Abstract
Objectives
To examine the association between gene variants of the detoxifying and antioxidant enzymes glutathione transferase M1 (GSTM1) and glutathione transferase A1 (GSTA1) and the extent of oxidative damage in patients with transitional cell carcinoma (TCC) of the urinary bladder.
Methods
GSTM1 deletion polymorphism was identified by polymerase chain reaction, and the restriction fragment length polymorphism method was used for the single nucleotide polymorphism of GSTA1. Enzyme immunoassay was used to determine markers of DNA (8-hydroxy-2′-deoxyguanosine, 8-OHdG) and lipid (8-epiprostaglandin F2α) oxidative damage in the urine of 80 TCC patients and 60 age-matched controls.
Results
Urinary 8-OHdG and 8-epi-prostaglandin F2α concentrations in TCC patients were significantly higher than in controls (P = 0.043 and 0.001, respectively). GSTM1 and GSTA1 polymorphisms influence vulnerability to both DNA and lipid oxidation, with the GSTM1-null gene variant having a more pronounced effect. A significant effect of combined GSTM1 and GSTA1 genotypes on the extent of oxidative damage was found only for 8-OHdG (P = 0.018). In addition, TCC patients with the most malignant tumors exhibited significantly higher frequencies of GSTM1-null or GSTA1-low activity genotypes, associated with a twofold increase in urinary 8-OHdG concentration (P = 0.044).
Conclusions
Our results suggest that absent GSTM1 or reduced GSTA1 antioxidant activity may increase the accumulation of oxidative DNA damage, thereby contributing to the malignant potential of TCC.
Introduction
The cytosolic glutathione transferase (GST) family of enzymes has attracted significant attention because of its association with various tumors, including bladder cancer.Citation1,Citation2 These enzymes are able to detoxify numerous genotoxic compounds and possess strong antioxidant activity toward reactive oxygen species (ROS).Citation3 Almost all members of the GST family exhibit genetic polymorphism, resulting in a complete lack or lowering of enzyme activity. Owing to a homozygous deletion for a 15-kb allele of the GSTM1 gene, approximately half of the population of different racial groups lacks active GSTM1 enzyme.Citation4 Among the GST polymorphisms of interest in genetic epidemiological studies, the role of GSTA1 polymorphism has emerged relatively recently. It is represented by three, apparently linked single nucleotide polymorphisms, resulting in differential expression with lower transcriptional activation of the variant GSTA1*B than the common GSTA1*A allele.Citation5 Besides the well-documented role of the GSTM1-null genotype in the risk of bladder cancer,Citation1,Citation6,Citation7 recent investigation indicates that the GSTA1-low activity genotype in combination with the GSTM1-null genotype significantly increases the risk of bladder cancer in smokers.Citation8 These results are biologically plausible in the light of the role of both GSTM1 and GSTA1 in the detoxification of carcinogens and free radicals in tobacco smoke.Citation3,Citation5,Citation9 Until now, the relationship between GST genotypes and oxidative DNA damage has been demonstrated in healthy GSTM1-null smokers.Citation9 Still, the assumption that GSTM1-null and/or GSTA1-low activity genotypes are associated with increased oxidative damage of macromolecules, with special emphasis on DNA, has to be addressed in conjunction with bladder cancer. In addition to the risk of cancer, these findings may help to further explain already known redox perturbations which contribute to the progression of transitional cell carcinoma (TCC) of the urinary bladder.Citation10–Citation12
With regard to bladder cancer, the data on byproducts of oxidative damage are limited. Using immunocytochemical analysis, Soini et al.Citation13 have shown that expression of nitrotyrosine, a marker of protein oxidative damage, as well as 8-hydroxy-deoxyguanosine (8-hydroxy-2′-deoxyguanosine, 8-OHdG), a reliable marker of oxidative DNA damage, in tumor tissue of the urinary bladder is associated with a poor prognosis in these patients. These results are in agreement with findings on the significant role of 8-OHdG as a potential biomarker of risk and prognosis in several solid tumors, including breast and non-small cell lung cancer.Citation14,Citation15 However, there are only few studies showing elevated urinary and serum 8-OHdG levels in bladder cancer,Citation16,Citation17 and the byproducts of oxidative damage to lipids, such as urinary isoprostanes, have not so far been determined.
