Abstract
Objective
An experimental animal model of contact dermatitis (CD) was used to investigate the effects of free and nanoencapsulated clobetasol propionate on the skin and on the oxidative profile of liver tissue.
Methods
Female Wistar rats were divided into six groups, each containing eight rats. The first group, control (C), was sensitized with solid vaseline. Group 2, (CD), was sensitized with 5% NiSO4. Groups 3 and 4 were sensitized with 5% NiSO4 and treated with free (FC) and nanoencapsulated (NC) clobetasol (0.42 mg/g), respectively, daily for 5 days. Group 5 was treated with nanoencapsulated clobetasol (0.42 mg/g) on days 1, 3, and 5 (C135) and group 6 received a hydrogel containing empty nanoparticles (NP) daily for 5 days. Thiobarbituric acid reactive substances (TBARS), carbonyl levels, non-protein sulfhydryl groups (NPSH) and catalase activity were measured in liver homogenates.
Results
A significant increase was observed in the levels of TBARS, NPSH, and catalase activity for the groups CD and NP.
Discussion
Our results suggest that both NiSO4 sensitization and NP administration induced oxidation of cellular lipids and activated the antioxidant enzyme catalase to protect from this damage. These results also indicated that daily treatment with the free and nanoencapsulated clobetasol, as well as treatment with the nanoencapsulated clobetasol every other day, were able to prevent these redox alterations and protect against histological damage.
Introduction
Contact dermatitis (CD) is a disorder that affects the skin, which is the body's first barrier against the physical and chemical agents present in the environment. According to the physiopathological mechanisms involved, there are two types of CD: irritative and allergic.Citation1,Citation2 Irritative CD is caused by the pro-inflammatory and noxious effects of xenobiotics (e.g. strong acid or alkali, soaps, detergents, solvents) that are able to activate skin immunity. Allergic contact dermatitis (ACD) requires the activation of antigen-specific acquired immunity, leading to the development of effector T cells that mediate cutaneous inflammation.Citation1–Citation3
ACD, also known as contact hypersensitivity (CHS), is an inflammatory reaction of the skin that is mediated by T cells and results from repeated contact with non-protein chemical substances known as haptens.Citation4 This is different from classical delayed hypersensitivity, which requires an intradermal shot of exogenous proteins.Citation1 In CHS, the dermatitis is triggered by the topical application of haptens, such as nickel, chrome, dinitrofluorobenzene (DNFB), trinitrochlorobenzene (TNCB), and oxazoline, which in turn sensitizes the epidermis.Citation4–Citation8
Clobetasol propionate is a synthetic glucocorticoid. It is widely used due its non-specific anti-inflammatory and immunosuppressive effects, which leads to vasoconstriction and decreased collagen synthesis.Citation9 It is administrated topically for the treatment of inflammatory disease, such as psoriasis, discoid lupus erythematosus, serious atopic dermatitis, neurodermatitis, scalp dermatoses, and other disorders that do not respond satisfactorily to less potent steroids.Citation10,Citation11 The minimization of systemic absorption by topical application of clobetasol should decrease the incidence of side effects, such as skin thinning, cellular atrophy, the appearance of veins, adrenal suppression, growth delay in children, weight gain, and glaucoma.Citation12,Citation13
Recently, there has been increased interest in the use of topical drug delivery systems (TDDSs), such as liposomes,Citation14,Citation15 solid lipid microparticles,Citation16 solid lipid nanoparticles,Citation17 and polymeric nanoparticles.Citation18,Citation19 Among these TDDSs, the polymeric nanoparticles have demonstrated many advantages, such as higher physicochemical stability compared with liposomes. This enhances the protective effect and diminishes the direct contact of the drug with the skin.Citation20–Citation22 These polymeric nanoparticles can modulate the release of the drug through the skin and its permeation into the different skin layers.Citation23,Citation24
Furthermore, polymeric nanoparticles may allow for the prolonged release of the drug.Citation25,Citation26 This characteristic is important in those cases where a high concentration of the drug may cause irritation. Therefore, polymeric nanoparticles can supply the skin with the drug and decrease the systemic absorption.Citation27 As result of their gradual drug release, polymeric nanocapsules have been extensively studied.Citation28,Citation29 Many researchers have administered topical medicines containing nanocapsules and found that the release of drug was reinforced by the gradual release of the drug into the skin.Citation18,Citation20,Citation30 Researchers investigated whether the drug could pass through the skin and determined that the majority of the applied drug was restricted to the stratum corneum upper layer and was therefore unable to cross the skin and reach the systemic circulation.Citation24,Citation31
Recently, our group developed a hydrogel nanomedicine containing clobetasol-loaded nanocapsules for topical administration. Our results showed that drug release from this hydrogel was prolonged when administered in this formulation. In addition, it was possible to improve the efficacy of the treatment of CD in an in vivo rat model.Citation32
As previously mentioned, clobetasol propionate induces epithelial changes and can also be absorbed and carried to the bloodstream. Once the drug has been absorbed, the liver is the major organ involved in its metabolism. The aim of this study is to establish an experimental model for CD in Wistar rats by sensitizing with nickel sulfate (NiSO4) and to determine the effect of treatment with free and nanoencapsulated clobetasol in this established model.
