Abstract
Objectives
We examined whether a single exposure of rats to water-immersion restraint stress (WIRS) induces oxidative stress in the thymus and spleen.
Methods
Vitamin E, ascorbic acid, reduced glutathione (GSH), and lipid peroxide (LPO) were assayed in the thymus and spleen of rats with and without 6 hours of WIRS.
Results
In unstressed rats, vitamin E, ascorbic acid, GSH, and LPO levels were higher in the thymus than in the spleen. Thymic ascorbic acid level was lower in stressed rats than in unstressed rats. Splenic ascorbic acid level was similar in both groups. Thymic and splenic GSH levels were lower in stressed rats than in unstressed rats but the reduced amount of GSH was lower in the spleen than in the thymus. Thymic vitamin E level was lower in stressed than in unstressed rats. Splenic vitamin E level was higher in stressed rats than in unstressed rats. Thymic and splenic LPO levels were higher in stressed rats than in unstressed rats but the increased amount of LPO was higher in the thymus than in the spleen.
Conclusion
It is indicated that a single expose of rats to WIRS induces oxidative stress more severely in the thymus than in the spleen.
Water-immersion restraint stress (WIRS) has been used frequently to induce stress-induced gastric mucosal lesions in experimental animals because WIRS induces stress-induced gastric mucosal lesions in a high reproducibility.Citation1 We have reported that decreases in the levels of non-enzymatic antioxidants such as ascorbic acid (or vitamin C), non-protein-SH including reduced glutathione (GSH), and vitamin E occurs with an increase in the level of lipid peroxide (LPO) in the damaged gastric mucosa of rats exposed to WIRS for 6 hours.Citation2 We have also reported that decreases in ascorbic acid and GSH levels occur with an increase in LPO level in the damaged liver of adult rats with 6 hours of WIRS and that cell damage and an increase in LPO level occur not only in the liver but also in the kidney, heart, and skeletal muscle of adult rats with 6 hours of WIRS, although a WIRS-induced change in vitamin E level differs among these tissues.Citation3,Citation4 It has been shown that a single pre-administration of vitamin C or vitamin E suppresses oxidative damage by attenuating increased lipid peroxidation and disrupted non-enzymatic antioxidant status in the gastric mucosa, liver, kidney, heart, and skeletal muscle of adult rats with 6 hours of WIRS.Citation4–Citation6 Thus, a single exposure of adult rats to WIRS induces oxidative damage in several tissues of the animals by impairing non-enzymatic antioxidant status in those tissues.
The thymus is a primary lymphoid organ with both endocrine and immune functions. The spleen is the largest secondary lymphoid organ which is responsible for initiating immune reactions to blood-born antigen and for filtering the blood of foreign material and old or damaged red blood cells. It has been reported that the immune functions in the lymphoid tissues of rats are particularly sensitive to a change in the antioxidant status and oxidative stress.Citation7 Al-OrfCitation8 has demonstrated that feeding of oxidized phosphatidylcholine to rats causes oxidative stress not only in the liver, kidney, heart, and lung, but also in the thymus and spleen and that the susceptibility to oxidative stress is higher in the thymus than in the spleen. Srikumar et al.Citation9 and Parathasarathy et al.Citation10 have reported that, in normal adult rats, GSH level is higher in the spleen than in the thymus, while vitamin C level is higher in the thymus than in the spleen, although the thymus has higher LPO concentration than the spleen. The same authors have also reported that superoxide dismutase (SOD) and catalase activities are similar in the thymus and spleen of normal adult rats, although glutathione peroxidase (GPX) activity is slightly higher in the spleen than in the thymus.Citation9,Citation10 In contrast, Ganesan et al.Citation11 have shown in normal adult rats that GSH and LPO levels and catalase and GPX activities are similar in the thymus and spleen, while SOD and glutathione S-transferase activities are lower in the thymus than in the spleen. Vitamin E is an important antioxidant to break the chain reaction of lipid peroxidation in cellular membranes. This vitamin exerts its antioxidant action more efficiently by interacting with ascorbic acid and GSH.Citation12 It has been shown in young rats that vitamin E level is higher in the spleen than in the liver and kidney, while the level of LPO is lower in the spleen than in the liver and kidney.Citation13 However, there is no information on whether vitamin E level is different between the spleen and thymus of normal adult rats.