In this study, we aimed to establish whether there is an association between GSTM1 and GSTA1 polymorphism and oxidative damage to DNA and lipids in patients with TCC of the urinary bladder. We hypothesized that a GSTM1-null and/or GSTA1-low activity genotype in patients with TCC results in increased oxidative damage that might contribute to a higher malignant potential of these tumors.
Materials and methods
Study subjects
In this hospital-based case–control study, we enrolled 80 patients (61 men and 19 women) newly diagnosed with TCC and 60 age-matched controls from the Clinics of Urology and Nephrology, Clinical Centre of Serbia, Belgrade. Pathological verification of TCC was carried out by routine urological practice, including endoscopic biopsy or surgical resection of urinary tract tumors followed by histopathological examination by board-certified pathologists. Inclusion criteria were no administration of systemic or intravesical immuno- or chemotherapy or surgical treatment at the time of sampling. The control group (38 men and 22 women) were individuals with nephrolithiasis admitted to the same hospital during the same time period and had no history of malignant diseases. Patients with TCC and the corresponding controls did not differ with respect to mean age and gender. All the participants provided written informed consent. The study protocol was approved by the Ethical Committee of the Medical Faculty, University of Belgrade, and the research was carried out in compliance with the Declaration of Helsinki.
After giving informed consent, each participant was interviewed by well-trained interviewers using a standard questionnaire to collect information including demographic characteristics, history of cigarette smoking, and lifetime occupational history. Smoking status was categorized into never smokers and ever-smokers according to the answer to the following question ‘Have you smoked at least 100 cigarettes in your life?’ Ever-smokers included light smoker (reported consuming between 1 and 10 cigarettes/day), moderate smoker (reported consuming between 11 and 19 cigarettes/day), heavy smoker (reported consuming 20 cigarettes or more per day), occasional smoker (respond ‘occasionally’ to the question ‘At the present time, do you smoke cigarettes every day, occasionally or not at all?’) and former smoker was not smoking at the time of the interview. The bladder cancer high-risk occupations were a priori defined and are listed elsewhere.Citation6 All patients were asked whether and for how long they had had these employments and patients were considered to be ‘exposed’ if they had occupied one or more of these jobs for ≥1 year in the past 10 years.
GST genotyping
Genomic DNA was isolated from whole blood using the QIAGEN QIAmp (Qiagen, Inc., Chatsworth, CA, USA) 96-spin blood protocol according to the manufacturer's instructions.
GSTM1 genotyping was performed by multiplex polymerase chain reaction (PCR) as described by Garcia-Closas et al.Citation18 Primers used were GSTM1 forward: 5′-GAA CTC CCT GAA AAG CTA AAGC-3′ and GSTM1 reverse: 5′-GTT GGG CTC AAA TAT ACG GTGG-3′. Exons 7 of CYP1A1 genes were co-amplified and used as an internal control using the following primers: CYP1A1 forward: 5′-GAA CTG CCA CTT CAG CTG TCT-3′ and CYP1A1 reverse: 5′-CAG CTG CAT TTG GAA GTG CTC-3′. The presence of a GSTM1-active genotype was detected by the band at 215 bp, as the assay does not distinguish heterozygous or homozygous wild-type genotypes. An internal positive control (CYP1A1) PCR product corresponded to 312 bp. Depending on their GSTM1 genotype, patients were dichotomized into the GSTM1 active or the GSTM1-null group.
GSTA1 C-69T polymorphism was determined by PCR–restriction fragment length polymorphism according to Ping et al.Citation19 Primers used were GSTA1 C-69T forward: 5′-TGT TGA TTG TTT GCC TGA AAT T-3′ and GSTA1 C-69T reverse: 5′-GTT AAA CGC TGT CAC CCG TCC T-3′. The presence of a restriction site resulting in only two fragments (481 and 385 bp) indicated a mutant allele (B/B), and if A/B polymorphism incurred then it resulted in three fragments of, respectively, 481, 385, and 96 bp.
