Abstract
Preterm delivery (PTD) is the leading cause of infant mortality and morbidity. However, the mechanism at the molecular level is still unknown. Placental inflammatory response and oxidative stress are associated with PTD. Thioredoxin-1 (TRX-1) regulates oxidative stress, inflammation, and the activities of transcription factors.
Objectives
The objective was to detect in placental tissues the expressions of TRX-1 and the TRX-1-related molecules: tumor necrosis factor-α (TNF-α), cyclooxygenase-2 (COX-2), thioredoxin-1-binding protein-2 (TBP-2), hypoxia inducible transcription factor 1α (HIF-1α), and forkhead box protein O3A (FoxO3A).
Methods
PTD was defined as gestation of <37 weeks and term delivery (TD) as ≥37 weeks. The expressions of TRX-1 and TRX-1-related molecules were examined in placental tissues by real-time polymerase chain rection and western blot.
Results
The expressions of TRX-1, TNF-α, COX-2, HIF-1α, and FoxO3A in the placenta of PTD were significantly higher as compared with TD, but no difference was observed in TBP-2 expression.
Discussion
These results indicate that TRX-1 may be adaptively induced by the effects of inflammation and oxidative stress, suggesting protective roles for TRX-1 against these effects in the placenta of PTD.
Introduction
Preterm delivery (PTD) is the delivery prior to 37 weeks of gestation. The frequency of PTD varies from 5 to 10% in the developed regions of the world, but it is much higher (40%) in certain poor areas.Citation1,Citation2 Although the survival rate for PTD has recently improved due to technological advances, PTD remains the leading cause of infant mortality and morbidity. It accounts for 75% of perinatal mortality.Citation3 The pathogenesis of PTD is multifactorial, associated with cervical insufficiency, hormonal imbalance, genetic predisposition, and altered immune surveillance.Citation4,Citation5
Approximately 45–50% of PTD is idiopathic or spontaneous, which is closely associated with intrauterine infection and/or inflammation.Citation6,Citation7 Inflammation induces oxidative stress by up-regulating proinflammatory cytokines and impairing the antioxidant defense systems. Although oxidative stress is involved in normal pregnancies and spontaneous parturition at term, there is growing evidence that oxidative stress has been implicated in the pathogenesis of many complications of human pregnancy, including miscarriage, pre-eclampsia, and preterm labor.Citation4,Citation8,Citation9 Moreover, oxidative stress occurs with the overproduction of reactive oxygen species (ROS), and placental antioxidant enzymes increase to compensate for the increase in oxidative stress.Citation10,Citation11 Antioxidant proteins, such as glutathione peroxidase, superoxide dismutase (SOD), thioredoxin, and thioredoxin reductase, participate in placental oxidative stress.Citation12–Citation14
Thioredoxin-1 (TRX-1) is a small molecular weight protein (12 kDa) with a conserved dithiol/disulphide active site, Cys-Gly-Pro-Cys.Citation15 TRX-1, also known as the ‘early pregnancy factor’ (EPF), modifies lymphocyte activity in pregnancy sera.Citation16 Genetic knockout of TRX-1 in mice is embryonically lethal.Citation17 In the placenta, TRX-1 may protect against lipopolysaccharide (LPS)-induced oxidative stress.Citation18 TRX-1 expression is reduced in pre-eclamptic placentaCitation19, but it has not been previously reported in human placenta of PTD. Therefore, the aim of this study is to examine the hypothesis that TRX-1 may be implicated in PTD.
Thioredoxin-1-binding protein-2 (TBP-2), also known as vitamin-D3 up-regulated protein-1 (VDUP1) or thioredoxin-interacting protein (TXNIP), has a molecular weight of 50 kDa. TBP-2 binds to reduced TRX-1 but not to oxidized TRX-1 or mutant TRX-1 in which two redox-active cysteine residues are substituted with serine.Citation20,Citation21 TBP-2 is the endogenous inhibitor of TRX-1 which binds to the reduced TRX-1 and inhibits TRX-1 activity.
The aim of this study was to examine the expression of TRX-1 in late gestation with other molecular signals expressed co-temporally.