It has been reported that exposure of adult rats to noise stress (100 dB, 4 hours/day) for 15 days induces oxidative stress in the thymus and spleen to a similar extent on the basis of noise stress-induced changes in the levels of LPO, GSH, and antioxidant enzymes such as SOD, catalase, and GPX in these tissues.Citation9 It has also been reported that exposure of adult rats to immobilization stress (6 hours/day) for 9 days causes an increase in LPO level and a decrease in total antioxidant activity in the spleen.Citation14 A recent report has shown that chronic exposure of adult rats to restraint stress (6 hours/day for 30 days) induces oxidative damage in the thymus and spleen through reductions of GSH level and SOD, catalase, GPX, and glutathione S-transferase activities and an increase in LPO level in the those tissues with an increase in serum corticosterone level.Citation11 To the best of our knowledge, however, there is no information on whether and how a single exposure of adult rats to WIRS as acute stress induces oxidative stress in the thymus and spleen.
In this study, therefore, we examined whether a single exposure of adult rats to WIRS induces oxidative stress in the thymus and spleen by impairing the status of non-enzymatic antioxidants such as ascorbic acid, GSH, and vitamin E in these tissues.
Materials and methods
Chemicals
l-Ascorbic acid, bovine serum albumin, 2,2′-bipyridyl, corticosterone, ethylenediaminetetraacetric acid (EDTA), 2-thiobarbituric acid (TBA), α-tocopherol (α-Toc), γ-tocohperol, and other chemicals were purchased from Wako Pure Chemical Ind., Ltd. (Osaka, Japan). All chemicals used were of reagent grade and were not further purified.
Animals
Male Wistar rats aged 3 weeks were purchased from Nippon SLC Co. (Hamamatsu, Japan). The animals were housed in plastic cages with soft chips in a ventilated animal room with controlled temperature (23 ± 2°C) and relative humidity (55 ± 5%) with 12 hours of light (7:00–19:00). The animals were maintained with free access to rat chow, a regular diet D1001 (Research Diets Inc., New Brunswick, NJ, USA), and tap water for 4 weeks. All animals received humane care in compliance with the Guidelines of the Management of Laboratory Animals in Fujita Health University. This animal experiment was approved by the Institutional Animal Care Committee and its approved protocol number was M 14–02.
Induction of WIRS
All 7-week-old rats used were starved for 24 hours before the onset of WIRS, but were allowed free access to tap water. The starved animals were retrained in wire stress cages and immersed up to the depth of the xiphoid process in a water bath (23°C) for 6 hours to induce WIRS, as described in our previous reports.Citation2–Citation6 The remaining starved animals without WIRS were further starved for 6 hours with free access to tap water.
Sample preparation
Immediately after the end of WIRS, all rats were weighed and then sacrificed under ether anesthesia at which time blood was collected from the inferior vena cava. Serum was obtained from the collected blood by centrifugation. Immediately after sacrifice, perfusion was conducted through the portal vein with ice-cold 0.9% NaCl to remove blood remaining in tissues and then thymus and spleen were removed. The removed thymus and spleen were washed in ice cold 0.9% NaCl, wiped on a paper filter, and then weighed. The obtained serum, thymus, and spleen were stored at −80°C until use.