Biomarkers of oxidative damage in urine
For the measurement of isoprostane 8-epi-prostaglandin F2α and 8-hydroxy-2′-deoxyguanosine, urine was collected from all patients and controls. Samples were centrifuged, aliquoted, and stored at −80°C. For the purpose of quantifying 8-OHdG and F2-IsoPs, results from spot urines correlated well with those from 24-hour collections, in part because of the low variation in excretion of these compounds over the course of a day; therefore, urines were collected from a single sample during the day rather than totals over a 24-hour period. 8-OHdG was determined by enzyme immunoassays (Bioxytech 8-OHdG-EIA; Oxis Research, Portland, OR, USA). In brief, 50 µl of primary monoclonal antibody and a 50-μl of sample/standard were added to microtiter plates pre-coated with 8-OHdG, incubated at 37°C for 1 hour and washed with phosphate-buffered saline (PBS). HRP-conjugated secondary antibody was then added, incubated at 37°C for 1 hour and washed with PBS. In the next step, 3,3′,5,5′-tetramethylbenzidine was added, incubated at 37°C for 1 hour. The reaction was terminated with 1 M phosphoric acid. Absorbance was read at 450 nm. For each set of measurements a calibration curve was set up using the six concentrations of urinary 8-OHdG standard concentrations. Intra-assay and inter-assay precision were measured as a part of the validation procedure. An intra-assay coefficient of variation was 4.7% and inter-assay 5.6%. The results were standardized against urinary creatinine concentrations and expressed in nanograms/milligrams of creatinine.
8-Epi-PGF2α was as well determined by enzyme immunoassays (Bioxytech Urinary 8-Epi-prostaglandin F2α; Oxis Research, Portland, OR, USA). The results were standardized against urinary creatinine concentrations and expressed in nanograms/milligrams of creatinine.
Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS, version 15.0; SPSS Inc, Chicago, IL, USA). The differences between groups were tested using the non-parametric Mann–Whitney and Kruskal–Wallis tests. We used a χ2 test to determine P values for differences in genotype frequencies. For each variable, values were expressed as median and confidence interval. A P value of <0.05 was considered statistically significant.
Results
The clinical characteristics of TCC patients and controls, together with the distribution of GST genotypes, are presented in . As expected, smoking prevalence and occupational exposure were higher among TCC patients than in controls (75% vs. 55% and 44% vs. 20%, respectively). The WHO grading of TCC patients was as follows: 24 patients were G1, 34 G2, and 22 G3. The frequencies of GSTA1-low activity and GSTM1-null genotype were higher in TCC patients than in controls, showing a common pattern already reported for GSTM1 and GSTA1 polymorphism ().
Table 1. Characteristics of transitional cell carcinoma patients and controls
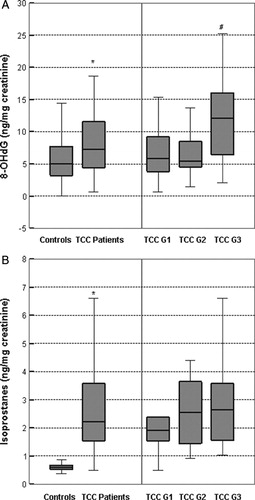
To estimate the oxidant-mediated DNA and lipid damage in TCC patients, we investigated the urinary concentrations of 8-OHdG (A) and 8-epi-prostaglandin F2α (B). Our patients showed increased oxidative damage to DNA and lipids compared with healthy individuals. Specifically, the median levels of oxidative damage biomarkers, 8-OHdG and 8-epi-prostaglandin F2α were significantly higher in TCC patients than in controls (7.22 (95% confidence interval, CI = 6.81–9.90) vs. 4.97 ng/mg creatinine (95% CI = 3.14–7.48); p = 0.043 and 2.21 (95% CI = 2.21–3.77) vs. 0.60 ng/mg creatinine (95% CI = 0.50–0.66); P = 0.001, respectively). When analyzed in terms of tumor grade, the highest 8-OHdG level was found in patients with grade 3 TCC (A). Specifically, the median 8-OHdG level in G3 patients was more than twice as high as in patients with G2 and G1 (12.11 (95% CI = 8.20–17.33) vs. 5.46 (95% CI = 4.49–7.21) and 5.85 ng/mg creatinine (95% CI = 5.01–8.86); p = 0.044). On the other hand, there was no association between isoprostane 8-epi-prostaglandin F2α levels and tumor grade in these patients (B). As shown, the median isoprostane level in G1, G2, and G3 TCC patients was 1.91 (95% CI = 1.54–3.15) vs. 2.54 (95% CI = 2.01–4.10) vs. 2.64 ng/mg creatinine (95% CI = 1.47–6.12), respectively (P = 0.477). Furthermore, in stratified analysis we found that the median levels of 8-OHdG and 8-epi-prostaglandin F2α did not differ by smoking exposure or occupational exposure (data not shown).