Materials and methods
Reagents
Nickel sulphate (Vetec, Brazil), solid vaseline (Embacaps, Brazil), Coomassie Brilliant Blue G-250 (Sigma-Aldrich, St Louis, MO, USA), clobetasol propionate was a gift from Neo Química (Goiás, Brazil). All other reagents used in the experiments were of analytical grade and of highest purity.
Animals
Female Wistar rats (280–300 g), with a mean age of 12 weeks, were obtained from the Central Animal House of the Federal University of Santa Maria (UFSM) for use in this study. They were housed five to a cage with a natural day/night cycle at a temperature of 22–24°C. They had access to water and standard chow ad libitum. All animal procedures were approved by the Animal Ethics Committee at UFSM.
Groups
The animals were divided into six groups of eight rats each. Group I: control (C), sensitized only with solid vaseline. Group II: CD, induced by sensitization with 5% NiSO4 dissolved in vaseline. Group III: CD treated with free clobetasol (FC; 0.42 mg/g) daily for 5 days. Group IV: CD treated with nanoencapsulated clobetasol (NC; 0.42 mg/g) daily for 5 days. Group V: CD treated with nanoencapsulated clobetasol (0.42 mg/g) on days 1, 3, and 5 (C135). Group VI: CD treated daily with the hydrogel containing empty nanoparticles (NP).
Induction of contact dermatitis and treatment with clobetasol
First, all the animals were trichotomized in the abdominal region. The control group was sensitized in the abdomen with vaseline only. Groups CD, FC, and NC were sensitized with 4 g of solid vaseline containing 5% NiSO4. Six days after sensitization, 6 g of solid vaseline containing 5% NiSO4 was applied to each ear that had already been trichotomized. Five applications of vaseline containing 5% NiSO4 were performed at 72 hours intervals. The CD group was anesthetized with isoflurane and euthanized 72 hours after the last application. The FC group was treated with 6 g of free clobetasol (0.42 mg/g) and the NC group was treated with 6 g of nanoencapsulated clobetasol at the same dose, daily for 5 days. Three days after treatment, the FC and NC groups were euthanized. The C135 group was treated with 6 g of nanoencapsulated clobetasol on days 1, 3, and 5. The NP group was treated daily with hydrogel containing empty nanoparticles. Three days after treatment, the C135 and NP groups were euthanized. After the animals were euthanized, the ears and liver were dissected and histological analyses and oxidative stress assays were performed. During the application of the 5% NiSO4, the dermal lesions were monitored daily by observation to evaluate any possible allergic reactions characteristic of CD.
Nanoparticles
Nanocapsules (NP) were prepared by interfacial deposition according to the preformed polymer method.Citation26,Citation33 First, an acetone solution containing 12.5 mg of drug, 250 mg of the polymer poly (ɛ-caprolactone), a mix of medium chain triglycerides (0.825 ml) and sorbitan monostearate (191.5 mg) was poured into an aqueous solution (134 ml) containing polysorbate 80 (191.5 mg). The solution was magnetically stirred for 10 minutes. Subsequently, the formulation was evaporated in a rotatory evaporator at 40°C and the final volume was adjusted to 25 ml.
