Abstract
Alcoholic liver disease is caused mainly by free radicals. Ascorbic acid (AA) and glutathione (GSH) are the major water-soluble antioxidants in the liver. The impact of AA supplementation on GSH, AA and activities of GSH-dependent enzymes in alcoholic guinea pigs was studied and was compared with alcohol abstention. Guinea pigs were administered ethanol at a dose of 4 g/kg body weight (b.wt)/day for 90 days. After 90 days, alcohol administration was stopped and one-half of the ethanol-treated animals were supplemented with AA (25 mg/100 g b.wt) for 30 days and the other half was maintained as the abstention group. There was a significant increase in the activities of alanine aminotransferase, aspartate aminotransferase, and gamma-glutamyl transpeptidase in the serum of the ethanol group. In addition, a significant decrease in the GSH content, activities of GSH peroxidase, GSH reductase, and increased activity of GSH-S-transferase were observed in the liver of the ethanol group. Histopathological analysis and triglycerides content in the liver of the ethanol group showed induction of steatosis. But AA supplementation and abstention altered the changes caused by ethanol. However, maximum protective effect was observed in the AA-supplemented group indicating the ameliorative effect of AA in the liver.
Introduction
It is well established that oxidative stress is one of the pathogenic mechanisms of alcoholic liver diseases. Oxidative stress is mainly caused by the generation of reactive oxygen species (ROS),Citation1 since physiological amounts of ROS are necessary for defence against infectious agents, cellular signalling systems, and induction of a mitogenic response.Citation2 Glutathione (GSH; l-glutamyl-l-cysteinyl-glycine), the most abundant antioxidant in liver cells, plays a major role in the defence against oxidative stress-induced cellular injury, and is essential for the maintenance of intracellular redox balanceCitation3 and the proper functioning of antioxidant enzymes. It is well documented that ethanol induces depletion of GSH content in the liver of alcoholics.Citation4,Citation5 In alcoholics, pools of mitochondrial GSH are depleted with the concomitant result that ROS damage can be exacerbated producing cell death and contributing to cirrhosis. The selective depletion of GSH in this organelle can sensitize hepatocytes to the oxidative effects of cytokines such as tumour necrosis factor.Citation6
High intracellular content of GSH in the liver is congruous with the detoxification functions of this organ. Depletion of GSH content in the liver can significantly disrupt liver function and in some instances can be conditionally lethal. Glutathione can exist intracellular in either an oxidized (GSSG) or reduced (GSH) state. Maintaining optimal GSH:GSSG ratios in the cell is critical to survival; hence, tight regulation of the system is imperative.Citation7 Production of GSH is considered as a crucial defence against oxidative damage and free radical generation.Citation8
Ascorbic acid (AA) plays an important physiological role in cells as a reducing agent, antioxidant, free radical scavenger, and enzyme cofactor and participates in multiple functions central to the physiology of cells.Citation9 Similarly, alcohol has been found to decrease the concentration of AA in rat and guinea pig livers, resulting in increased lipid peroxidation and liver injury.Citation10,Citation11
Previous studies reported that GSH deficiency was accompanied by a marked decrease in the tissue levels of AA, indicating either a role for GSH in the metabolism of AA or that AA is used in reactions that normally use GSH.Citation9 Earlier studies from our laboratory reported the beneficial effect of AA in ameliorating the toxicity induced by alcohol.Citation10,Citation12,Citation13 Hence, in the present study, we evaluated the effect of the supplementation of AA on the ethanol-induced impaired GSH metabolism in guinea pigs and compared it with the ethanol abstention group. The guinea pig was chosen as the animal model since, like man, it cannot synthesize AA due to the deficiency of the key enzyme gulonolactone oxidase and its metabolism of ethanol more closely resembles human alcohol metabolism than other animal species.Citation14 Hence, the results of this study can be easily extrapolated to humans.