Figure 1. Levels of 8-OHdG (A) and 8-epi-prostaglandin F2α (B) in controls and TCC patients, as well as in TCC patients stratified to tumor grade. The top and bottom lines represent the lowest and highest values, the lower line of the box represents the first quartile (Q1), the upper line represents the third quartile box (Q3), and the line in the box represents the median. The 8-OHdG concentration is expressed as ng/mg creatinine. *P < 0.05, according to controls; #P < 0.05, according to grades 2 and 1.
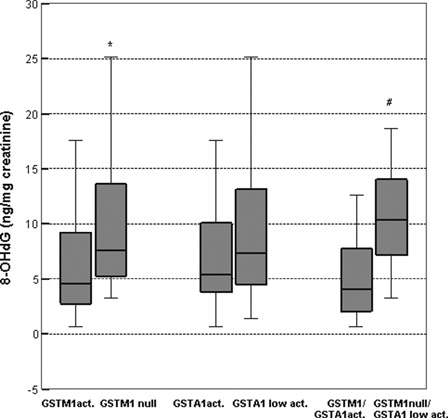
Results on the degree of oxidative DNA damage in TCC patients stratified according to GST genotype are presented in . Levels of 8-OHdG were significantly higher in patients with GSTM1-null genotype than in those with GSTM1-active genotype (7.60 (95% CI = 7.86–12.52) vs. 4.58 ng/mg cretainine (95% CI = 4.38–7.64); P = 0.001) (). However, no such association was found for 8-OHdG levels in patients with GSTA1-low activity genotype versus more active GSTA1 carriers (7.43 (95% CI = 7.01–10.19) vs. 5.38 ng/mg creatinine (95% CI = 4.62–11.21); P = 0.281) (). The most pronounced effect on 8-OHdG levels was observed in patients with combined GSTM1-null/GSTA1-low activity genotypes. In these patients, urinary excretion of 8-OHdG was twice as high as in carriers of both fully active genes (10.40 (95% CI = 8.50–12.99) vs. 4.03 ng/mg creatinine (95% CI = 2.43–9.24); P = 0.018). No association was found with urinary isoprostane 8-epi-prostaglandin F2α levels and GSTM1 or GSTA1 genotypes (data not shown).
Figure 2. Levels of 8-OHdG in TCC patients stratified according to GSTM1, GSTA1, and combined GSTM1/GSTA1 genotypes. The top and bottom lines represent the lowest and highest value, the lower line of the box represents the first quartile (Q1), upper line represents the third quartile box (Q3), and the line in the box represents the median. The 8-OHdG concentration is expressed as ng/mg creatinine. Active genotype refers to at least one active allele present, null genotype refers if no active alleles present, low activity genotype if at least one lower activity allele present. *P < 0.05, according to GSTM1 active genotype; #P < 0.05, according to both active GSTM1 and GSTA1 genotypes.
The distribution of GSTM1 and GSTA1 genotypes according to the malignant potential of TCC is presented in . Based on our results, GSTM1-null genotype was related to the grade of the tumor. Specifically, 77% of G3 TCC patients are carriers of the GSTM1-null genotype, whereas 62% of G2 patients and only 38% of G1 patients carry the GSTM1-null genotype (P = 0.021). A similar pattern of changes was observed for the less active GSTA1 genotype according to tumor grade, but still did not reach statistical significance (P = 0.056).
Table 2. GSTM1 and GSTA1 genotype distributions in transitional cell carcinoma patients according to tumor grade
Comment
In this study, we showed that patients with TCC of the urinary bladder exhibit increased urinary levels of the byproducts of oxidative DNA and lipid damage. Oxidative DNA damage in these patients correlates with GSTM1-null or GSTA1-low activity genotype, either alone or in combination. GSTM1-null and GSTA1-low activity genotype, as well as the extent of oxidative DNA damage, is associated with a higher malignant potential of TCC.