Hydrogels
Carbopol Ultrez® (Lubrizol Corporation, Lakeland Boulevard Wickliffe, Ohio, USA) 10 NF was used as the polymer to prepare the hydrogels (0.5% acrylic acid polymer). It was dispersed in the nanoparticle suspensions containing clobetasol propionate, resulting in a drug concentration of 0.05%. The dispersion was neutralized with 0.2% triethanolamine to obtain an adequate semisolid formulation for skin application. Imidazolidinyl urea (0.6%) was added as a preservative. Following the same methodology, we also prepared hydrogels containing empty nanoparticles. The hydrogel containing free clobetasol propionate was prepared using a hydroethanolic solution of the drug (0.5 mg/ml) instead of the nanoparticle suspension.Citation32 These hydrogels had physicochemical and rheological characteristics that were optimized for skin administration. The drug formulation was verified by HPLC before use in the animal model.Citation32
Histopathological analysis of ear tissue
To validate this exposure model, a histopathological analysis of the ear tissue was performed. Samples of ex vivo ear tissue were collected and fixed in a 10% formalin solution and then dehydrated and embedded in paraffin. This was followed by sectioning and histological staining with hematoxylin and eosin (H&E).Citation34 The slides were observed in an optical microscope (×400) to check for possible changes in the ear tissue that were indicative of CD induction.
Liver homogenization
Liver tissue was homogenized (1:10 weight/volume) with 50 mM Tris–hydrochloride buffer pH 7.5 and centrifuged at 2000 rpm for 10 minutes. The supernatant was used in the subsequent biochemical assays.
Thiobarbituric reactive substances
As an index of lipid peroxidation, we used thiobarbituric acid reactive substances (TBARS) formation during an acid-heating reaction as described previouslyCitation35 with some modifications. In brief, 200 µl of homogenized tissue supernatant (1:10 w/v) samples were mixed with 500 µl of 2.5 M acetic acid pH 3.4, 500 µl of 0.8% thiobarituric acid, 200 µl of 8.1% sodium dodecyl sulfate (SDS) and 100 µl of distilled water. This mixture was then heated in a boiling water bath for 120 minutes. TBARS were determined by the absorbance at 532 nm and were expressed as malondialdehyde equivalents (nmol MDA/ml).
Carbonyl proteins
Carbonyl proteins were measured by reaction with 2,4-dinitrophenylhydrazine (DNPH) following the method previously described.Citation36 Assays were performed in duplicate for both the DNPH-treated samples and the blanks. A 1-ml aliquot of the homogenized tissue supernatant (1:10 w/v) sample containing approximately 6 mg of protein was placed in each of four tubes. The sample tubes received 200 µl of 10 mM 2,4-DNPH while the blanks received the same volume of 2 N HCl. The reaction was left in the dark at room temperature for 60 minutes. The samples were vortexed every 15 minutes and then 500 µl of 3% SDS, 2 ml of ethanol, and 2 ml of heptane were added to each tube. After vortexing, the tubes were centrifuged at 3000 rpm for 15 minutes. The pellets were washed in ethanol/ethyl acetate (1:1). Following this wash, 1 ml of 3% SDS was added to all the tubes and the absorbance was read at 370 nm. To calculate the concentration of carbonyl proteins (nmol carbonyl/mg of protein), a delta value was obtained by subtracting the blanks from the samples, dividing by 0.022 (extinction coefficient) and multiplying by the quantified proteins (mg/ml).
Tissue non-protein thiols
Tissue non-protein thiols were determined as described previously.Citation37 In brief, an aliquot of the homogenized tissue supernatant (1:10 w/v) was diluted (1:1) with 10% trichloroacetic acid, vortexed, and centrifuged at 2000 rpm for 10 minutes. Subsequently, the supernatant was reacted with 250 µM DTNB in a final volume of 2 ml and the absorbance was read at 412 nm. A standard curve was constructed with 0.5 mM cysteine to calculate the total sulfhydryl groups in the samples.
Catalase activity
The homogenized tissue supernatant (1:10 w/v) was further diluted by 1:60 and used to determine the catalase (CAT) activity. In brief, enzymatic activity was determined by the method of AebiCitation38 by measuring the rate of catalysis of 30 mM hydrogen peroxide (H2O2) at 240 nm in 50 mM potassium phosphate buffer at pH 7.0.
Protein determination
Protein was measured by the Coomassie blue methodCitation39 using serum albumin as standard.
Statistical analysis
The statistical analysis was performed using one-way analysis of variance (ANOVA), followed by the Newman–Keuls multiple comparison test. P < 0.05 was considered to represent a significant difference among the analyses. All data were expressed as mean ± standard error of the mean (SEM).