Materials and methods
Placental biopsies
Fifty-eight pregnant women were recruited for this study and divided into two groups: (i) 29 cases with PTD (29–37 weeks, average age 28.7 ± 5.2 years, average gestational age 34.2 ± 2.0 weeks) and (ii) 29 cases with term delivery (TD) (≥37 weeks, average age 28.7 ± 2.9 years, average gestational age 38.7 ± 1.4 weeks). There was no significant difference in the average age of pregnant women between PTD and TD (P > 0.05). All patients were hospitalized in the First People's Hospital of Yunnan Province, Kunming, China. Pregnant women with chronic hypertension, renal disease, diabetes mellitus, pre-eclampsia, major fetal anomalies, infection, fever, or intrauterine fetal death were excluded from the study. All patients underwent normal vaginal delivery without surgery. All of the placental biopsies were collected from patients who agreed to donate their placenta to the hospital. The ethics committee of the First People's Hospital of Yunnan Province approved this study and all procedures were performed in accordance with the Code of Ethics of the World Medical Association.
Placental biopsies were collected from the same location (the maternal surface side of the placenta to a depth of approximately 1 cm and about 500 mg) immediately after delivery. Abnormal areas and infarcts were avoided. Biopsies were immediately frozen in liquid nitrogen and stored at –80°C for later biochemical analysis.
Quantitative reverse transcriptase-polymerase chain reaction
Total RNA was extracted from 0.1 g placental tissues using a Trizol reagent kit (CWBIO Corporation, Beijing, China) and converted to cDNA using the RevertAid™ First Strand cDNA Synthesis Kit (Fermentas, Walldorf Baden, Germany). The product was analyzed using a Prism 7300 Sequence Detection System (Applied Biosystems, Foster, CA, USA). The following primer pairs were selected for quantitative real-time polymerase chain reaction (PCR): human TRX-1 forward: 5′-AAG CCT TGG ACG CTG CAG-3′, reverse: 5′-CAT CCT GAC AGT CAT CCA CAT CTA CT-3′, probe: TGA TCA AGC CTT TCT TTC ATT CCC TCT C; human TBP-2 forward: 5′- GGA TCC CAG CAG TGC AAA C-3′, reverse: 5′-AAG CCG AAC TTG TAC TCA TAT TTG T-3′, probe: AGT ACC TGC GCT ATG AAG ACA CGC TT; human GAPDH forward: 5′-CAA GGC TGA GAA CGG GAA G-3′, reverse 5′-GGT GAA GAC GCC AGT GGA CT-3′, probe: ATC CCA TCA CCA TCT TCC AGG AGC G; human hypoxia inducible transcription factor 1α (HIF-1α) forward: 5′-ACA GCA GCC AGA CGA TCA TGC AG-3′, reverse: 5′-AAC TGG TCA GCT GTG GTA ATC CAC T-3′; human cyclooxygenase-2 (COX-2) forward: 5′-TGC ATT CTT TGC CCA GCA CT-3′, reverse: 5′-AAA GGC GCA GTT TAC GCT GT-3′; human tumor necrosis factor- α (TNF-α) forward: 5′-GCC CAG GCA GTC AGA TCA TC-3′, reverse: 5′-CGG TTC AGC CAC TGG AGC T-3′. Reaction mixtures containing Premix Ex Taq™ (TaKaRa code: DRR039) and SYBR Green (TaKaRa code: DRR041; TaKaRa Biotechnology, Dalian, China) were prepared according to the manufacturer's protocol. GAPDH was used as an internal standard for all samples.
