Abstract
Background
In knee surgery a tourniquet is usually applied in order to provide a bloodless surgical field. This study aims to compare, in a tourniquet-induced ischemia–reperfusion model, the glutathione oxidation and lipid peroxidation in two different blood sources: an operated knee drainage tube or the antecubital vein.
Methods
Thirty-two patients undergoing total knee replacement were studied. We measured reduced glutathione (GSH), oxidized glutathione (GSSG), malondialdehyde (MDA) and other metabolic parameters in the blood from the operated knee (17 patients), or from the antecubital vein (15 patients) before tourniquet inflation (T0) and after tourniquet release at different times: 3, 10, and 60 minutes (T1, T2, and T3, respectively).
Results
We have found an early increase of approximately 100% in GSSG and MDA blood levels coinciding with a decrease in GSH levels at T1–T3 in blood from the operated knee. These changes were also observed in blood from the antecubital vein at T1 and T2, but at lower levels than in blood from the operated knee.
Conclusions
There is an intense glutathione oxidation and lipid peroxidation in the operated knee after tourniquet deflation. The surgical drainage is a good source of blood for the determination of biomarkers in a tourniquet-induced ischemia–reperfusion model, because changes are observed more early and it is more direct than another source of systemic blood.
Introduction
In orthopaedic surgery of arms and legs, particularly in total knee replacement, a tourniquet is usually applied in order to provide a bloodless surgical field. This results in ischemia–reperfusion (I–R) injury when blood perfusion is newly established with metabolic and hemodynamic responses to the tourniquet release. The pathogenesis of I–R damage is directly related with local and systemic inflammatory response and the production of radical oxygen species (ROS), including superoxide anions (O2˙−), hydroxyl radicals (OH˙), hypochlorous acid (HOCl), hydrogen peroxide (H2O2), and nitric oxide-derived peroxynitrite (ONOO−).Citation1 Determination of products of oxidative damage in biological systems is important to understand the role of ROS in disease states. Oxidative stress is defined as a disturbance between the prooxidant and the antioxidant balance and results in cell injury by oxidation of proteins, lipids, and DNA. Reduced glutathione (GSH) is a tripeptide (γ-Glu-Cys-Gly) and the most abundant thiol antioxidant in all living mammalian cells. However, the oxidized glutathione (GSSG), its disulfide form, is present in minimal amounts compared with the reduced form. The glutathione blood level is a good indicator of oxidative stress and may reflect glutathione redox status in other less accessible tissues. Measurement of GSH and GSSG in blood and the oxidized glutathione/total glutathione ratio are considered good indicators of oxidative stress in several physiological and pathological situations.Citation2,Citation3 Lipids peroxidation is a chain reaction leading to oxidation of polyunsaturated fatty acids which break the structure of biological membranes and produce toxic metabolites such as malondialdehyde (MDA) and other stable degradation products of lipid peroxides, which play an important role in reperfusion damage because they act locally but also act in other distant organs as lungs and circulatory system.Citation4–Citation11 Also recently have been reported two studies on tourniquet-induced I–R injury during lower extremity surgery, showing DNA damage in peripheral blood lymphocytes.Citation12,Citation13
So far all studies on oxidative stress in patients undergoing knee surgery have used the systemic blood, usually from an arm vein or the radial artery. For the first time we have performed an in situ oxidative stress study of blood collected from the surgical drainage catheter placed in the operated knee and we have compared this one with oxidative stress levels in the systemic blood from the antecubital vein. Therefore, blood was taken directly from the reperfused leg after tourniquet release. The aim of this work was to compare in situ (the operated knee) blood levels of GSH, GSSG, oxidized glutathione/total glutathione ratio and MDA with those of the systemic blood (an antecubital vein).
Materials and methods
Study design and patients
It is an observational study with 32 patients undergoing total knee replacement. These patients were randomly allocated to one of the two groups, according to the blood samples sources: directly from a drainage tube placed in the operated knee following tourniquet release (group K, n = 17 patients) or from an antecubital vein (group V, n = 15 patients). The biochemical analyst of blood samples was blinded to the patient group allocation. The study was conducted according to the Declaration of Helsinki and it was approved by the hospital ethics committee. Informed written consent was obtained. Thirty-two patients with ASA I-III (American Society of Anaesthesiology patient classification status) scheduled for elective total knee replacement surgery were recruited. Patients with malignant disease, active infection, or preoperative antioxidant therapy were excluded.