Results
Histopathological analysis of ear tissue
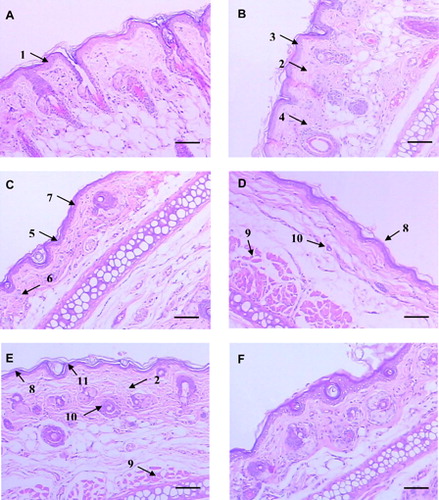
The H&E histopathological analyses of the experimental groups are described in . The tissue in the control group was preserved, with no sign of lesions. The Malpighian extract presented three or four layers of cells (arrow no.1), absence of lymphocytes and four well-developed hair follicles per high power field (HPF) (A). Dermal edema (arrow no. 2), acanthotic Malpighian layers (up to five layers of cells – arrow no. 3) and moderate lymphocytic infiltrates (up to six lymphocytes per HPF – arrow no. 4) were observed in the CD group (B). The FC group had a shortening of the cornea (one or two layers of keratinocytes – arrow no. 5) and granulomatous layers, a decrease of lymphocytic infiltrates (up to three lymphocytes per HPF), reduction in the number and thickness of hair follicles (two per HPF) and Malpighian layer atrophy (C). Both the NC and C135 groups showed Malpighian layer atrophy; however, in a lesser degree (two to three layers of keratinocytes), an increased number of hair follicles (three per HPF), and muscular layer with increased thickness when compared with group FC (D and E). The empty nanoparticles (NP) group showed normal epidermal thickness (three or four layers of cells), absence of dermal edema, and usual number and thickness of hair follicles (four per HPF) (F).
Figure 1. Histopathological analysis of ear tissue, H&E. Scale bars: 100 µm. (A) Control: normal histology of rat ear tissue with the absence of inflammatory infiltrate and edema. Basal layer and epidermal cell maturation preserved (1). (B) NiSO4 sensitized; contact dermatitis characteristics such as dermal edema (2), acanthotic Malpighian layer (3) and moderate lymphocytic infiltrate (4). (C) NiSO4 sensitized and treatment with free clobetasol; shortening of the cornea and thickness of the granulomatous layer (5), decrease of lymphocytic infiltrate (6), and Malpighian layer atrophy (7). (D) NiSO4 sensitized and daily treatment with nanostructured clobetasol; Malpighian layer (8), muscular (9) and fur atrophy (10). (E) NiSO4 sensitized and alternately treated with clobetasol (days 1, 3, and 5); Malpighian layer (8), muscular (9) and fur atrophy (10), desquamation of the cornea layer (11) and edema (2). (F) Nanoparticles treatment: absence of atrophy.
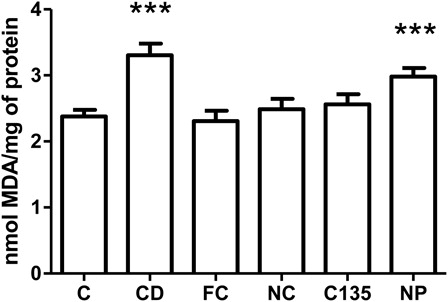
TBARS levels
TBARS content was measured as an index of lipid peroxidation in liver homogenates. One-way ANOVA showed a statistically significant difference among the groups [F(5, 42) = 7.62, P < 0.001] (). Subsequently, post hoc analysis showed an increased lipid peroxidation in the liver of groups CD and NP compared with C, FC, NC, and C135 (P < 0.001).
Figure 2. TBARS levels in livers from rats with NiSO4-induced contact dermatitis treated with free and nanostructured clobetasol. C, control group; CD, contact dermatitis; FC, CD treated with free clobetasol; NC, CD treated with nanostructured clobetasol; C135, CD treated with nanostructured clobetasol on days 1, 3, and 5; NP, empty nanoparticles. Bars represent mean ± SEM for eight animals in each group. ANOVA Newman–Keuls multiple comparison test. ***P < 0.001 compared with C, FC, NC, and C135.
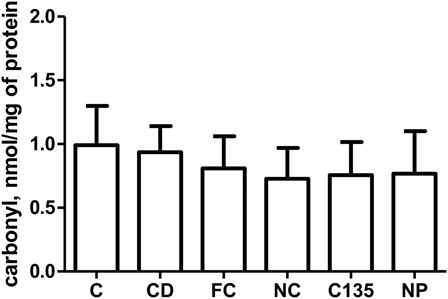
Carbonyl levels
Carbonyl groups were measured as an index of protein oxidation products in liver homogenates. Statistical analysis did not reveal any difference in the protein oxidation products in the liver tissue among the groups [F (5, 42) = 0.14, P > 0.05] ().