Western blotting
Placental tissues from 58 pregnant women were homogenized and lysed in a solubilizing solution (150 mM NaCl, 0.5% NP-40, 10 mM Tris-HCl, pH 7.2, 0.1 mM PMSF, 2 µg/ml aprotinin, and 200 µg/ml NaN3) on ice for 1 hour and centrifuged at 20 600 g (Allegra® X-22 Series Benchtop Centrifuge, Beckman Coulter, Fullerton, CA, USA) for 15 minutes. The protein concentration in the supernatant was determined using the Bio-Rad Bradford protein assay (Bio-Rad Laboratories, Hercules, CA, USA). Equal amounts of protein from each sample (10 µg for TRX-1, 20 µg for COX-2, and forkhead box protein O3A (FoxO3A)) were subjected to electrophoresis in 15% (TRX-1) and 10% (COX-2 and FoxO3A) sodium dodecyl sulfate polyacrylamide gels under reducing conditions and transferred with polyvinylidene fluoride membranes (Millipore Corporation, Billerica, MA, USA) using a humid transfer machine. Membranes were incubated in a blocking solution (PBS-T [Tween-20-phosphate buffered saline] containing 10% powdered non-fat milk and 0.1% Tween-20) at 4°C overnight followed by incubation in the same solution containing the primary antibody (anti-human TRX-1, dilution ratio 1:5000 (Redox Bioscience, Inc., Kyoto, Japan); anti-human COX-2, dilution ratio 1:1000 (Santa Cruz Biotechnology, CA, USA); anti-human FoxO3A, dilution ratio 1:5000, (Epitomics, CA, USA)) at room temperature for 70 minutes. Membranes were washed in TBS-T and incubated with a horseradish peroxidase-conjugated secondary antibody (KPL, Gaithersburg, MD, USA) at room temperature for 1 hour. The immunoreactive bands were visualized using an enhanced chemiluminescence system (Millipore Corporation) according to the manufacturer's instructions. Each sample was normalized to β-actin. Image analysis software (ImageJ) assessed band intensity and provided the quantitative analysis.
Statistical analysis
Data are expressed as the means ± SD. Statistical analysis was performed using SPSS 16.0 (SPSS, Chicago, IL, USA) software. A one-way analysis of variance followed by a post hoc comparisons test was performed. Comparisons between PTD and TD were analysed by multiple-samples comparison. Differences were considered statistically significant at either *P < 0.05, **P < 0.01, or ***P < 0.001.
Results
Placental inflammation in PTD placenta
Although the mechanism of PTD remains unknown, it has been reported that the placental inflammation is associated with the premature onset of labor.Citation22 Therefore, we first examined whether inflammation existed in PTD. TNF-α was detected by using real-time PCR. The mRNA level of TNF-α was higher in PTD than TD (P < 0.05; A), which suggested that inflammation was involved in the pathogenesis of PTD. This result is consistent with previous studies.Citation23,Citation24
Figure 1. The expressions of TNF-α mRNA levels and COX-2 mRNA levels and protein in placenta of PTD (n = 29) and TD (n = 29). (A) The mRNA levels of TNF-α in placenta of PTD and TD by real-time PCR. (B) The mRNA levels of COX-2 in placenta of PTD and TD by real-time PCR. (C) The expression of COX-2 protein in placenta of PTD and TD by western blot. The relative protein density was normalized to β-actin. Bars indicate the mean value ± SD. Asterisks indicate statistical significance (*P < 0.05, ***P < 0.001). PTD, preterm delivery; TD, term delivery.
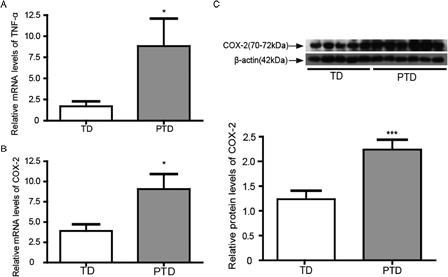
COX-2 is an inducible enzyme that is up-regulated by the inflammatory responses. It is involved in LPS- and glucocorticoid-induced PTD.Citation25–Citation27 Further examination of the expression of COX-2 in the placenta of PTD showed that the levels of COX-2 mRNA and protein in PTD were higher compared with that in TD (B and 1C). This result further demonstrated that inflammation occurred in the placenta of PTD in the present study.
TRX-1 expression in PTD placenta
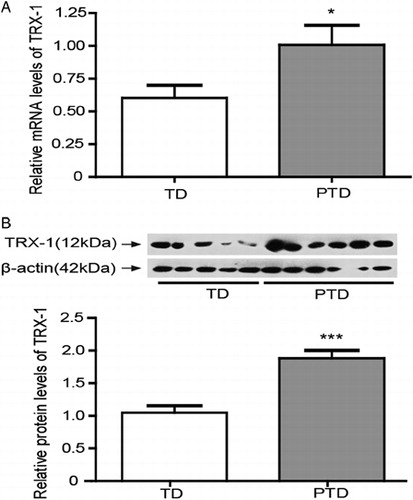
TRX-1, also known as the ‘EPF’, modifies lymphocyte activity in pregnancy sera.Citation16 TRX-1 expressions in PTD and TD placenta were examined, and the results showed that TRX-1 mRNA and protein levels were significantly increased (P < 0.05) in PTD compared to TD (A and 2B).