Anesthetic procedures
All patients were anesthetized by spinal anesthesia using a 25-gauge Whitacre pencil point needle in the L3–L4 inter-space with 2–2.5 ml of 0.5% hyperbaric bupivacaine and Fentanyl 20 µg. Postoperative analgesia was made by femoral nerve catheter infusion (levobupivacaine 0.125%) or patient-controlled analgesia with intravenous morphine (1 mg/ml). Patients received oxygen (2 l/min) by nasal cannulae during surgery and for 2 hours postoperatively. A pneumatic tourniquet was inflated to 250 mm Hg to interrupt arterial blood flow to the limb during surgery. Blood pressure, heart rate, and peripheral oxygen saturation were monitored in the operating room.
Blood collection
Blood was collected in a tube BD Vacutainer® tubes LH PST™ II with lithium heparin. The patients were divided into two groups: K and V. In a group (K), prior to inflation of the tourniquet, one basal blood sample was collected from a foot dorsal vein (T0). When the surgery finished and the tourniquet was released, three blood samples were collected from the surgical drainage tube placed by the surgeon during the surgery in the operated knee: 3 (T1), 10 (T2), and 60 minutes (T3) after tourniquet release. In the other group (V), similarly one basal blood sample was collected from a foot dorsal vein (T0), and when the surgery finished, and the tourniquet was released, three blood samples were collected from an antecubital vein at the same times as mentioned before (T0, T1, T2, and T3).
Malondialdehyde determination
The blood samples (1 ml) to determine MDA (CAS 542-78-9) were centrifuged at 3000 rpm for 12 minutes. Plasma was separated and stored at −80°C until analysis. Lipid peroxidation was measured in plasma using a high-performance liquid chromatography (HPLC) method which determines the MDA formed from lipid peroxides. Lipoperoxides are hydrolyzed by boiling in diluted phosphoric acid (CAS 7664-38-2). Malondialdehyde reacts with thiobarbituric acid (TBA) (CAS 504-17-6) to form an adduct MDA (TBA)2. The protein-free extract was fractioned by a HPLC method to separate the MDA-(TBA)2 adduct from interfering chromogens and quantified spectrophotometrically with UV light at 532 nm. The calibration curve was assayed using tetramethoxypropane (CAS 102-52-3) which undergoes hydrolysis to liberate stoichiometric amounts of malondialdehyde.Citation14
Reduced glutathione determination
Blood samples (1 ml) to determine GSH (CAS 70-18-8) were centrifuged at 3000 rpm for 12 minutes. Plasma was separated and stored at −80°C until analysis. GSH was measured spectrophotometrically using glutathione-S-transferase (CAS 50812-37-8) (EC 2.5.1.18) assay. In brief, the reaction between chlorodinitrobenzene (CAS 50812-37-8) and GSH in 0.1 M potassium phosphate (CAS 7778-77-0 and 7758-11-0) buffer pH 7.0, catalyzed by glutathione-S-transferase is followed at 340 nm. This method is specific for GSH measurement due to the specificity of glutathione-S-transferase for glutathione.Citation15
Oxidized glutathione determination
Blood samples (0.5 ml) to determine GSSG (CAS 27025-41-8) are treated with 0.5 ml of perchloric acid (CAS 7601-90-3) 6% to precipitate proteins. Containing N-ethylmaleimide (CAS 128-53-0) 20 mM as a GSH quenching agent in order to prevent GSH oxidation during sample preparation and bathophenanthroline disulfonic acid (CAS 52746-49-3) 1 mM as a metal chelator. Blood samples are then derivatized and analyzed by a HPLC method with detection at 365 nm, which was developed to measure GSSG in presence of a large excess of reduced glutathione.Citation2
The oxidized glutathione/total glutathione ratio was expressed as a percentage and was calculated as [2GSSG/(GSH + 2GSSG)].100, which shows the glutathione redox status and is a good index of oxidative stress.