Assays of serum and tissue components
Serum corticosterone was assayed by the fluorometric method of Guillemin et al.Citation15 Serum glucose was assayed using a commercial kit of Glucose C-II Test Wako (Wako Pure Chemicals Ind., Ltd). Serum LPO was assayed by the fluorometric TBA method of YagiCitation16 using tetramethoxypropane as a standard. Serum ascorbic acid was assayed by the colorimetric bipyridyl method of Zannoni et al.Citation17 using l-ascorbic acid as a standard. Serum GSH was assayed using a commercial GSH assay kit of BIOXYTECH GSH-400 (OxisResearch, Portland, OR, USA). Vitamin E was assayed by the high-performance liquid chromatographic method with electrochemical detection using γ-tocopherol as an internal standard as described in our previous report.Citation18 Thymus and spleen tissues were homogenized in nine volumes of ice cold 0.05 M Tris–HCl buffer (pH 7.4) containing 1 mM EDTA using a Handy Microhomogenizer, Physcotron NS-310E (Micotech Co., Funahashi, Japan). The prepared tissues were sonicated on ice for 60 seconds using a Handy Sonic model UR-20P (Tomy Seiko Co., Tokyo, Japan). Ascorbic acid, GSH, and vitamin E in thymus and spleen tissue samples were assayed by the same methods as used for the assays of serum ascorbic acid, GSH, and vitamin E, respectively. LPO in thymus and spleen tissue samples was assayed by the colorimetric TBA method of Ohkawa et al.Citation19 using tetramethoxypropane as a standard except that I mM EDTA was added to the reaction mixture. The concentration of LPO in serum and tissues is expressed as that of malondialdehyde (MDA) equivalents. The concentration of vitamin E in serum and tissues is expressed as that of α-Toc. Protein in thymus and spleen tissue samples was assayed using a commercial kit of Protein Assay Rapid Kit Wako (Wako Pure Chemicals Ind., Ltd.). Bovine serum albumin was used as a standard in this protein assay.
Statistical analysis
All results obtained are expressed as the mean ± SD. The statistical analyses of the data were performed using a computerized Excel statistical package. The data on serum corticosterone, glucose, LPO, ascorbic acid, GSH, and vitamin E and tissue weight in stressed and unstressed groups were statistically analyzed using Student's t-test. The data on LPO, ascorbic acid, GSH, and vitamin E in the thymus and spleen of stressed and unstressed rats were compared by two-way analysis of variance and Sheffe post hoc test for multiple comparisons. The significance level was set at P < 0.05.
Results and discussion
The effects of stress are mainly due to the activation of both the hypothalamic-pituitary-adrenocortical (HPA) axis and sympathetic nervous system (SNS). Serum corticosterone and glucose concentrations in rats with 6 hours of WIRS were significantly higher than those in rats without WIRS; stressed rats had 2.9- and 2.4-fold higher serum corticosterone and glucose concentrations, respectively, than unstressed rats (A and B). These results are well consistent with those reported previously.Citation4 It has been shown that plasma epinephrine and norepinephrine levels are increased via activation of the SNS in fasted rats with 6 hours of WIRS.Citation20 It has also been shown that hyperglycemia in fasted rats with immobilization stress is mediated by the elevation of epinephrine in the adrenal medulla through activation of the SNS.Citation21 Thus, stress-induced activation of the HPA axis and SNS was observed in the fasted rats with 6 hours of WIRS
Figure 1. Effect of WIRS on serum corticosterone (A), glucose (B), and LPO (C) concentrations in rats. Each value is a mean ± SD (n = 5 for rats without WIRS; n = 8 for rats with WIRS). *Significantly different from rats without WIRS (P < 0.05).
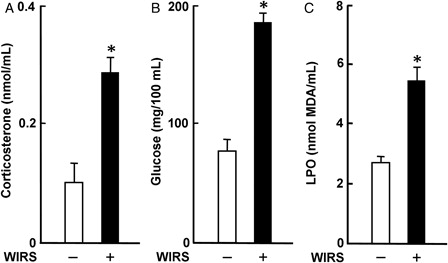
Serum LPO concentration in rats with WIRS was significantly higher than that in rats without WIRS; stressed rats had a 2.1-fold higher serum LPO concentration than unstressed rats (C). This result is well consistent with that reported previouslyCitation4–Citation6 and suggests the systemic occurrence of oxidative stress in rats with 6 hours of WIRS.
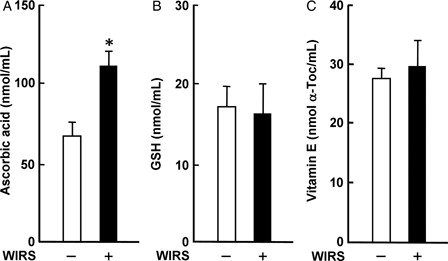
Serum ascorbic acid concentration in rats with WIRS was significantly higher than that in rats without WIRS; stressed rats had a 1.7-fold higher serum ascorbic acid concentration than unstressed rats (A). In contrast, there were no significant differences in serum GSH and vitamin E concentrations between rats with and without WIRS (B and C). The increase in serum ascorbic acid concentration in rats with 6 hours of WIRS was consistent with the result shown in our previous report.Citation6 It has been suggested that the increase in serum ascorbic acid concentration in rats with 6 hours of WIRS could be due to an enhanced release of ascorbic acid from the damaged liver.Citation6
Figure 2. Effect of WIRS on serum ascorbic acid (A), GSH (B), and vitamin E (C) concentrations in rats. Each value is a mean ± SD (n = 5 for rats without WIRS; n = 8 for rats with WIRS). *Significantly different from rats without WIRS (P < 0.05).