Materials and methods
Animals
Male guinea pigs (Cavia porcellus) (NIH coloured) were purchased from Veterinary College, Mannuthy, Thrissur, Kerala, India. Weight-matched animals were selected (average initial weight of 400 ± 25 g) and housed individually in wire-netted cages with room temperature maintained at 25 ± 1°C and light and dark cycles of 12 hours. The study protocol was approved by our Institutional Animal Ethics Committee. Animals were handled using laboratory animal welfare guidelines. Guinea pigs were fed with guinea pig pellets (Sai Feeds Bangalore, India). Food and water were given ad libitum.
Experimental design
Animals (n = 36) were first divided into two groups and maintained for 90 days as follows: control (C) (n = 18) and ethanol group (E) (n = 18) (4 g/kg body weight (b.wt)/day). After 90 days, ethanol administration was stopped and six animals from both groups were killed after overnight fasting. Blood and liver samples were collected for biochemical analysis. The control group was used only to evaluate whether 90 days of ethanol feeding induced liver toxicity in ethanol (E) group by analysis of liver toxicity markers alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gamma glutamyl transpepetidase (GGT) in the serum (). All other parameters were analysed only in the ethanol group. The remaining animals in the control group were divided into two groups and maintained for 30 days as follows:
Table 1. Activities of ALT, AST, and GGT in serum after 90 days of ethanol feeding
Group I – Control (C) (n = 6) (normal diet)
Group II – Control + ascorbic acid (C + AA) (n = 6) (AA 25 mg/100 g b.wt/day)
The remaining animals in the ethanol group were divided into two groups and maintained for 30 days as follows:
Group III – Abstention (A) (n = 6) (normal diet)
Group IV – Ethanol + ascorbic acid (E + AA) (n = 6) (AA 25 mg/100 g b.wt/day).
This is schematically presented in .
Ethanol (Merck, Mumbai, India) diluted with distilled water (1:1) and AA (Sigma-Aldrich, St Louis, MO, USA) freshly dissolved in distilled water were given orally by an intragastric tube. These doses were selected from our previous studies.Citation12,Citation13 The activity of GGT was measured once in every week during the reversal period. After 30 days of treatment, all the animals were fasted overnight, anaesthetized with ketamine hydrochloride injection and sacrificed. The liver was removed and washed with phosphate-buffered saline, and transferred into ice-cold containers. Blood was collected in heparinized tubes for various biochemical analyses.
Biochemical parameters
Toxicity markers ALT and AST in the serum were assayed by the method of Reitman and Frankel as described by Wooten.Citation15 GGT in the serum was assayed using the method by Szasz.Citation16 The activity of GSH peroxidase (GPX) was determined by the method of Rotruck et al.Citation17 The activity of GSH reductase (GR) was determined by the procedure of David and Richard.Citation18 GSH transferase (GST) was assayed by using the method of Habig et al.Citation19 by 1-chloro-2,4-dinitrobenzene as substrate. GSH content was estimated by the method of Patterson and Lazarow.Citation20 Triglycerides (TGs) was estimated by the method of Van Handel and Zilver Smith.Citation21 Total proteins in tissue were estimated by the method of Lowry et al.Citation22
High-performance liquid chromatography analysis of AA in serum
One part serum with four parts 6% metaphosphoric acid was mixed in a polypropylene storage vial. The vial contents were vortexed and centrifuged at 10 000 × g for 15 minutes at 4°C and the supernatant was used for analysis. HPLC analysis was done by the Shimadzu Prominence SCL-20AHT (Shimadzu, Kyoto, Japan) system and the separation of AA was done by isocratic gradient elution using a Luna 5S NH2 100A column (Phenomenex, Torrance, USA). The mobile phase was 50 mM phosphoric acid (H3PO4) (eluent A, pH 2.5), acetonitrile (eluent B) in 1:1 ratio, the total flow rate was 1.0 ml per minute and the time of analysis was 15 minutes. The detector's wavelengths were set at 268 nm. The injection volume was 20 µl and the temperature of the column was thermostated at 40°C.