Susceptibility to TCC depends on both the expression profile of the enzymes involved in biotransformation of carcinogens and the cumulative/additive effects of cigarette smoke and occupational exposure, as well-documented TCC risk factors.Citation20 Almost all compounds known to be risk factors for bladder tumors are also important sources of free radicals, which exert their effects either directly, like those present in cigarette smoke, or during the metabolism of carcinogenic agents, such as polycyclic aromatic hydrocarbons (PAHs).Citation20,Citation21 In particular, the formation of DNA adducts due to increased ROS generation during the conversion of PAHs to quinones is well documented.Citation22 It can be assumed that during malignant transformation a significant perturbation of cellular redox status occurs, which can result in ROS-mediated DNA damage, gene mutations and structural alterations to the DNA. Moreover, it is important to note that oxidative stress interacts not only with initiation, but also with the promotion and progression of cancer. In the promotion stage, ROS can contribute to abnormal gene expression and modification of signaling pathways, thereby resulting in an increase of cell proliferation or a decrease in apoptosis. Finally, oxidative stress may also participate in the progression stage of the cancer process by adding further DNA alterations that in turn could lead to a more malignant phenotypic behavior of tumors.Citation23,Citation24
As the most widely used fingerprint of radical attack towards DNA, 8-OHdG has been strongly implicated in the progression of carcinogenesis. For example, in breast carcinoma 8-OHdG has been reported to be increased 8–17-fold in breast primary tumors compared with non-malignant breast tissue.Citation25 Our results on significantly higher urinary 8-OHdG concentrations in TCC patients in comparison with healthy controls are in agreement with the recent study of Chung,Citation17 which examined 8-OHdG levels in bladder cancer patients in relation to arsenic exposure. In addition to DNA damage, increased lipid peroxidation in TCC patients was also found, i.e. patients exhibit increased urinary concentrations of isoprostanes, the most reliable markers of lipid oxidative damage. It is important to note that 8-OHdG and isoprostanes are biomarkers of systemic oxidative stress in humans. However, it is tempting to speculate whether in the clinical setting of TCC these oxidative byproducts also reflect local oxidative stress in neoplastic cells. It is likely that, for example, oxidative bursts of macrophages and neutrophils, oncological treatments and various inflammatory conditions may affect both serum and urine 8-OHdG or isoprostane concentrations. However, considering recent imunohistochemical findings on the expression of 8-OHdG in tumor uroepithelial cells,Citation13 it seems reasonable to assume that 8-OHdG and isoprostanes could also reflect oxidative damage in TCC cells. Furthermore, we determined these oxidative byproducts at the time of diagnosis, before surgical or other treatment, such as BCG instillation, which could interfere with the obtained values.Citation26
In this study, we outlined the putative significance of both GSTM1 and GSTA1 genotypes in oxidative stress in TCC patients, investigating the influence of their deletion and low activity on the degree of oxidative damage of macromolecules. We showed that TCC patients with the GSTM1-null genotype have higher urinary 8-OHdG levels and are more vulnerable to oxidative DNA damage. Furthermore, a combined effect of GSTM1-null and GSTA1-low activity genotype on DNA oxidative damage was demonstrated in these patients. Our data on the correlation between GSTM1-null and GSTA1-low activity genotype and increased oxidative DNA damage prove that increased vulnerability of DNA represents the molecular basis for higher susceptibility of carriers of these genotypes to TCC. Because both enzymes are catalytically active towards free radicals and PAHs metabolites,Citation4,Citation5 it seems reasonable to assume that greater damage was observed for combined GSTM1-null/GSTA1-low activity genotype. The antioxidant and detoxifying activities of both GSTM1 and GSTA1 enzymes could contribute to the protection of DNA from oxidative and genotoxic damage, especially with respect to the GSTM1 isoenzyme expressed by uroepithelial cells.Citation2,Citation27,Citation28
Finally, our study demonstrates, for the first time, the significant impact of both GSTM1 and GSTA1 polymorphisms on the malignant potential of TCC, in that the frequency of GSTM1-null or GSTA1-low activity genotypes was significantly higher in TCC patients with advanced histological grade. These results are similar to the association found between GSTM1-null genotype and tumor grade in patients with renal cell carcinoma.Citation29 Our findings further imply the important role of GSTM1 and GSTA1 gene variants in protection from DNA damage, based on the highest levels of 8-OHdG in G3 TCC patients. What is more, at the molecular level this explains the influence of GST genotype on the prognosis of TCC patients.Citation30 In this respect, the results of Nørskov et al.Citation30 on the general population have shown that exact copy number variation (CNV) in GSTM1 gene predicts the 5-year survival of bladder cancer in a gene-dose-dependent manner, with GSTM1-null genotype having the highest risk. Thus, the cumulative 5-year survival after bladder cancer diagnosis decreased with decreasing GSTM1 copy number.Citation30
A limitation of our study is that the number of TCC patients was relatively small. The methods used to determine biomarkers of DNA and lipid oxidative damage were not standardized, and the values obtained for 8-OHdG and isoprostanes may also be affected by the major confounders, such as age and smoking status. However, this was intended to be a pilot study, as there are no data on the prognostic value of investigated oxidative stress markers. Nevertheless, based on our positive findings, further studies with larger numbers of TCC patients warranted.
