake in humans. Free Radic Res 1996;25:1–32.
- Jayaprakash GK, Rao LJ. Phenolic constituents from lichen parmontrema stuppeum. Food Control 2000;56:1018–22.
- Mizuoka H, Shikata N, Yang J, Takasu M, Inoue K, Tsubura A. Biphasic effect of colchicine on acute liver injury induced by carbon tetrachloride or by dimethylnitrosamine in mice. J Hepatol 1999;1:825–33.
- Recknagel RO. A new direction in the study of carbon tetrachloride hepatotoxicity. Life Sci 1983;33:401–8.
- Braca A, Tommasi DT, Bari LD, Pizza C, Politi M, Morelli I. Antioxidant principles from Bauhinia terapotensis. J Nat Prod 2001;64:892–5.
- Halliwell B, Gutteridge JM, Aruoma OI. The deoxyribose method: a simple ‘test tube’ assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem 1987;165:215–9.
- Oyaizu M. Studies on product of browning reaction prepared from glucose amine. Jpn J Nut 1986;44:307–15.
- Reitman S, Frankel AS. A colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminases. Am J Clin Path 1975;28:56–63.
- King JC, Van D (Ed.). The dehydrogenases or oxidoreductases – lactate dehydrogenase. In: Practical clinical enzymology. London: Nostrand Co; 1965. p. 83–93.
- Lowry OH, Rosenbrough NJ, Farr AI, Randall RJ. Protein estimation with the folin phenol reagent. J Biol Chem 1951;193:265–75.
- Nichans WG, Samuelson D. Formation of malondialdehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur J Biochem 1968;6:126–30.
- Sharma N, Trikha PA, Raisuddin SM. Inhibition of benzo[a]pyrene- and cyclophosphamide-induced mutagenicity by cinnamomum cassia. Mutat Res 2001;480–481:179–88.
- Haque RH, Parvez B, Pandey S, Sayeed S, Ali I, Raisuddin SM. Aqueous extract of walnut protects mice against cyclophosphamide induced biochemical toxicity. Hum Exp Toxicol 2003;22:473–80.
- Claiborne A, Greenwald RA (Ed.). Catalase activity. In: CRC handbook of methods of oxygen radicals research. Boca Raton: CRC; 1985. p. 283–4.
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gel. Anal Biochem 1971;44:276–87.
- Bhushan SS, Rao MJ, Saxena AK, Qazi GN. A novel lignin composition from Cedrus deodara induces apoptosis and early nitric oxide generation in human leukemia Molt-4 and HL-60 cells. Nitric Oxide 2006;14:72–88.
- Lee JC, Kim H, Kim R, Jang J. Antioxidant property of an ethanol extract of the stem of Opuntia ficus-indica var. Saboten. J Agric Food Chem 2002;50:6490–6.
- Chen CW, Ho CT. Antioxidant properties of polyphenols extracted from green and black tea. J Food Lipids 1995;2:35–46.
- Tanaka M, Kuie CW, Nagashima Y, Taguchi T. Applications of antioxidative Maillard reaction products from histidine and glucose to sardine products. Nippon Suisan Gakkaishi 1988;54:1409–14.
- Jorgensen LV, Madsen HL, Thomsen MK, Dragster LO, Skibsted LH. Regulation of phenolic antioxidants from phenoxyl radicals: an ESR and electrochemical study of antioxidant hierarchy. Free Radic Res 1999;30:207–20.
- Fernandez MA, De LH, Garcia BM, Saenz MD, Villar AT. New insights into the mechanism of action of the anti inflammatory triterpene lupeole. J Pharm Pharmacol 2003;53:1533–9.
- Patocka J. Biologically active pentacyclic triterpenes and their current medicine signification. J Appl Biomed 2003;1:7–12.
- Roginsky V, Lissi EA. Review of methods to determine chain-breaking antioxidant activity in food. Food Chem 2005;92:235–54.
- Shigenori K, Masa T, Yasuyuki S, Masayo S, Misun K, Tsutomu N. Antioxidant activities of polyphenols in carob pods. Agric Food Chem 2002;50:373–7.
- Gordon MH, BJF (Ed.). The mechanism of antioxidant action in vitro. In: Food antioxidants. Elsevier applied science. Amsterdam: Elsevier; 1990. p. 1–18.
- Duh PD, Tu YY, Yen GC. Antioxidant activity of the aqueous extract of harn jyur (Chrysanthemum morifolium Ramat). Lebensmittel-Wissenschaft Technologie 1999;32:269–77.
- Johnson DE, Kroening C. Mechanism of early carbon tetra chloride toxicity in cultured rat hepatocytes. Pharmacol Toxicol 1998;83:231–9.
- Faroon O, De RC, Smith LT. Carbon tetrachloride: health affects toxicokinetics, human exposure and environmental fate. Toxicol Ind Health 1994;10:4–20.
- Zimmerman HJ, Seeff LB. Enzymes in hepatic disease in diagnostic enzymology. Pharmacologyonline 1970;1:365–74.
- Kamiyama T, Sato C, Liu J. Role of lipidperoxidation in acetaminophen induced hepatotoxicity; comparison with carbon tetrachloride. Toxicol Lett 1993;66:7–12.
- Tappel AC. Lipid peroxidation damage to cell components. Fed Proc 1973;32:1870–4.
- Kalpowitz N, Aw TY, Simon FR, Stolz A. Drug induced hepatotoxicity. Ann Int Med 1986;104:826–39.