Figure 2. The expressions of TRX-1 in placenta of PTD (n = 29) and TD (n = 29). (A) The mRNA levels of TRX-1 in placenta of PTD and TD by real-time PCR. (B) The expression of TRX-1 protein in placenta of PTD and TD by western blot. The relative protein density was normalized to β-actin. Bars indicate the mean value ± SD. Asterisks indicate statistical significance (*P < 0.05, ***P < 0.001). PTD, preterm delivery; TD, term delivery.
The expressions of TRX-1-associated molecules (TBP-2, HIF-1α, and FoxO3A)
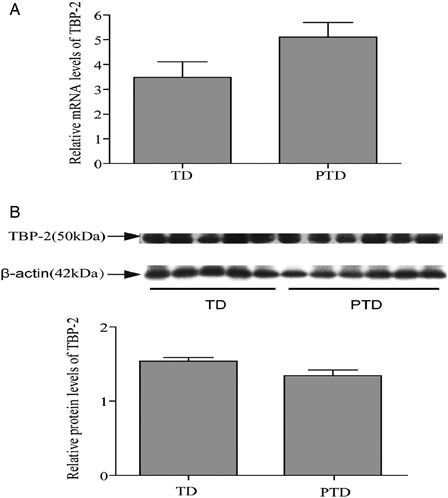
Although TRX-1 can be induced by inflammation and oxidative stress, it was needed to detect the mechanisms or roles of TRX-1 induction in PTD. TBP-2, as an endogenous inhibitor of TRX-1, was detected using real-time PCR and western blot. The results showed that there were no significant differences (P > 0.05) in placental TBP-2 mRNA and protein levels between PTD and TD (A and 3B).
Figure 3. The expression of TBP-2 in placenta of PTD and TD (n = 29) and TD (n = 29). (A) The mRNA levels of TBP-2 in placenta of PTD and TD by real-time PCR. (B) The expression of TBP-2 protein in placenta of PTD and TD by western blot. The relative protein density was normalized to β-actin. Bars indicate the mean value ± SD. PTD, preterm delivery; TD, term delivery.
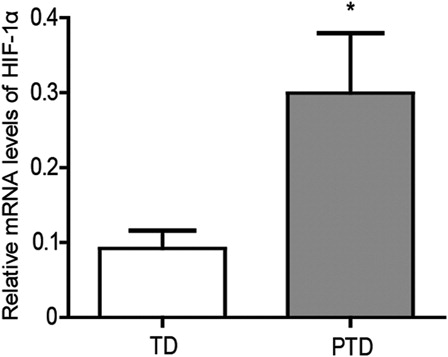
Oxidative stress usually occurs in hypoxic conditions. As a result, the expression of HIF-1α in the placenta was detected, and the HIF-1α mRNA levels were found to be significantly higher in PTD as compared with TD (P < 0.05; ). Therefore, the increase in HIF-1α expression may be associated with placental oxidative stress in PTD.
Figure 4. The mRNA levels of HIF-1α in placenta of PTD (n = 29) and TD (n = 29) by real-time PCR. Bars indicate the mean value ± SD. Asterisks indicate statistical significance (*P < 0.05). PTD, preterm delivery; TD, term delivery.
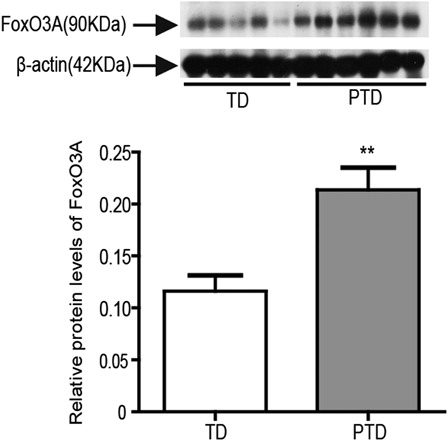
The overexpression of FoxO3A reduces the abnormal accumulation of ROS, enhances cellular resistance to oxidative stress, and increases antioxidant gene expression.Citation28 Thus, we further examined FoxO3A expression in the placenta of PTD and found that FoxO3A expression was significantly higher in the placenta of PTD than TD (P < 0.01; ). The increased expression of FoxO3A may play a role in resisting placental oxidative stress of PTD by inducing TRX-1 expression.