Figure 3. Carbonyl levels in livers from rats with NiSO4-induced contact dermatitis treated with free and nanostructured clobetasol. C, control group; CD, contact dermatitis; FC, CD treated with free clobetasol; NC, CD treated with nanostructured clobetasol; C135, CD treated with nanostructured clobetasol on days 1, 3, and 5; NP, empty nanoparticles. Bars represent mean ± SEM for eight animals in each group. ANOVA Newman–Keuls multiple comparison test.
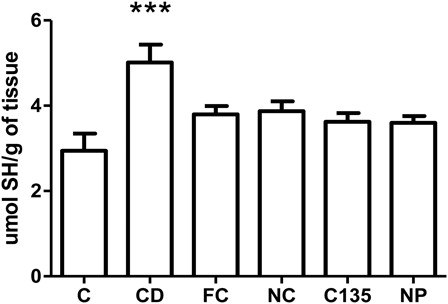
Non-protein sulfhydryl group levels
Non-protein sulfhydryl group (NPSH) is represented predominantly by reduced glutathione (GSH) and serve as an index of non-enzymatic antioxidant defense. One-way ANOVA showed a statistically significant difference among the groups [F (5, 42) = 5.57, P < 0.001] (). Post hoc analysis demonstrated an increased level of NPSH in the liver tissue of group CD compared with all the groups (P < 0.001).
Figure 4. Non-protein sulfhydryl group levels in livers from rats with NiSO4-induced contact treated with free and nanostructured clobetasol. C, control group; CD, contact dermatitis; FC, CD treated with free clobetasol; NC, CD treated with nanostructured clobetasol; C135, CD treated with nanostructured clobetasol on days 1, 3, and 5; NP, empty nanoparticles. Bars represent mean ± SEM for eight animals in each group. ANOVA Newman—Keuls multiple comparison test. ***P < 0.001 compared with all the groups.
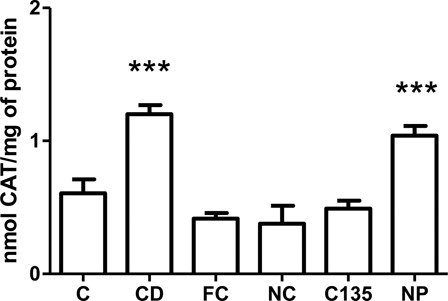
Catalase activity
CAT belongs to the enzymatic antioxidant defense system and is one of the most outstanding endogenous antioxidants. One-way ANOVA indicated significant difference among the groups [F (5.42) = 17.32, P < 0.001]. Subsequently, post hoc analysis showed an increased CAT activity in the liver of groups CD and NP compared with C, FC, NC and C135 (P < 0.001) ().
Figure 5. Catalase activity in livers from rats with NiSO4-induced contact dermatitis treated with free and nanostructured clobetasol. C, control group; CD, contact dermatitis; FC, CD treated with free clobetasol; NC, CD treated with nanostructured clobetasol; C135, CD treated with nanostructured clobetasol on days 1, 3, and 5; NP, empty nanoparticles. Bars represent mean ± SEM for eight animals in each group. ANOVA Newman–Keuls multiple comparison test. ***P < 0.001 compared with C, FC, NC, and C135.
Discussion
In this study, we induced CD in Wistar rats by sensitization with nickel sulfate (NiSO4) and then investigated the effects of free and nanoencapsulated clobetasol propionate on histological and oxidative stress parameters in the liver.
The stratum corneum is the outermost layer of the skin and is an obstacle to the absorption of many drugs used for dermatological therapy. To overcome this obstacle, nanostructured systems have been developed that can act over a large surface area and provide homogeneous release of drug into the skin surface.Citation18 In addition, topical application of these systems favors the retention of nanoparticles on both the skinCitation40 and the hair follicle. Studies have shown that the hair follicle plays an important role in the penetration of substances through the skin when the substances are associated with nanostructures.Citation41
First, to verify this experimental model of CD in rats, we proceeded with a histopathological analysis of ear specimens from control and CD groups to confirm the diagnosis of a skin inflammatory reaction. In the control group, no pathological alterations were detected in the tissue, while in the sensitized group, pathological alterations were observed. This verified the efficacy of this experimental model. We also observed, via histology, that treatment with both free (FC) and nanoencapsulated drug (NC, C135) reversed the damage caused by NiSO4. It is important to note that the NC group showed more promising results than the FC group. This was most likely due to the gradual release of the drug. Furthermore, the NP group presented results similar to the CD group, which may be an inherent property of the nanoparticles because CD was also induced in this group.