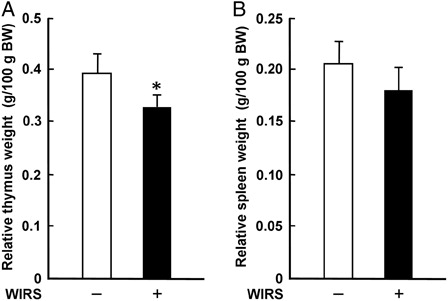
It has been demonstrated that glucocorticoids secreted from the adrenal gland via activation of the HPA axis cause thymic involution with a profound reduction of thymic mass and volume through induction of thymocyte apoptosis under stress conditions.Citation22 Although the mechanism(s) for splenic involution under stress conditions has not been clarified enough yet, it is known that splenic involution occurs later than thymic involution in mice with various periods of immobilization stress.Citation23 The relative thymus weight in rats with WIRS was significantly lower than that in rats without WIRS; the relative thymus weight in stressed rats was 83% of that in unstressed rats (A). There was no significant difference in the relative spleen weight between stressed and unstressed rats, although the relative spleen weight tended to be lower in stressed rats than in unstressed rats (B). These results indicate that, in rats with 6 hours of WIRS, the sensitivity to stress response is higher in the thymus than in the spleen.
Figure 3. Effect of WIRS on the relative weights of the thymus (A) and spleen (B) in rats. The weights of the removed thymus and spleen are expressed as the relative tissue weight based on 100 g body weight (BW). Each value is a mean ± SD (n = 5 for rats without WIRS; n = 8 for rats with WIRS). *Significantly different from rats without WIRS (P < 0.05).
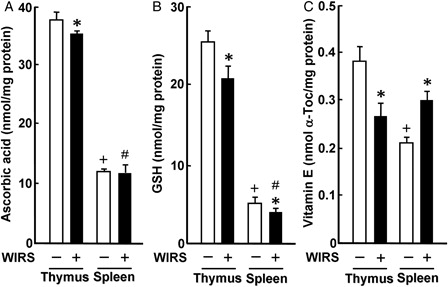
In rats without WIRS, ascorbic acid, GSH, and vitamin E concentrations were approximately 3-, 5-, and 2-fold higher in the thymus than in the spleen, respectively (). The result on the difference in ascorbic acid concentration between the thymus and spleen of unstressed rats was similar to that reported by Srikumar et al.Citation9 or Parathasarathy et al.Citation10 However, the result on the difference in GSH concentration between the thymus and spleen of unstressed rats was reverse to the result reported by Parathasarathy et al.Citation10 The reason for this discrepancy as to the difference in GSH concentrations between the thymus and spleen of unstressed rats is unknown at present, although there were differences in age and feeding condition in the experiment using male Wistar rats between this study and the study of Parathasarathy et al.Citation10 Thymic ascorbic acid concentration in rats with WIRS was significantly less than that in rats without WIRS, while there was no significant difference in splenic ascorbic acid concentration between stressed and unstressed rats (A). Thymic and splenic GSH concentrations in stressed rats were significantly lower than those in unstressed rats; thymic and splenic GSH concentrations in stressed rats were 82 and 77% of those in unstressed rats, respectively (b). However, the reduced amount of GSH was significantly much larger in the thymus than in the spleen (P < 0.05) (B). Thymic vitamin E concentration was significantly lower in stressed rats than in unstressed rats; thymic vitamin E concentration in stressed rats was 69% of that in unstressed rats (C). In contrast, splenic vitamin E concentration was significantly higher in stressed than in unstressed rats; stressed rats had a 1.4-fold higher splenic vitamin E concentration than unstressed rats (C). Thus, a single exposure of rats to 6 hours of WIRS was found to impair the thymic non-enzymatic antioxidant status more severely than the splenic non-enzymatic antioxidant status. The increase in vitamin E concentration found in the spleen of rats with 6 hours of WIRS may be the phenomenon of adaptation to oxidative stress in the tissue. It has been shown that 6 hours of WIRS causes an increase in vitamin E concentration in the kidney and heart, but not the liver and skeletal muscle, of rats.Citation4 However, the mechanism(s) for the increase in vitamin E concentration in the spleen as well as the kidney and heart of rats by exposure to WIRS should be further studied.