Figure 5. The protein expression of FoxO3A in placenta of PTD (n = 29) and TD (n = 29) by western blot. The relative protein density was normalized to β-actin. Bars indicate the mean value ± SD. Asterisks indicate statistical significance (0.001 < **P < 0.01). PTD, preterm delivery; TD, term delivery.
Discussion
As reported previously, the pathogenesis of PTD is closely associated with inflammation.Citation29 TNF-α is an inflammatory stressor. As the proinflammatory cytokine, TNF-α could induce oxidative stress in various cells and provoke a variety of biological effects on the placenta.Citation23,Citation24,Citation30 COX-2 is also a factor in the inflammatory response and is involved in LPS and glucocorticoid-induced PTD, and it is responsible for prostaglandin (PG) production during TD and PTD.Citation26,Citation27,Citation31,Citation32 In this study, we demonstrated the increasing expressions of TNF-α and COX-2, as shown in A–1C. These results indicate that inflammation is involved in PTD. The increase of TNF-α and COX-2 could stimulate the synthesis and release of PG, which ultimately leads to uterine contraction and irreversible cervical changes.Citation33 COX-2 is also known to be involved in the initiation and augmentation of parturition.Citation34 Therefore, the increase of TNF-α and COX-2 is associated with PTD of the fetus.
Although infection and/or inflammation are the main pathological processes, placental oxidative stress has been implicated in the pathogenesis of many complications of human pregnancy, including miscarriage, pre-eclampsia, and PTD. TRX-1 is one of the major components of the thiol-reducing system and is important for the regulation of the redox balance.Citation35 TRX-1 has an essential role in maintaining their surface thiol density as the principal mechanism for antioxidative defense, and is sensitive to proinflammatory stimuli, mainly TNF-α. Increased TRX-1 production in the naturally occurring human regulatory T cells confers enhanced tolerance to oxidative stress.Citation36
TRX-1 is localized in the cytosol of normal early and term placenta.Citation37–Citation39 The expression of TRX-1 protein and mRNA levels were increased in PTD placenta in the present study (). The increase of TRX-1 may play roles in protecting the placenta from oxidative stress and inflammation.
TBP-2 expression is regulated by environmental conditions, various stresses and inflammation, and its expression affects cellular growth and apoptosis.Citation20,Citation21,Citation40 We examined TBP-2 expression in the placenta of PTD and found that TBP-2 mRNA and protein levels in placenta were not significantly different between the groups (A and 3B), suggesting that TRX-1 may not be regulated by TBP-2 in PTD placenta.
The expression of TRX-1 can be induced by various stimulations through AP-1, antioxidant responsive element, and cAMP-responsive element sites in the TRX promoter.Citation41 The forkhead-box (Fox) gene family of transcription factors, which includes more than 100 members, includes FoxO3A that binds to the TRX-1 promoter to induce TRX-1 expression through the AMP-activated protein kinase pathway during oxidative stress responses.Citation42 Thus, it was important to further examine the expression of FoxO3A. This study is the first peer-reviewed report of an increase in FoxO3A in the placenta of PTD (). Mammalian FoxO proteins play numerous roles in cellular proliferation/arrest, survival/death, metabolism, and autophagy. FoxO3A-null mice exhibit age-dependent infertility due to abnormal ovarian follicular development, leading to degeneration.Citation43,Citation44 FoxO3A functions as a major regulator of oxidative stress, and this transcription factor increases oxidative stress resistance by the up-regulation of mitochondrial SOD and peroxisomal catalase.Citation45,Citation46 FoxO3A is expressed in human placentaCitation47 and our data demonstrate that the increased expression of FoxO3A might be associated with oxidative stress in PTD placenta.