Clobetasol propionate is a synthetic topical glucocorticoid; however, it may have several adverse side effects when used for an extended period of time. It can also be absorbed through the skin into the circulatory system.Citation42
After this experimental procedure was proven to be effective in inducing CD, several oxidative stress parameters were evaluated in the liver tissue. These parameters include measuring the levels of malondialdehyde (MDA), carbonyl and NPSH content and the antioxidant activity of the enzymes superoxide dismutase and catalase.
Levels of MDA are positively related to lipid peroxidation and it is a commonly used biomarker.Citation43,Citation44 Sensitization with NiSO4 resulted in damage to the liver tissue as indicated by an increased level of MDA. This also resulted in an increase in NPSH and in the antioxidant activity of catalase. These results indicate that the NiSO4 effects were not only restricted to the ear tissue but also affected the liver tissue, as indicated by alterations in several of the oxidative stress parameters. Increased MDA levels are known to be related to plasma membrane instability in the face of reactive oxygen (ROS) and reactive nitrogen species generation. The increased catalase activity is in agreement with the lipid peroxidation data. This increase in catalase activity may be an adaptive mechanism to scavenge the ROS.
As many authors have described, there are two distinct phases in cutaneous hypersensitivity. First, in the sensitization phase, there is an increase in the number of T cells in the lymph nodes.Citation3,Citation45 Second, in the induction phase, chemokines are synthesized, endothelial cells and mast cells are activated, and there is an infiltration of polymorphonuclear cells.Citation46 The T cells interact with cutaneous antigen-presenting cells. Therefore, activated CD8+ cytotoxic T cells produce type 1 cytokines, such as IFN-γ and chemokines that induce the activation of cutaneous cells. They also induce the apoptosis of keratinocytes and allow for the recruitment of a cellular infiltrate that is characteristic of cutaneous hypersensitivity.Citation47,Citation48 A number of skin diseases are thought to be associated with oxidative stress, including psoriasis, cutaneous vasculitis, and CD, both during the early pre-immunological phase following exposure to contact allergens that readily auto-oxidize and also during the later stages of inflammatory cell infiltration.Citation49–Citation51
In this study, an increase in oxidative damage markers in livers from the CD group was observed. These animals had a partial recovery when they were treated with free and nanoencapsulated clobetasol. According to these results, it is hypothesized that cutaneous lesions may be related to the redox imbalance observed in the hepatic tissue and may possibly be mediated by the immune response. Furthermore, the administration of clobetasol propionate, both in the free and nanostructured formulations, was protective as observed by histopathology and oxidative stress parameters. This indicates that this drug has both topical and systemic effects.
Conclusion
In conclusion, our findings reveal that the sensitization of rats with NiSO4 was able to break the redox balance in the liver tissue and cause tissue damage. We also showed that treatment with both free and nanoencapsulated clobetasol propionate formulations was effective in preventing this damage, as measured by oxidative stress and histological observation.
Acknowledgements
This study was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Rio Grande do Sul (FAPERGS) and Fundação Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil.
References
- Fyhrquist-Vanni N, Alenius H, Lauerma A. Contact dermatitis. Dermatol Clin 2007;25(4):613–23, x.
- Slodownik D, Lee A, Nixon R. Irritant contact dermatitis: a review. Australas J Dermatol 2008;49(1):1–9; quiz 10–1.
- Saint-Mezard P, Rosieres A, Krasteva M, Berard F, Dubois B, Kaiserlian D, et al. Allergic contact dermatitis. Eur J Dermatol 2004;14(5):284–95.
- Lepoittevin JP, Leblond I. Hapten-peptide T cell interactions: molecular basis for the recognition of haptens by T lymphocytes. Eur J Dermatol 1997;7(3):151–4.
- Asano Y, Makino T, Norisugi O, Shimizu T. Occupational cobalt induced systemic contact dermatitis. Eur J Dermatol 2009;19(2):166–7.
- Nicolas JF, Testud F, Vocanson M. Sensibilisation versus tolerance dans l'eczema de contact. Ann Dermatol Venereol 2008;135:733–6.