Histopathological analysis of liver
The liver tissues were rapidly dissected out and fixed by immersion at room temperature in 10% formalin solution. For the histological examinations, paraffin-embedded tissue sections of liver (4–6 µm thick) were stained with haematoxylin–eosin (H&E). The tissue samples were then examined and photographed under a light microscope for observation of structural abnormality.Citation23
Statistical analysis
The results were analysed using a statistical programme SPSS/PC+, Version 11.5 (SPSS Inc., Chicago, IL, USA). A one-way analysis of variance was employed for comparison among the groups. Duncan's post hoc multiple comparison tests were used to determine significant differences among groups: P < 0.05 was considered to be significant.
Results
The activities of liver toxicity markers ALT, AST, and GGT in the serum () were significantly increased in the ethanol group when compared with the control and C + AA groups. Our results showed that the activity of ALT, AST, and GGT () were significantly decreased in the abstention and E+AA-treated group, but the E+AA group showed more decrease in their activity than the abstention group.
Table 2. Toxicity markers
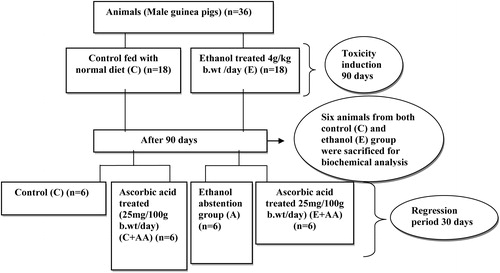
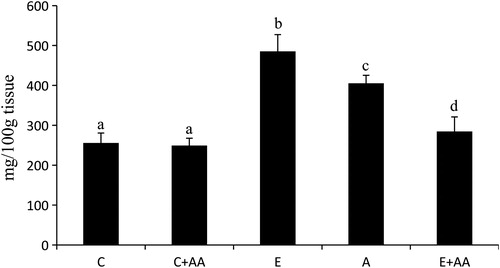
The GSH content in the liver was significantly decreased in the ethanol-treated groups (), when compared with the control and C+AA group. In the abstention group slight increase was observed in the GSH content, in comparison with the ethanol group. But in the E+AA group, GSH content was significantly increased in comparison with the ethanol and abstention groups ().
Figure 2. Graphical representation of reduced GSH content in liver. Values are expressed as mean ± SEM of six guinea pigs in each group. Values not sharing a common superscript differ significantly at P < 0.05.
The activities of GSH-dependent enzymes, GPX and GR (), in the liver were significantly decreased in the ethanol group in comparison with the control and C+AA groups. In the abstention group, the activities of these enzymes were increased in comparison with the ethanol group. But more significant increase was observed in AA-treated E+AA groups in comparison with the ethanol and abstention groups.
Table 3. Activity of GSH-dependent antioxidant enzymes in liver
The activity of GSH-dependent detoxifying enzyme GST was significantly increased in the ethanol-treated group in comparison with the control and C+AA groups (). When compared with the ethanol group, its activity was significantly decreased in the abstention and E+AA groups. But more decreased GST activity was observed in the E+AA group, in comparison with the abstention group.
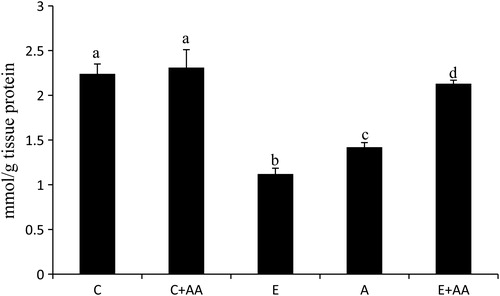
The AA content in the serum was analysed (A and B) and it was found that its level was significantly decreased in the ethanol and abstention groups in comparison with the control group. But in the C+AA and E+AA groups, AA content was significantly increased in comparison with the control, ethanol, and abstention groups. The increase in the E+AA group was less than that of the C+AA group.
Figure 3. (A) HPLC chromatogram of ascorbic acid in serum. (a) Control, (b) C+AA, (c) ethanol, (d) abstention, and (e) E+AA. The retention time was 2.2 minutes. (B) Graphical representation of ascorbic acid content in serum. Values are expressed as mean ± SEM of six guinea pigs in each group. Values not sharing a common superscript letter differ significantly at P < 0.05.