Besides TRX-1, many proteins are also induced by oxidative stress, such as angiogenic molecules.Citation4,Citation8,Citation9 HIF-1α, an angiogenic molecule, is a redox sensitive transcription factor and was detected by reverse transcriptase (RT)-PCR. Our results showed a significant increase in the expression of HIF-1α in the placenta of PTD compared with those of TD (). In normal pregnancy, a low oxygen environment in the placenta is physiological and necessary in the first trimester, but it is pathological and associated with common complications of pregnancy in later gestation.Citation48 HIF-1α could be expressed during early pregnancy, but its amount decreases with gestational age.Citation49 Therefore, hypoxia is implicated as a key regulator of placental morphogenesis and function.Citation49,Citation50 HIF-1α was induced by the oxidative stress occurred in the PTD placenta. TRX-1 regulates the activation of transcription factors, including HIF-1α. In addition, TRX-1 enhances HIF-1α activity and protein expression under both normoxic and hypoxic conditions. In contrast, a redox-inactive TRX (C32S/C35S) markedly decreases HIF-1α protein levels.Citation51 In current clinical development, an inhibitor of TRX-1 has been found to decrease tumor levels of the HIF-1α transcription factor.Citation52 Thus, HIF-1α may be induced by the redox response in the PTD placenta and regulated by TRX-1. In addition, TNF-α and interleukin-1β have been shown to stabilize HIF-1α protein, and thus HIF-1α can be recruited by tissue inflammation.Citation53–Citation55
In conclusion, this study showed that TNF-α, COX-2, FoxO3A, TRX-1, and HIF-1α were significantly increased in the human placenta of PTD. These results suggest that oxidative stress and inflammation are involved in the pathological process of PTD.
Acknowledgements
This study was supported by The Candidates of Young and Middle Aged Academic Leaders of Yunnan Province 2006PY01–07, 2008PY045. Key Laboratory of Medical Neurobiology of Kunming University of Science and Technology.
References
- Menon R. Spontaneous preterm birth, a clinical dilemma: etiologic, pathophysiologic and genetic heterogeneities and racial disparity. Acta Obstet Gynecol Scand 2008;87(6):590–600.
- Creasy RK. Preventing preterm birth. N Engl J Med 1991;325(10):727–9.
- Medina TM, Hill DA. Preterm premature rupture of membranes: diagnosis and management. Am Fam Physician 2006;73(4):659–64.
- Sakata M, Sado T, Kitanaka T, Naruse K, Noguchi T, Yoshida S, et al. Iron-dependent oxidative stress as a pathogenesis for preterm birth. Obstet Gynecol Surv 2008;63(10):651–60.
- Myatt L, Cui XL. Oxidative stress in the placenta. Histochem Cell Biol 2004;122(4):369–82.
- Romero R, Oyarzun E, Mazor M, Sirtori M, Hobbins JC, Bracken M. Meta-analysis of the relationship between asymptomatic bacteriuria and preterm delivery/low birth weight. Obstet Gynecol 1989;73(4):576–82.
- LeBlanc MM, Giguere S, Lester GD, Brauer K, Paccamonti DL. Relationship between infection, inflammation and premature parturition in mares with experimentally induced placentitis. Equine Vet J 2012; 44 Suppl:418–14.
- Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, Petraglia F. Inflammation and Pregnancy. Reprod Sci 2009;16(2):206–15.
- Mocatta TJ, Winterbourn CC, Inder TE, Darlow BA. The effect of gestational age and labour on markers of lipid and protein oxidation in cord plasma. Free Radic Res 2004;38(2):185–91.
- Qanungo S, Mukherjea M. Ontogenic profile of some antioxidants and lipid peroxidation in human placental and fetal tissues. Mol Cell Biochem 2000;215(1–2):11–9.
- Burton GJ, Jauniaux E. Oxidative stress. Best Pract Res Clin Obstet Gynaecol 2011;25(3):287–99.
- Sahlin L, Ostlund E, Wang H, Holmgren A, Fried G. Decreased expression of thioredoxin and glutaredoxin in placentae from pregnancies with pre-eclampsia and intrauterine growth restriction. Placenta 2000;21(7):603–9.
- Clifton VL, Vanderlelie J, Perkins AV. Increased anti-oxidant enzyme activity and biological oxidation in placentae of pregnancies complicated by maternal asthma. Placenta 2005;26(10):773–9.
- Vanderlelie J, Venardos K, Clifton VL, Gude NM, Clarke FM, Perkins AV. Increased biological oxidation and reduced anti-oxidant enzyme activity in pre-eclamptic placentae. Placenta 2005;26(1):53–8.
- Holmgren A. Redox regulation by thioredoxin and thioredoxin reductase. Biofactors 2000;11(1–2):63–4.