- Saloga J, Knop J, Kolde G. Ultrastructural cytochemical visualization of chromium in the skin of sensitized guinea pigs. Arch Dermatol Res 1988;280(4):214–9.
- Krasteva M, Kehren J, Ducluzeau MT, Sayag M, Cacciapuoti M, Akiba H, et al. Contact dermatitis I. Pathophysiology of contact sensitivity. Eur J Dermatol 1999;9(1):65–77.
- Wiedersberg S, Leopold CS, Guy RH. Bioavailability and bioequivalence of topical glucocorticoids. Eur J Pharm Biopharm 2008;68(3):453–66.
- Gagliardi L, Orsi D, Giudice M, Gatta F, Porra R, Chimenti P, et al. Development of a tandem thin-layer chromatography-high-performance liquid chromatography method for the identification and determination of corticosteroids in cosmetic products. Analytica Chimica Acta 2002;457(2):187–98.
- Reepmeyer J, Revelee L, Vidavsky I. Detection of clobetasol propionate as an undeclared steroid in zinc pyrithione formulations by high- performance liquid chromatography with rapid-scanning ultraviolet spectroscopy and mass spectrometry. J Chromatogr A 1998;828(1–2):239–46.
- Hengge UR, Ruzicka T, Schwartz RA, Cork MJ. Adverse effects of topical glucocorticosteroids. J Am Acad Dermatol 2006;54(1):1–15; quiz 6–8.
- Fang JY, Shen KL, Huang YB, Wu PC, Tsai YH. Evaluation of topical application of clobetasol 17-propionate from various cream bases. Drug Dev Ind Pharm 1999;25(1):7–14.
- Honeywell-Nguyen P, Bouwstra J. Vesicles as a tool for transdermal and dermal delivery. Drug Discov Today, Technol 2005;2(1):67–74.
- Ramon E, Alonso C, Coderch L, de la Maza A, Lopez O, Parra JL, et al. Liposomes as alternative vehicles for sun filter formulations. Drug Deliv 2005;12(2):83–8.
- Tursilli R, Piel G, Delattre L, Scalia S. Solid lipid microparticles containing the sunscreen agent, octyl-dimethylaminobenzoate: effect of the vehicle. Eur J Pharm Biopharm 2007;66(3):483–7.
- Muller RH, Radtke M, Wissing SA. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Deliv Rev 2002;54( Suppl. 1):S131–55.
- Alvarez-Roman R, Naik A, Kalia YN, Guy RH, Fessi H. Enhancement of topical delivery from biodegradable nanoparticles. Pharm Res 2004;21(10):1818–25.
- Wissing SA, Muller RH. Solid lipid nanoparticles as carrier for sunscreens: in vitro release and in vivo skin penetration. J Control Release 2002;81(3):225–33.
- Alvarez-Roman R, Barre G, Guy RH, Fessi H. Biodegradable polymer nanocapsules containing a sunscreen agent: preparation and photoprotection. Eur J Pharm Biopharm 2001;52(2):191–5.
- Perugini P, Simeoni S, Scalia S, Genta I, Modena T, Conti B, et al. Effect of nanoparticle encapsulation on the photostability of the sunscreen agent, 2-ethylhexyl-p-methoxycinnamate. Int J Pharm 2002;246(1–2):37–45.
- Vettor M, Perugini P, Scalia S, Conti B, Genta I, Modena T, et al. Poly(D,L-lactide) nanoencapsulation to reduce photoinactivation of a sunscreen agent. Int J Cosmet Sci 2008;30(3):219–27.
- Ourique AF, Melero A, Silva CD, Schaefer UF, Pohlmann AR, Guterres SS, et al. Improved photostability and reduced skin permeation of tretinoin: development of a semisolid nanomedicine. Eur J Pharm Biopharm 2011;79(1):95–101.
- Alves MP, Scarrone AL, Santos M, Pohlmann AR, Guterres SS. Human skin penetration and distribution of nimesulide from hydrophilic gels containing nanocarriers. Int J Pharm 2007;341(1–2):215–20.
- Marchiori ML, Lubini G, Dalla Nora G, Friedrich RB, Fontana MC, Ourique AF, et al. Hydrogel containing dexamethasone-loaded nanocapsules for cutaneous administration: preparation, characterization, and in vitro drug release study. Drug Dev Ind Pharm 2010;36(8):962–71.
- Fontana MC, Coradini K, Guterres SS, Pohlmann AR, Beck RC. Nanoencapsulation as a way to control the release and to increase the photostability of clobetasol propionate: influence of the nanostructured system. J Biomed Nanotechnol 2009;5(3):254–63.