Figure 4. Effect of WIRS on ascorbic acid (A), GSH (B), and vitamin E (C) concentrations in the thymus and spleen of rats. Each value is a mean ± SD (n = 5 for rats without WIRS; n = 8 for rats with WIRS). *Significantly different from rats without WIRS (P < 0.05). +Significantly different from the thymus of rats without WIRS (P < 0.05). #Significantly different from the thymus of rats with WIRS (P < 0.05).
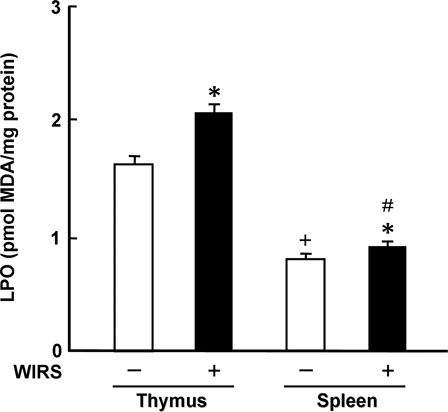
In rats without WIRS, thymic LPO concentration was approximately 2-fold higher than splenic LPO concentration (). This result was similar to the result reported by Srikumar et al.Citation9 or Parathasarathy et al.Citation10 Thymic and splenic LPO concentrations in rats with WIRS were significantly higher than those in rats without WIRS; stressed rats had 1.3- and 1.1-fold higher thymic and splenic LPO concentrations than unstressed rats, respectively (). However, the increased amount of LPO was significantly much larger in the thymus than in the spleen (P < 0.05) (). These results suggest that the severity of oxidative stress is larger in the thymus than in the spleen of rats with WIRS. Furthermore, one can think that this difference in the severity of oxidative stress between the thymus and spleen of rats with WIRS is associated with the above-described difference in impaired non-enzymatic antioxidant status between the two tissues. It has been reported that when mice receive a single dose of lipid hydroperoxide orally, increased LPO concentration in the thymus is higher than that in the spleen at 24 hours after the administration, while a reduction of the thymus weight is larger than that of the spleen weight at the same time point.Citation24 Therefore, there is a possibility that enhanced lipid peroxidation contributes to the above-described reduction of the relative thymus weight in rats with 6 hours of WIRS.
Figure 5. Effect of WIRS on LPO concentration in the thymus and spleen of rats. Each value is a mean ± SD (n = 5 for rats without WIRS; n = 8 for rats with WIRS). *Significantly different from rats without WIRS (P < 0.05). +Significantly different from the thymus of rats without WIRS (P < 0.05). #Significantly different from the thymus of rats with WIRS (P < 0.05).
In conclusion, the results obtained in this study indicate that a single expose of adult rats to WIRS induces oxidative stress more severely in the thymus than in the spleen by impairing non-enzymatic antioxidant status in these tissues in a different manner.
References
- Takagi K, Okabe S. The effects of drugs on the stress ulcer in rats. Jpn J Pharmacol 1968;18:9–18.
- Ohta Y, Kamiya Y, Imai Y, Arisawa T, Nakano H. Role of gastric mucosal ascorbic acid in gastric mucosal lesion development in rats with water immersion restraint stress. Inflammopharmacology 2005;13:249–59.
- Ohta Y, Chiba S, Tada M, Imai Y, Kitagawa A. Development of oxidative stress and cell damage in the liver of rats with water-immersion restraint stress. Redox Rep 2007;12:139–47.