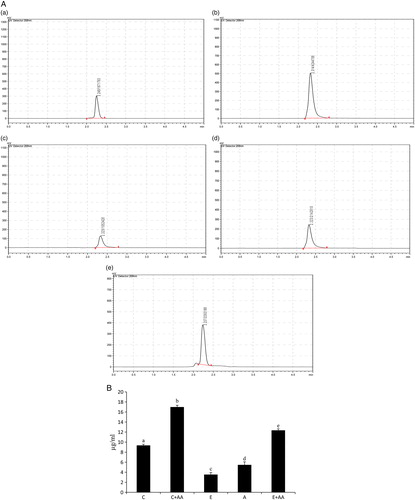
The concentration of TGs () was enhanced significantly in the liver of the ethanol group in comparison with the control and C+AA groups. When compared with the ethanol group, TGs content was significantly decreased in the abstention and E+AA groups. But the decrease was more pronounced in the AA-supplemented E+AA group.
Figure 4. Graphical representation of TGs content in liver. Values are expressed as mean ± SEM of six guinea pigs in each group. Values not sharing a common superscript letter differ significantly at P < 0.05.
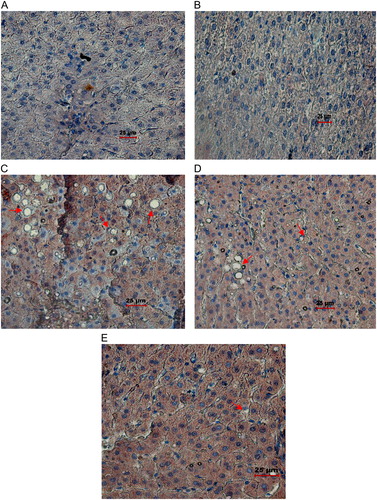
Histopathological analysis of the liver of the ethanol group (C) showed marked fat accumulation, mild inflammation, and necrosis in comparison with the control and C+AA groups. But in the abstention group, marked fatty changes were attenuated to moderate steatosis with mild inflammation and necrosis in comparison with the ethanol group. However, in the AA-supplemented E+AA group (E), liver exhibited less steatosis, inflammation, and necrosis.
Figure 5. Histopathological#analysis of liver (magnification ×40). (A) Control liver tissue with normal hepatocyte architecture. (B) C+AA group liver tissue with normal hepatocyte architecture. (C) Ethanol group, showing altered hepatocyte architecture with vascular steatosis and inflammation. (D) Abstention group showing altered hepatocyte architecture with vascular steatosis and inflammation. (E) E+AA liver tissue showing nearly normal histological features with reduced amount of steatosis and inflammation.
Discussion
Alcohol toxicity is one of the world's major health problems as a significant number of people are affected due to several fatal diseases caused by alcohol.Citation24 Alcohol-induced oxidative stress leads to a decrease in the intracellular antioxidative capacity of the liver cells including small molecular antioxidants (GSH and AA) and antioxidant enzymes.Citation5,Citation25 In ethanol-induced liver toxicity, both GSH and AA were simultaneously decreased and it indicates that AA plays some role in the metabolism of GSH. Therefore, supplementation with exogenous antioxidants has been an attractive approach to prevent or reduce ethanol-induced hepatotoxicityCitation26,Citation27 and maintain normal levels of GSH in the liver. Hence, we studied the impact of AA supplementation (after 90 days of ethanol feeding) on GSH levels and GSH-dependent enzymes in comparison with the ethanol abstention group.