Conclusions
To our knowledge, this is the first study showing a significant impact of both GSTM1 and GSTA1 polymorphisms on the malignant potential of TCC. The results obtained demonstrated that increased urinary 8-OHdG levels correlate with GSTM1-null and GSTA1-low activity genotype in TCC patients. Moreover, increased DNA vulnerability may represent the molecular basis for the higher susceptibility to TCC of carriers of these genotypes.
Support/financial disclosures
None.
Acknowledgements
This work was supported by a Grant 175052 from the Serbian Ministry of Science. The authors thank Charlesworth Publishing Services for language editing.
References
- McGrath M, Michaud D, De Vivo I. Polymorphisms in GSTT1, GSTM1, NAT1 and NAT2 genes and bladder cancer risk in men and women. BMC Cancer 2006;6:239–47.
- Simic T, Savic-Radojevic A, Pljesa-Ercegovac M, Matic M, Mimic-Oka J. Glutathione S-transferases in kidney and urinary bladder tumors. Nat Rev Urol 2009;6:281–9.
- Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol 2005;45:51–88.
- Hayes JD, Strange RC. Glutathione S-transferase polymorphisms and their biological consequences. Pharmacology 2000;61:154–66.
- Coles FB, Kadlubar FF. Human alpha class glutathione S-transferases: genetic polymorphism, expression, and susceptibility to disease. In: , Sies H, Lester P, (eds.) Glutathione transferases and gamma-glutamyl transpeptidases, methods enzymology. London: Elsevier Academic Press; 2005. p. 9–42.
- Engel LS, Taioli E, Pfeiffer R, Garcia-Closas M, Marcus PM, Lan Q, et al. Pooled analysis and meta-analysis of glutathione S-transferase M1 and bladder cancer: a HuGE review. Am J Epidemiol 2002;156:95–109.
- Garcia-Closas M, Malats N, Silverman D, Dosemeci M, Kogevinas M, Hein DW, et al. NAT2 slow acetylation, GSTM1 null genotype, and risk of bladder cancer: results from the Spanish Bladder Cancer Study and meta-analyses. Lancet 2005;366:649–59.
- Matic M, Pekmezovic T, Djukic T, Mimic-Oka J, Dragicevic D, Krivic B, et al. GSTA1, GSTM1, GSTP1 and GSTT1 polymorphisms and susceptibility to smoking-related bladder cancer: a case-control study. Urol Oncol, accepted for publication.
- Palma S, Cornetta T, Padua L, Cozzi R, Appolloni M, Ievoli E, et al. Influence of glutathione S-transferase polymorphisms on genotoxic effects induced by tobacco smoke. Mutat Res 2007;633:1–12.
- Simic T, Mimic-Oka J, Savic-Radojevic A, Opacic M, Pljesa M, Dragicevic D, et al. Glutathione S-transferase T1–1 activity upregulated in transitional cell carcinoma of urinary bladder. Urology 2005;65:1035–40.
- Savic-Radojevic A, Mimic-Oka J, Pljesa-Ercegovac M, Opacic M, Dragicevic D, Kravic T, et al. Glutathione S-transferase-P1 expression correlates with increased antioxidant capacity in transitional cell carcinoma of the urinary bladder. Eur Urol 2007;52:470–7.
- Badjatia N, Satyam A, Singh P, Seth A, Sharma A. Altered antioxidant status and lipid peroxidation in Indian patients with urothelial bladder carcinoma. Urol Oncol 2010;28:360–7.
- Soini Y, Haapasaari KM, Vaarala MH, Turpeenniemi-Hujanen T, Kärjä V, Karihtala P. 8-hydroxydeguanosine and nitrotyrosine are prognostic factors in urinary bladder carcinoma. Int J Clin Exp Pathol 2011;4:267–75.