- Ohta Y, Kaida S, Chiba S, Tada M, Teruya A, Imai Y, et al. Involvement of oxidative stress in increases in the serum levels of various enzymes and components in rats with water-immersion restraint stress. J Clin Biochem Nutr 2009;45:1–8.
- Ohta Y, Imai Y, Kaida S, Kamiya Y, Kawanishi M, Hirata I. Vitamin E protects against stress-induced mucosal lesions in rats more effectively than vitamin C. Biofactors 2010;36:60–9.
- Kaida S, Ohta Y, Imai Y, Kawanishi M. Protective effect of ascorbic acid against oxidative damage in the liver of rats with water-immersion restraint stress. Redox Rep 2010;15:11–9.
- Gu J-Y, Wakizono Y, Sunada Y, hung P, Nonaka M, Sugano M, et al. Dietary effect of tocopherols and tocotorienols on the immune function of spleen and mesenteric lymph node lymphocytes in Brown Norway rats. Biosci Biotechnol Biochem 1999;63:1697–702.
- Al-Orf SM. Effect of oxidized phosphatidylcholine on biomarkers of oxidative stress in rats. Ind J Clin Biochem 2011;26:154–60.
- Srikumar R, Parathasarathy NJ, Mainkandan S, Narayanan GS, Sheeladevi R. Effect of Triphala on oxidative stress and on cell-mediated immune response against noise stress in rats. Mol Cell Biochem 2006;283:67–74.
- Parathasarathy NJ, Kumar RS, Manikandan S, Devi RS. Methanol-induced oxidative stress in rat lymphoid organs. J Occup Health 2006;48:20–27.
- Ganesan B, Anandan R, Lakshmanan PT. Studies on the protective effects of betaine against oxidative damage during experimentally induced restraint stress in Wistar albino rats. Cell Stress Chaperones 2011;16:641–52.
- Liebler DC. The role of metabolism in the antioxidant function of vitamin E. Crit Rev Toxicol 1993;23:147–69.
- Azhar S, Cao L, Reaven E. Alteration of the adrenal antioxidant defense system during aging in rats. J Clin Invest 1995;96:1414–24.
- Lee CY, Cheng HM, Sim SM. Mulberry leaves protect rat tissues from immobilization stress-induced inflammation. Biofactors 2007;31:25–33.
- Guillemin R, Clayton GW, Lipsomb HS, Smith JG. Fluorometric measurement of rat plasma and adrenal corticosterone concentration. A note on technical details. J Lab Clin Med 1959;53:830–2.
- Yagi K. A simple fluorometric assay for lipoperoxides in blood sample. Biochem Med 1976;15:131–8.
- Zannoni V, Lynch M, Goldstein S, Sato PA. A rapid micromethod for the determination of ascorbic acid in plasma and tissue. Biochem Med 1974;11:41–8.
- Kamiya Y, Ohta Y, Imai Y, Arisawa T, Nakano H. A critical role of gastric mucosal ascorbic acid in the progression of acute gastric mucosal lesions induced by compound 48/80. World J Gastroenterol 2005;11:1324–32.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979;95:351–8.
- Arakawa H, Kodama H, Matsuoka N, Yamaguchi I. Stress increases plasma enzyme activity in rats: differential effects of adrenergic and cholinergic blockers. J Pharmacol Exp Ther 1997;280:1296–303.
- Yamada F, Inoue S, Saitoh T, Tanaka K. Glucoregulatory hormones in the immobilization stress-induced increase of plasma glucose in fasted and fed rats. Endocrinology 1993;132:2199–205.
- Bejelaković G, Stojanovic I, Jetvtovic-Stoimenov T, Pavlović D, Kocić G, Kamenov B, et al. Thymus as a target of glucocorticoid action: what are the consequence of glucocorticoids thymectomy? J Basic Clin Physiol Pharmacol 2009;20:99–125.
- Domínguez-Gerpe L, Rey-Méndez M. Time-course of the murine lymphoid tissue involution during and following stressor exposure. Life Sci 1997;61:1019–27.
- Oarada M, Ito E, Terao K, Miyazawa T, Fujimoto K, Kaneda T. The effect of dietary lipid hydroperoxide on lymphoid tissues in mice. Biochim Biophys Acta 1988;960:229–35.