Alcohol-induced liver toxicity was evidenced by the increased activities of liver toxicity markers, ALT, AST, and GGT, in the serum. The supplementation of AA reduced the alcohol-induced hepatotoxicity in the E+AA group more than in the abstention group, suggesting the role played by AA in the faster reduction of alcoholic hepatotoxicity. This is reflected in the reduced activities of ALT, AST, and GGT in the serum. This is in line with the previous reports that AA reduces alcohol-induced toxicity.Citation12,Citation13,Citation28
GSH is a powerful nucleophile, critical for cellular protection activities such as detoxification of ROS, conjugation and excretion of toxic molecules, and control of inflammatory cytokine cascade.Citation29 The free radicals increased the lipid peroxidation and GSSG, and subsequently promoted liver cell apoptosis. Our findings are consistent with other published reports which showed that both AA and GSH concentration were decreased during ethanol ingestion.Citation30,Citation31 In addition, an earlier study from our laboratory reported that GSH content was increased in experimental animals ingested with ethanol for 30–45 days.Citation10,Citation13 The enhanced GSH content observed may be due to the feedback activation of GSH synthesis.Citation13 The induction of GSH synthesis might have been an adaptive step to combat alcohol-induced oxidative stress. But in the present study GSH content was lower in the liver of 90 days ethanol-fed guinea pigs. The prolonged exposure to alcohol might have caused reduction in the initial induction of GSH synthesis. This is in line with previous studies which indicated that GSH content was decreased in alcohol-induced liver toxicity.Citation4,Citation5 The reduction in GSH content can be linked to the reduced activities of GR and also the altered GSH metabolism due to onset of liver fibrosis after 90 days of ethanol feeding. The reduced levels of GSH may be explained on the basis of its utilization in scavenging the free radicals, oxidation of GSH to its oxidized form by GPX in detoxification of hydrogen peroxide, suppression of GSH synthesis by ethanol,Citation32 and increased activity of GSH-degrading enzyme GGT in liver. The increased activity of GGT induces extracellular GSH breakdown and GGT activity might be in great demand if intracellular GSH is depleted by ethanol toxicity.Citation33 Levels of both AA and GSH were significantly increased in the abstention and E+AA groups. But more significant increase was observed in the AA-treated group, in comparison with the abstention group. Thus, AA not only decreases oxidants but also increases concentrations of GSH in the liver. This is further supported by the activity of GSH-dependent enzymes like GPX, GR, and GST in the liver.
Decreased activities of GSH-dependent enzymes in the liver of rats that ingested alcohol could be due to either free radical-dependent inactivation of enzyme or depletion of its co-substrates, that is GSH and NADPH.Citation34 Our results are also in line with the above observations. The activities of enzymes (GPX, GR, and GST) were significantly reduced in the ethanol group. This is in agreement with reports which indicate that chronic ethanol administration reduced the activities of scavenging enzymes.Citation35 The reduced activity of GPX is due to the deficiency of GSH for antioxidant functions of GPX. The GSH peroxidises (selenium-dependent and independent) are responsible for the enzymatic reduction of peroxides. The NADPH-dependent GR converts GSSG back to GSH after the action of GPX on free radicals, and so almost all intracellular GSH is reduced. GST plays an essential role in the liver by eliminating toxic compounds by conjugating them with GSH. Increased GST activity and decreased GR activity followed by thiol depletion are important factors sustaining a pathogenic role for oxidative stress.Citation36 But AA supplementation offered protection rather than ethanol abstention by increased activities of GPX, GR, and reducing the activity of GST.
Chronic alcohol consumption increases hepatic accumulation of fat, largely TGs, and leads to hepatic steatosis, which is the earliest and most common response to heavy alcohol intake. Alcohol-induced fat accumulation in hepatocytes may result from increase of TG synthesis, inhibition of fatty acid oxidation, and excessive oxidative stress. Hepatic steatosis may render the liver more susceptible to oxidative damages, in particular to endotoxins,Citation37 which promote the pathogenesis of alcoholic hepatitis or liver fibrosis.Citation38 In agreement with the above observations, TGs content significantly increased in the liver of the ethanol group and it was further supported by histopathological analysis of the liver of the ethanol group. Therefore, recovery from fatty liver would help decrease susceptibility to liver fibrosis. But in the AA-treated group, TGs content was significantly reduced in the liver and histopathological analysis of the liver showed that AA reversed steatosis induced by alcohol. In addition to this, we demonstrated that AA was significantly decreased in ethanol-treated guinea pigs and it was reversed in the AA-treated group.