The other clinical markers of tissue damage
We also measured myoglobin blood levels and other clinical markers of tissue damage by I–R such as lactate, pH, base excess, bicarbonate ion, lactate with an ABL 88 Flex apparatus (Radiometer, Copenhagen, Denmark).
Statistical analysis
Results are expressed as mean ± standard deviation. Differences between groups were analyzed with two-tailed Mann–Whitney U test. One-way analysis of variance and the Wilcoxon test were used to interpret the results within the groups. P values of <0.05 were considered statistically significant. The Statistical Package for the Social Sciences® (version 11.0) was used for analysis (SPSS, Chicago, IL, USA).
Results
The two groups were comparable in demographic and physical data. No differences were found between the two groups in tourniquet time, operative heart rate and mean arterial pressure at any of the observation times (see ). There were no differences between the two studied groups in peripheral oxygen saturation (97–99%) and total administration of colloids-crystalloids (average 1500 ml).
Table 1. Demographic and physical data
shows an increase in the oxidative stress marker levels in blood from the two groups at different observation times. However, we have found in blood drained from the operated knee a great and early increase (T1) of approximately 100% in GSSG and MDA blood levels (P < 0.001), coinciding with a decrease in GSH levels of about 33 and 50% in T1 and T2, respectively (P < 0.001 both) after tourniquet deflation. These cited changes were significantly greater in group K (blood taken from the operated knee) than in group V (blood taken from the antecubital vein), particularly in the first 3 (T1) and 10 minutes (T2) after tourniquet deflation and leg reperfusion (see ).
Figure 1. Time course of oxidized glutathione (GSSG) blood levels in patients undergoing total knee replacement. Values expressed as mean ± SD. Differences were analyzed with two-tailed tests for non-parametric data: Mann–Whitney U and Wilcoxon tests. P values of <0.05 were considered statistically significant. Patients were divided into two groups: group K, n = 17 (blood collected from a drainage tube placed in the operated knee), and group V, n = 15 (blood collected from a antecubital vein). Time course study: before surgery (T0) and after tourniquet deflation at different times: 3, 10, and 60 minutes (T1, T2, and T3, respectively). Statistical differences were observed in the same group (T0 vs. T1 or T2 or T3): *P < 0.05; **P < 0.01; ***P < 0.001. Statistical differences were observed between both groups (K vs. R): †P < 0.05; ††P < 0.01; †††P < 0.001.
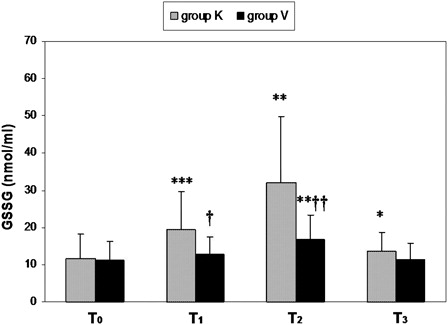
Table 2. Time course of glutathione oxidation and lipid peroxidation marker levels in blood in patients undergoing total knee replacement
shows significant changes of metabolic parameter levels and myoglobin in blood from the two groups of patients at different observation times. We observed a metabolic acidosis with decreased bicarbonate ion levels and base excess, accompanied by increased lactate levels as an early effect of I–R in the operated leg. However, those changes were significantly higher in group K than in group V (P < 0.001) at all times. Also we have found an increase in myoglobin blood levels in the two groups (P < 0.001 both), which was higher in group K than in group V (P < 0.05).
Table 3. Time course of metabolic parameter and myoglobin in blood
Discussion
Our study shown for the first time as the drainage tube placed in the operated knee was used for an in situ measurement of oxidative stress markers levels and others metabolic metabolites in blood after tourniquet deflation. This method is more sensitive, direct, and quicker than using the systemic blood from a peripheral artery or vein as the source for detection of changes in blood marker levels. In our opinion, this method is interesting for future studies on I–R and also for the application of preconditioning techniques in patients undergoing orthopedic surgery. In this regard and recently has been published a study on the protective effect of preconditioning by ischemia reperfusion in arthroscopic knee surgeryCitation16. Our method could have been useful and interesting in this type of study.