- Clarke FM, Orozco C, Perkins AV, Cock I, Tonissen KF, Robins AJ, et al. Identification of molecules involved in the ‘early pregnancy factor’ phenomenon. J Reprod Fertil 1991;93(2):525–39.
- Matsui M, Oshima M, Oshima H, Takaku K, Maruyama T, Yodoi J, et al. Early embryonic lethality caused by targeted disruption of the mouse thioredoxin gene. Dev Biol 1996;178(1):179–85.
- Ejima K, Koji T, Nanri H, Kashimura M, Ikeda M. Expression of thioredoxin and thioredoxin reductase in placentae of pregnant mice exposed to lipopolysaccharide. Placenta 1999;20(7):561–6.
- Shibata E, Ejima K, Nanri H, Toki N, Koyama C, Ikeda M, et al. Enhanced protein levels of protein thiol/disulphide oxidoreductases in placentae from pre-eclamptic subjects. Placenta 2001;22(6):566–72.
- Patwari P, Higgins LJ, Chutkow WA, Yoshioka J, Lee RT. The interaction of thioredoxin with Txnip. Evidence for formation of a mixed disulfide by disulfide exchange. J Biol Chem 2006;281(31):21884–91.
- Junn E, Han SH, Im JY, Yang Y, Cho EW, Um HD, et al., Vitamin D3 up-regulated protein 1 mediates oxidative stress via suppressing the thioredoxin function. J Immunol 2000;164(12):6287–95.
- Arias F, Victoria A, Cho K, Kraus F. Placental histology and clinical characteristics of patients with preterm premature rupture of membranes. Obstet Gynecol 1997;89(2):265–71.
- Vince G, Shorter S, Starkey P, Humphreys J, Clover L, Wilkins T, et al. Localization of tumour necrosis factor production in cells at the materno/fetal interface in human pregnancy. Clin Exp Immunol 1992;88(1):174–80.
- Hunt JS, Chen HL, Hu XL, Tabibzadeh S. Tumor necrosis factor-alpha messenger ribonucleic acid and protein in human endometrium. Biol Reprod 1992;47(1):141–7.
- Roos KL, Simmons DL. Cyclooxygenase variants: the role of alternative splicing. Biochem Biophys Res Commun 2005;338(1):62–9.
- Shoji T, Yoshida S, Mitsunari M, Miyake N, Tsukihara S, Iwabe T, et al. Involvement of p38 MAP kinase in lipopolysaccharide-induced production of pro-and anti-inflammatory cytokines and prostaglandin E(2) in human choriodecidua. J Reprod Immunol 2007;75(2):82–90.
- Zhu XO, Yang Z, Guo CM, Ni XT, Li JN, Ge YC, et al. Paradoxical stimulation of cyclooxygenase-2 expression by glucocorticoids via a cyclic AMP response element in human amnion fibroblasts. Mol Endocrinol 2009;23(11):1839–49.
- Li J, Du W, Maynard S, Andreassen PR, Pang Q. Oxidative stress-specific interaction between FANCD2 and FOXO3a. Blood 2010;115(8):1545–8.
- Dollner H, Vatten L, Halgunset J, Rahimipoor S, Austgulen R. Histologic chorioamnionitis and umbilical serum levels of pro-inflammatory cytokines and cytokine inhibitors. BJOG 2002;109(5):534–9.
- Almasry SM, Eldomiaty MA, Elfayomy AK, Habib FA. Expression pattern of tumor necrosis factor alpha in placentae of idiopathic fetal growth restriction. J Mol Histol 2012; DOI 10.1007/s10735-012-9410-6.
- Kniss DA. Cyclooxygenases in reproductive medicine and biology. J Soc Gynecol Investig 1999;6(6):285–92.
- Roos KL, Simmons DL, Cyclooxygenase variants: the role of alternative splicing. Biochem Biophys Res Commun 2005;338(1):62–9.
- Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med 2000;342(20):1500–7.
- Burdon C, Mann C, Cindrova-Davies T, Ferguson-Smith AC, Burton GJ. Oxidative stress and the induction of cyclooxygenase enzymes and apoptosis in the murine placenta. Placenta 2007;28(7):724–33.
- Yodoi J, Nakamura H, Masutani H. Redox regulation of stress signals: possible roles of dendritic stellate TRX producer cells (DST cell types). Biol Chem 2002;383(3–4):585–90.