Conclusion
From these results, we concluded that AA plays a vital role in maintaining GSH levels. Guinea pigs are genetically unable to synthesize AA; therefore, a great depletion of AA and GSH occurs in guinea pigs after ethanol treatment same as in the case of human beings. Hence, supplementation of AA at the dose of 25 mg/100 g body weight caused faster restoration of GSH and GSH-dependent enzymes, and reduced ethanol-induced steatosis in the liver of ethanol-treated guinea pigs. This suggests that AA should be supplemented to alcoholics in de-addiction centres for the speedy reversal of alcohol-induced hepatotoxicity.
Acknowledgement
This research work is fully supported by the Indian Council of Medical Research (ICMR), India. Letter no. 52/16/2008 BMS.
References
- Albano E. Oxidative mechanisms in the pathogenesis of alcoholic liver disease. Mol Aspects Med 2008;29:9–16.
- Marian V, Dieter L, Jan M, Mark TDC, Milan M, Joshua T. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007;39:44–84.
- Dey A, Cederbaum AI. Alcohol and oxidative liver injury. Hepatology 2006;43:S63–74.
- Neuman MG, Shear NH, Bellentani S, Tiribelli C. Role of cytokines in ethanol-induced cytotoxicity in vitro in Hep G2 cells. Gastroenterology 1998;115:157–66.
- Zhong JQ, Chen Z, Yong XL, Jae-Young J, Se-Kwon K, Won-Kyo J. Protective effects of emodin and chrysophanol isolated from marine fungus Aspergillus sp. on ethanol-induced toxicity in HepG2/CYP2E1 cells. Evid Based Complement Alter Med 2011, doi:10.1155/2011/452621.
- Fernandez-Checa JC, Kaplowitz N, Garcia-Ruiz C, Colell A. Mitochondrial glutathione: importance and transport. Semin Liver Dis 1998;18:389–401.
- Danyelle MT, Kenneth DT, Haim T. The importance of glutathione in human disease. Biomed Pharmacother 2003;57:145–55.
- Tandon SK, Singh S, Prasad S. Reversal of cadmium induced oxidative stress by chelating agent, antioxidant or their combination in rat. Toxicol Lett 2003;145:211–7.
- Viviana M. Vitamin C is an essential antioxidant that enhances survival of oxidatively stressed human vascular endothelial cells in the presence of a vast molar excess of glutathione. J Biol Chem 2007;282:15506–15.
- Suresh MV, Lal JJ, Ranjitkumar CV, Indira M. Impact of ascorbic acid supplementation on alcohol-induced oxidative stress in guinea pigs. Toxicol Lett 1999;104:221–9.
- Chakraborthy S, Nandi A, Mukhopadhyay M, Mukhopadhyay CK, Chatterjee IB. Ascorbate protects guinea pigs tissues against lipid peroxidation. Free Radic Biol Med 1994;16:417–26.
- Suresh MV, Lal JJ, Sreeranjitkumar CV, Indira M. Ascorbic acid metabolism in rats and guinea pigs after the administration of ethanol. Comp Biochem Physiol Part C 1999;124:175–9.
- Sivaram AG, Suresh MV, Indira M. Combined effect of ascorbic acid and selenium supplementation on alcohol-induced oxidative stress in guinea pigs. Comp Biochem Physiol C Toxicol Pharmacol 2003;134:397–401.
- Zahlten RN, Nejtek ME, Jacobson JC. Ethanol metabolism in guinea pig: ethanol oxidation and its effect on NAD/NADH ratios, oxygen consumption, and ketogenesis in isolated hepatocytes of fed and fasted animals. Arch Biochem Biophys 1982;13:200–23.
- Wooten IDP. Microanalysis in medical biochemistry. 4th ed. London: J & A Churchill Ltd; 1964. p. 140.
- Szasz G. A kinetic photometric method for serum gamma glutamyl transpeptidase. Clin Chem 1969;15:24–6.