I–R injury induced by tourniquet application in knee surgery is an excellent model for the study of massive amounts of ROS produced and early released into the systemic circulation after reperfusion by tourniquet deflation.Citation14 Furthermore, a local oxidative injury abruptly occurs too, involving protein, lipids, and DNA, although not always clinically significant. In the last 10 years, it has been shown in several papers made in patients undergoing knee surgery with tourniquet ischemia, increases of lipid peroxidation markers in systemic blood (venous or arterial), measuring the concentration of TBARS (TBA reacting substance), MDA, F2-isoprostanes, or isofurans ischemia.Citation4–Citation11,Citation17–Citation19
The main finding for our study was that reduced glutathione and polyunsaturated fatty acids were oxidized very quickly with an increase in a 100% of GSSG and MDA blood levels, but coinciding with a decrease in GSH levels at T1–T3 in blood drained from operated knee (group K). These increases in GSSG and MDA blood levels were also observed in peripheral blood from a forearm vein (group V) at T1 and T2, but at lower levels than in blood from the operated knee. Our method of determination of GSSG and MDA levels using HPLC, has been used previously by our team on several works related to oxidative stress during colon surgeryCitation20,Citation21 and is characterized by its high specificity in comparison with other methods previously used in other studies related to this topic. It has been the first time that we measured the levels of GSH and GSSG in the blood from the reperfused leg after deflating the tourniquet, which is used as a good index of oxidative stress in pathological situations. So it has also increased very significantly in blood from operated knee drainage the oxidized glutathione/total glutathione ratio, indicating a large oxidation and consumption of GSH (the most abundant intracellular antioxidant) and a large generation of GSSG (disulfide form) which shows exactly the glutathione redox status in the operated knee. Prior to our findings, only one study showed changes in the glutathione levels in quadriceps muscle biopsy, before and 24 hours after tourniquet deflation, indicating oxidative stress in patients undergoing knee surgery.Citation22
Lactate levels, pH, base excess, and bicarbonate ion changed significantly in both groups after tourniquet release as a result of anaerobic metabolism and indicating tissue damage by ischemia. However, changes in group K were much more important than in group V for the same reason as the changes described in the oxidative damage markers, therefore with more true and better reflect the reality in the reperfused leg.
In conclusion, we have shown that knee surgery with tourniquet is the ideal model to study the phenomenon of I–R, which allows us to detect quickly enough, an in situ increase of oxidative stress marker levels in blood drained directly from the operated knee. Therefore, we have found for the first time that levels of oxidative stress markers are higher in blood from the reperfused leg than in blood from peripheral blood. In view of these results and as prevention, we should minimize the maximum ischemic time in knee surgery with tourniquet.
Acknowledgements
The authors thank Prof. Dr José Viña and Maria Dolores Royo (Department of Physiology, University of Valencia) for his generous facilities and her skilful technical assistance, respectively. They also thank Koen J. Mateboer and Cristina Soler Sanchez for proofreading the English manuscript.
References
- Collard CD, Gelman S. Pathophysiology, clinical manifestations, and prevention of ischemia-reperfusion injury. Anesthesiology 2001;94:1133–8.
- Asensi M, Sastre J, Pallardó FV, García de la Asunción J, Estrela JM, Viña J. A high performance liquid chromatography method for measurement of glutathione in biological samples. Anal Biochem 1994;217:323–28.
- Asensi M, Sastre J, Pallardó FV, Lloret A, Lehner M, García de la Asunción J, Viña J. Ratio of reduced to oxidized glutathione as indicator of oxidative stress status and DNA damage. Methods Enzymol 1999;299:267–76.
- Adachi J, Kurisaki E, Kudo R, Nakagawa K, Hatake K, Hiraiwa K, et al. Enhanced lipid peroxidation in tourniquet-release mice. Clin Chim Acta 2006;371:79–84.
- Carmo-Araujo EM, Dal-Pai-Silva M, Dal-Pai V, Cecchini R, Anjos FA. Ischaemia and reperfusion effects on skeletal muscle tissue: morphological and Histochemical studies. Int J Exp Pathol 2007;88:147–54.
- Saricaoglu F, Dal D, Salman E, Atay ÖA, Doral MN, Salman MA, et al. Effect of low-dose N-acetyl-cysteine infusion on tourniquet-induced ischaemia reperfusion injury in arthroscopic knee surgery. Acta Anesthesiol Scand 2005;49:847–51.