- Mougiakakos D, Johansson CC, Jitschin R, Bottcher M, Kiessling R. Increased thioredoxin-1 production in human naturally occurring regulatory T cells confers enhanced tolerance to oxidative stress. Blood 2011;117(3):857–61.
- Padmini E, Lavanya S. HSP70-mediated control of endothelial cell apoptosis during pre-eclampsia. Eur J Obstet Gynecol Reprod Biol 2011;156(2):158–64.
- Ejima K, Nanri H, Toki N, Kashimura M, Ikeda M. Localization of thioredoxin reductase and thioredoxin in normal human placenta and their protective effect against oxidative stress. Placenta 1999;20(1):95–101.
- Ejima K, Nanri H, Araki M, Koji T, Shibata E, Kashimura M, et al. Expression of mitochondrial thioredoxin-dependent antioxidant protein, SP-22, in normal human and inflammatory mouse placentae. Placenta 2000;21(8):847–52.
- Nishiyama A, Masutani H, Nakamura H, Nishinaka Y, Yodoi J. Redox regulation by thioredoxin and thioredoxin-binding proteins. IUBMB Life 2001;52(1–2):29–33.
- Bai J, Nakamura H, Kwon YW, Hattori I, Yamaguchi Y, Kim YC, et al. Critical roles of thioredoxin in nerve growth factor-mediated signal transduction and neurite outgrowth in PC12 cells. J Neurosci 2003;23(2):503–9.
- Li XN, Song J, Zhang L, LeMaire SA, Hou X, Zhang C, et al. Activation of the AMPK-FOXO3 pathway reduces fatty acid-induced increase in intracellular reactive oxygen species by upregulating thioredoxin. Diabetes 2009;58(10):2246–57.
- Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science 2003;301(5630):215–8.
- Hosaka T, Biggs WH, Tieu D, Boyer AD, Varki NM, Cavenee WK, et al., Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc Natl Acad Sci USA 2004;101(9):2975–80.
- Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, et al. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature 2002;419(6904):316–21.
- Nemoto S, Finkel T. Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science 2002;295(5564):2450–2.
- Lappas M, Lim R, Riley C, Menon R, Permezel M. Expression and localisation of FoxO3 and FoxO4 in human placenta and fetal membranes. Placenta 2010;31(12):1043–50.
- Pringle KG, Kind KL, Sferruzzi-Perri AN, Thompson JG, Roberts CT. Beyond oxygen: complex regulation and activity of hypoxia inducible factors in pregnancy. Hum Reprod Update 2010;16(4):415–31.
- Rajakumar A, Conrad KP. Expression, ontogeny, and regulation of hypoxia-inducible transcription factors in the human placenta. Biol Reprod 2000;63(2):559–69.
- Semenza GL. HIF-1 and mechanisms of hypoxia sensing. Curr Opin Cell Biol 2001;13(2):167–71.
- Welsh J, Wietzke JA, Zinser GM, Smyczek S, Romu S, Tribble E, et al. Impact of the Vitamin D3 receptor on growth-regulatory pathways in mammary gland and breast cancer. J Steroid Biochem Mol Biol 2002;83(1–5):85–92.
- Kim YH, Coon A, Baker AF, Powis G. Antitumor agent PX-12 inhibits HIF-1alpha protein levels through an Nrf2/PMF-1-mediated increase in spermidine/spermine acetyl transferase. Cancer Chemother Pharmacol 2011;68(2):405–13.
- Jung YJ, Isaacs JS, Lee S, Trepel J, Neckers L. IL-1beta-mediated up-regulation of HIF-1alpha via an NFkappaB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB J 2003;17(14):2115–7.
- Jung Y, Isaacs JS, Lee S, Trepel J, Liu ZG, Neckers L. Hypoxia-inducible factor induction by tumour necrosis factor in normoxic cells requires receptor-interacting protein-dependent nuclear factor kappa B activation. Biochem J 2003;370(Pt 3):1011–7.
- Scortegagna M, Cataisson C, Martin RJ, Hicklin DJ, Schreiber RD, Yuspa SH, et al. HIF-1alpha regulates epithelial inflammation by cell autonomous NFkappaB activation and paracrine stromal remodeling. Blood 2008;111(7):3343–54.