- Rotruck JT, Pope AL, Ganther HE. Selenium: biochemical roles as a component of glutathione peroxidase. Science 1973;179:588–90.
- David M, Richard JS. Glutathione reductase. In: , Bergmeyer HU, Bergmeyer J (eds.) Methods of enzymatic analysis. Academic Press, New York. 1983. p. 258–65.
- Habig WH, Mabst MJ, Jokoby WB. Glutathione S-transferase: the first enzymatic step in mercapturic acid formation. J Biol Chem 1974;249:7130–39.
- Patterson JW, Lazarow A. Determination of glutathione. In: , Glick D, (ed.), Methods of biochemical analysis. Interscience, New York. 1955. p. 259–78.
- Van Handel L, Zilver Smith DB. Micro method for direct determination of serum triglycerides. J Lab Clin Med 1957;1550:152–62.
- Lowry OH, Rosebrough NJ, Farr AL, Randell RJ. Protein measurement with the fall in phenol reagent. J Biol Chem 1951;193:265–75.
- Piyush D, Mool KT, Kirtikar S, Sonal S, Jasvindar KG, Rimi S. Hepatoprotective effect of stem of Musa sapientum Linn in rats intoxicated with carbon tetrachloride. Ann Hepatol 2011;10:333–9.
- Fleming MF, Manwell LB, Barry KL, Johnson K. At-risk drinking in an HMO primary care sample: prevalence and health policy implications. Am J Public Health 1998;88:90–3.
- Lecomte E, Herbeth B, Pirollet P, Chancerelle Y, Arnaud J, Musse N. Effect of alcohol consumption on blood antioxidant nutrients and oxidative stress indicators. Am J Clin Nutr 1994;60:255–61.
- Dhiman RK, Chawla YK. Herbal medicines for liver diseases. Dig Dis Sci 2005;50:1807–12.
- Xu BJ, Zheng YN, Sung CK. Natural medicines for alcoholism treatment: a review. Drug Alcohol Rev 2005;24:525–36.
- Subir KD, Vasudevan DM. Protective effect of silymarin, a milk thistle (Silybium marianum) derivative on ethanol induced oxidative stress in liver. Indian J Biochem Biophys 2006;43:306–11.
- Brown LA, Harris FL, Ping XD, Gauthier TW. Chronic ethanol ingestion and the risk of acute lung injury: a role for glutathione availability? Alcohol 2004;33:191–7.
- Fernandez V, Videla IA. Effect of acute and chronic ethanol ingestion on the content of reduced glutathione on various tissues of the rat. Experientia 1981;37:392–4.
- Jaya DS, Augstine J, Menon VP. Role of lipid peroxides, glutathione and antiperoxidative enzymes in alcohol and drug toxicity. Indian J Exp Biol 1993;31:453–9.
- Subir KD, Vasudevan DM. Monitoring oxidative stress in patients with non-alcoholic and alcoholic liver diseases. Indian J Clin Biochem 2005;20:24–8.
- Whitfield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci 2001;38:263–355.
- Subir KD, Vasudevan DM. Effect of ethanol on liver antioxidant defence systems: a dose dependent study. Indian J Clin Biochem 2005;20:79–83.
- Senthil KR, Ponmozhi M, Viswanathan P, Nalini N. Activity of Cassia auriculata leaf extract in rats with alcoholic liver injury. J Nutr Biochem 2003;14:452–8.
- Neil Kaplowitz MD. The importance and regulation of hepatic glutathione. Yale J Biol Med 1981;54:497–502.
- Bhagwandeen BS, Apte M, Manwarring L, Dickeson J. Endotoxin induced hepatic necrosis in rats on an alcohol diet. J Pathol 1987;152:47–53.
- Sung HK, Jae HC, Choon WK, Sang GK. Combined metadoxine and garlic oil treatment efficaciously abrogates alcoholic steatosis and CYP2E1 induction in rat liver with restoration of AMPK activity. Chem Biol Interact 2007;169:80–90.