- Matsui A, Ikeda T, Enomoto K, Hosoda K, Nakashima H, Omae K, et al. Increased formation of oxidative DNA damage, 8-hydroxy-2′-deoxyguanosine, in human breast cancer tissue and its relationship to GSTP1 and COMT genotypes. Cancer Lett 2000;151:87–95.
- Shen J, Deininger P, Hunt JD, Zhao H. 8-Hydroxy-2′-deoxyguanosine (8-OH-dG) as a potential survival biomarker in patients with nonsmall-cell lung cancer. Cancer 2007;109:574–50.
- Chiou CC, Chang PY, Chan EC, Wu TL, Tsao KC, Wu JT. Urinary 8-hydroxydeoxyguanosine and its analogs as DNA marker of oxidative stress: development of an ELISA and measurement in both bladder and prostate cancers. Clin Chim Acta 2003;334:87–94.
- Chung CJ, Huang CJ, Pu YS, Su CT, Huang YK, Chen YT, et al. Urinary 8-hydroxydeoxyguanosine and urothelial carcinoma risk in low arsenic exposure area. Toxicol Appl Pharmacol 2008;226:14–21.
- Garcia-Clocas M, Kelsey KT, Hankinson SF, Spiegelman D, Springer K, Willett WC, et al. Glutathione S-transferase mu and theta polymorphisms and breast cancer susceptibility. J Natl Cancer Inst 1999;91:1960–4.
- Ping J, Wang H, Huang M, Liu ZS. Genetic analysis of glutathione S-transferase A1 polymorphism in the Chinese population and the influence of genotype on enzymatic properties. Toxicol Sci 2006;89:438–43.
- Filiadis I, Hrouda D. Genetic factors in chemically-induced transitional cell bladder cancer. BJU Int 2000;86:794–801.
- Valavanidis A, Vlachogianni T, Fiotakis K. Tobacco smoke: involvement of reactive oxygen species and stable free radicals in mechanisms of oxidative damage, carcinogenesis and synergistic effects with other respirable particles. Int J Environ Res Public Health 2009;6:445–62.
- Yu H, Xia Q, Yan J, Herreno-Saenz D, Wu YS, Tang IW, et al. Photoirradiation of polycyclic aromatic hydrocarbons with UVA light - a pathway leading to the generation of reactive oxygen species, lipid peroxidation, and DNA damage. Int J Environ Res Public Health 2006;3:348–54.
- Halliwell B. Oxidative stress and cancer: have we moved forward? Biochem J 2007;401:1–11.
- Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked?. Free Radic Biol Med 2010;49:1603–16.
- Malins DC, Haimanot R. Major alterations in the nucleotide structure of DNA in cancer of the female breast. Cancer Res 1991;51:5430–2.
- Mitropoulos D, Deliconstantinos G, Zervas A, Giannopoulos A, Kyriakou G, Dimopoulos C. Oxidative stress of red blood cells during Bacillus Calmette-Guerin intravesical instillations. In Vivo 2000;14:721–4.
- Matic M, Simic T, Dragicevic D, Mimic-Oka J, Pljesa-Ercegovac M, Savic-Radojevic A. Isoenzyme profile of glutathione transferases in transitional cell carcinoma of upper urinary tract. Transl Res 2010;155:256–62.
- Pljesa-Ercegovac M, Savic-Radojevic A, Dragicevic D, Mimic-Oka J, Matic M, Sasic T, et al. Enhanced GSTP1 expression in transitional cell carcinoma of urinary bladder is associated with altered apoptotic pathways. Urol Oncol 2011;29:70–7.
- De Martino M, Klatte T, Schatzl G, Remzi M, Waldert M, Haitel A, et al. Renal cell carcinoma Fuhrman grade and histological subtype correlate with complete polymorphic deletion of glutathione S-transferase M1 gene. J Urol 2010;183:878–83.
- Nørskov MS, Frikke-Schmidt R, Bojesen SE, Nordestgaard BG, Loft S, Tybjærg-Hansen A. Copy number variation in glutathione-S-transferase T1 and M1 predicts incidence and 5-year survival from prostate and bladder cancer, and incidence of corpus uteri cancer in the general population. Pharmacogenomics J 2011;11:292–9.