- Ertuk E, Cecik B, Geze S, Kosucu M, Coskun I, Eroglu A, et al. Comparison of the effect of propofol and N-acetyl cysteine in preventing ischemia-reperfusion injury. Eur J Anaesthesiol 2009;26:279–84.
- Mas E, Barden AE, Corcoran TB, Phillips M, Jackson Roberts L, Mori TA. Effects of spinal or general anesthesia on F2-isoprostanes and isofurans during ischemia/reperfusion of the leg in patients undergoing knee replacement surgery. Free Radic Biol Med 2011;50:1171–6.
- Girardis M, Milesi S, Donato S, Raffaeli M, Spasiano A, Antonuto G, et al. The hemodynamic and metabolic effects of tourniquet application during knee surgery. Anesth Analg 2000;91:727–31.
- Lee JY, Kim CJ, Chung MY. Effect of high-dose vitamin C on oxygen free radical production and myocardial enzyme after tourniquet ischemia reperfusion injury during bilateral total knee replacement. J Int Med Res 2010;38:1519–29.
- Li-na L, Liang-rong W, Wang-tie W, Lie-lie J, Xi-yue Z, Liu-pu Z, et al. Ischemic preconditioning attenuates pulmonary dysfunction after unilateral thigh tourniquet-induced ischemia-reperfusion. Anesth Analg 2010;111:539–43.
- Karhalil B, Polat S, Senkoylu A, Bölükbaşi S. Evaluation of DNA damage after tourniquete-induced ischaemia/reperfusion injury during lower extremity surgery. Injury 2010;41:758–62.
- Lialiaris T, Kouskoukis A, Tiaka E, Digkas E, Beletsiotis B, Vlasis K, et al. Cytogenetic damage after ischemia and reperfusion. Genet Test Mol Biomark 2010;14 (4):471–5.
- Wong SH, Knight JA, Hopfer SM, Zaharia O, Leach CN, Sunderman FW Lipoperoxides in plasma as measured by liquid-chromatographic separation of malondialdehyde-thiobarbituric acid adduct. Clin Chem 1987;33:214–20.
- Brigelius R, Merckel C, Akerboon T, Sies H. Identification and quantification of glutathione in hepatic protein mixed disulfides and its relationship to glutathione disulfide. Biochem Pharmacol 1983;32:2529–34.
- Koca K, Yurttas Y, Cayci T, Bilgic S, Kaldirim U, Durusu M, et al. The role of preconditioning and N-acetylcysteine on oxidative stress resulting from tourniquet-induced ischemia-reperfusion in arthroscopic knee surgery. J Trauma Inj Infect Crit Care 2011;70(3):717–23.
- Cheng Y-J, Chien C-T, Chen C-F. Oxidative stress in bilateral total knee replacement, under ischemic tourniquet. J Bone Joint Surg 2003;85-B:679–82.
- Aldemir O, Celebi H, Cevik C, Duzgun E. The effects of propofol or halothane on free radical production after tourniquet induced ischemia-reperfusion injury during knee arthroplasty. Acta Anesthesiol Scand 2001;45:1221–5.
- Cheng YJ, Wang YP, Chien CT, Chen CF. Small-dose propofol sedation attenuates the formation of reactive oxygen species in tourniquet-induced ischemia-reperfusion injury under spinal anesthesia. Anesth Anal 2002;94:1617–20.
- García-de-la-Asunción J, Belda FJ, Greif R, Barber G, Viña J, Sastre J. Inspired supplemental oxygen reduces markers of oxidative stress during elective colon surgery. Br J Surg 2007;41:475–7.
- García-de-la-Asunción J, Barber G, Rus D, Perez-Griera J, Belda FJ, Martí F, et al. Hyperoxia during colon surgery is associated with a reduction of xanthine oxidase activity and oxidative stress in colonic mucosa. Redox Rep 2011;16:121–8.
- Westman B, Weidenhielm L, Rooyackers O, Fredricksson K, Wernerman J, Hammarqvist F. Knee replacement surgery as a human clinical model of the effects of ischemia/reperfusion upon skeletal muscle. Clin Sci 2007;113:313–8.