- Mandawgade SD, Patravale VB. Development of SLNs from natural lipids: application to topical delivery of tretinoin. Int J Pharm 2008;363(1–2):132–8.
- de Jalon EG, Blanco-Prieto MJ, Ygartua P, Santoyo S. PLGA microparticles: possible vehicles for topical drug delivery. Int J Pharm 2001;226(1–2):181–4.
- Jenning V, Gysler A, Schafer-Korting M, Gohla SH. Vitamin A loaded solid lipid nanoparticles for topical use: occlusive properties and drug targeting to the upper skin. Eur J Pharm Biopharm 2000;49(3):211–8.
- Luengo J, Weiss B, Schneider M, Ehlers A, Stracke F, Konig K, et al. Influence of nanoencapsulation on human skin transport of flufenamic acid. Skin Pharmacol Physiol 2006;19(4):190–7.
- Siqueira NM, Contri RV, Paese K, Beck RC, Pohlmann AR, Guterres SS. Innovative sunscreen formulation based on benzophenone-3-loaded chitosan-coated polymeric nanocapsules. Skin Pharmacol Physiol 2011;24(3):166–74.
- Fontana MC, Rezer JF, Coradini K, Leal DB, Beck RC. Improved efficacy in the treatment of contact dermatitis in rats by a dermatological nanomedicine containing clobetasol propionate. Eur J Pharm Biopharm 2011;79(2):241–249.
- Fessi H, Devissaguet J, Puisieux F. Procédé de préparation de systèmes colloïdaux dispersibles d'une substance sous forme de nanocapsules. European Patent No 0274961-A1. 1988.
- Tolosa EMC, Rodrigues CJ. Manual de técnicas para histologia normal e patológica. 2nd ed. São Paulo: Manole; 2003.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979;95(2):351–8.
- Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, et al. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 1990;186:464–78.
- Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys 1959;82(1):70–7.
- Aebi H. Catalase in vitro. Methods Enzymol 1984;105:121–6.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–54.
- Rao G, Murthy R. Evaluation of liposomal clobetasol propionate topical formulation for intra-dermal delivery. Indian J Pharm Sci 2000;62(6):459–62.
- Lademann J, Richter H, Teichmann A, Otberg N, Blume-Peytavi U, Luengo J, et al. Nanoparticles–an efficient carrier for drug delivery into the hair follicles. Eur J Pharm Biopharm 2007;66(2):159–64.
- ANVISA. Bula do profissional da saúde: bulário eletrônico da ANVISA. 2011.
- Grotto D, Valentini J, Boeira S, Paniz C, Maria L, Vicentini J, et al. Avaliação da estabilidade do marcador plasmático do estresse oxidativo - malondialdeído. Química Nova 2008;31(2):275–9.
- Jentzsch AM, Bachmann H, Furst P, Biesalski HK. Improved analysis of malondialdehyde in human body fluids. Free Radic Biol Med 1996;20(2):251–6.
- Blauvelt A, Hwang ST, Udey MC. Allergic and immunologic diseases of the skin. J Allergy Clin Immunol 2003;111 (2 Suppl.):S560–70.
- Watanabe H, Unger M, Tuvel B, Wang B, Sauder DN. Contact hypersensitivity: the mechanism of immune responses and T cell balance. J Interferon Cytokine Res 2002;22(4):407–12.
- Cavani A, Albanesi C, Traidl C, Sebastiani S, Girolomoni G. Effector and regulatory T cells in allergic contact dermatitis. Trends Immunol 2001;22(3):118–20.
- Fehr BS, Takashima A, Matsue H, Gerometta JS, Bergstresser PR, Cruz PD Contact sensitization induces proliferation of heterogeneous populations of hapten-specific T cells. Exp Dermatol 1994;3(4):189–97.
- Fuchs J, Freisleben HJ, Packer L. Antioxidants in the skin. Boca Raton, Florida: CRC Press; 1992.
- Picardo M, Zompetta C, Grandinetti M, Ameglio F, Santucci B, Faggioni A, et al. Paraphenylenediamine, a contact allergen, induces oxidative stress and ICAM-1 expression in human keratinocytes. Br J Dermatol 1996;134:681–5.
- Sharkey P, Eddy DJ, Burrows D, McCaigue MD, Bell AL. A possible role for superoxide production in the pathogenesis of contact dermatitis. acta dermato-venereologica. 1990;71:156–9.