Abstract
Objectives
The aim of this study was to determine the relationship between the antioxidant profile of anesthetics and its relation to total antioxidant capacity (TAC) of plasma in children who underwent tourniquet-induced ischemia-reperfusion (IR) injury during extremity operations.
Methods
Children were randomized into three groups: general inhalational anesthesia with sevoflurane (group S), total intravenous anesthesia (TIVA) with propofol (group T), and regional anesthesia (group R). Venous blood samples were obtained before peripheral nerve block and induction of general anesthesia (baseline), 1 minute before tourniquet release (BTR), and 5 and 20 minutes after tourniquet release (ATR). Plasma TAC as well as antioxidant potential of propofol, thiopental, and bupivacaine were measured using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay.
Results
Plasma TAC in group T was increased significantly at 20 minutes ATR in comparison with basal and BTR values, and also was significantly higher in comparison with plasma TAC in groups S and R measured at the same time point. The radical scavenging activity of anesthetics in vitro indicated that only propofol possessed a significant antioxidative activity in the reaction with DPPH radical in comparison with thiopental and bupivacaine.
Discussion
These data confirm that TIVA with propofol attenuates oxidative stress related to tourniquet-induced ischaemia-reperfusion injury in children.
Introduction
Tourniquet application in extremity surgery is the prerequisite to provide a bloodless environment during surgery. The blood flow in the ischemic limb is restored after surgery by releasing the tourniquet. The use of tourniquet thereby comes with the risk of ischemia-reperfusion (IR) injury. Mechanisms underlying IR injury have been studied extensively and are known to engage a spectrum of pathways.Citation1 Since surgical trauma not only intensifies the oxidative stress by generating reactive oxygen species (ROS), but also weakens the biological defense system against ROS attack, the effects of anesthesia techniques on IR injury are of considerable scientific and clinical interest because anesthetics with anti-oxidant properties or free radical-scavenging activity could offer considerable protection and benefits in this setting.Citation2
Propofol anesthesia seems to enhance the antioxidant capacity against tourniquet-induced IR injury.Citation3 Intravenous anesthetic propofol (2,6 diisopropylphenol) appears to inhibit lipid peroxidation secondary to oxidative stress in in vitro studiesCitation4 and to reduce lipid peroxidation during skeletal muscle IR injury.Citation2–Citation5 Propofol also has antiinflammatory properties, decreasing production of proinflammatory cytokines,Citation6 decreasing NFkB expression,Citation7 reducing inducible nitric oxide synthase activity, and inhibiting neutrophil infiltration.Citation8
Free radical-scavenging effects of barbiturates, particularly thiopental, likely contribute to the well-validated neuroprotective effects of these drugs, but there is insufficient evidence to support significant scavenging effects during IR injuries in other tissues.Citation9,Citation10
Many studies have shown the protective effects of volatile anestetic sevoflurane (1,1,1,3,3,3-hexafluoro-2-(fluoromethoxy)propane) on the myocardium after ischemia.Citation11,Citation12 The authors analyzed a possible protective effect of sevoflurane and found out that sevoflurane reduced glycocalyx shedding in the postischemic coronary bedCitation13 and inhibited mitochondrial permeability transition pore.Citation14 Clinical studies are now warranted to define the role of volatile anesthetics preconditioning in a non-cardiac tissue which, if conclusive, would be of significant relevance for the reduction of perioperative ischemic organ damage and associated morbidity and mortality.
Some studies reported less hemodynamic and metabolic changes related to tourniquet application in patients undergoing regional anesthesia compared with general anesthesia.Citation15 Furthermore, local anesthetics inhibit migration, enzyme release, and O2•− generation of polymorphonuclear leukocytesCitation16 and attenuate inflammatory response, lipid peroxidation, oxidative injury, and apoptosis.Citation17
In adults, various comorbid conditions could overlap and contribute to oxidative stress. Thus, studies conducted in children may provide more accurate results and improve our daily practice in a manner to that the best choice of anesthetics for our patients could be established.
This prospective study was carried out to investigate the relationship between the antioxidant profile of anesthetics and its relation to total antioxidant capacity (TAC) of plasma in children who underwent tourniquet-induced IR injury during extremity operations.
Methods
After obtaining the Ethics Committee approval (No. 01-1674, March 13 2008, according to the Article 37 of the Faculty of Medicine in Nis Ethics Committee Regulation) and written informed consent from the parents, we studied 45 patients ASA I or II, 8–17 years of age, undergoing orthopedic procedures that required bloodless limb surgery. Children and adolescents were randomized to sevoflurane (n = 15), propofol (n = 15), or peripheral nerve block (n = 15) group. All patients were premedicated with midazolam. In the sevoflurane group (group S) general anesthesia was induced with thiopental (5 mg/kg), with alfentanyl (25 mcg/kg), and maintained with sevoflurane inhalation (3–4 vol %). In the propofol group (group T), anesthesia was induced with propofol (2.5 mg/kg) with alfentanyl (25 mcg/kg), followed by continuous infusion of propofol at a rate of 10 mg/kg/h, reduced to 8 and 6 mg/kg/h, respectively, at 10 minutes intervals. The maintenance dose was adjusted to clinical signs and anticipated demand, according to the total intravenous anesthesia (TIVA) concept. Atracurium (0.6 mg/kg) was given to facilitate tracheal intubation in sevoflurane and propofol group and the lungs were ventilated with 65% nitrogen in oxygen. Rescue analgesia was provided by single bolus doses of alfentanyl (10 mcg/kg). In the regional anesthesia group (group R), patients received peripheral nerve blocks using bupivacaine 0.25% (volume adjusted to the type of block and patient's weight). Peripheral nerves were identified using peripheral nerve stimulator. Despite the proper psychological preparation, some children in the group R required additional sedation with midazolam. During lower limb operations, one arm was used for blood sampling and the other for intravenous fluid infusions. During upper limb operations, contralateral arm was used for intravenous fluid and propofol infusion and the dorsal vein of the foot was used for blood sampling. The tourniquet was applied at a pressure approximately twice the systolic arterial pressure. No blood transfusions were used; the fluid deficits were corrected with lactated Ringer's solution during the operation. Using heparin-locked intravenous catheters, sequential venous blood samples were obtained at four time points: before peripheral nerve block and induction of general anesthesia (baseline), 1 minute before tourniquet release (BTR), and 5 and 20 minutes after tourniquet release (ATR).
Measurement of plasma total antioxidant capacity
The total antioxidant capacity (TAC) of plasma as well as free radical-scavenging activities of anesthetics were measured using 2,2-diphenyl-1-picrylhydrazyl (DPPH).
The reduction of DPPH, as indicated below, is followed by monitoring the decrease in its absorbance at a characteristic wavelenght during the reaction. In its radical form, DPPH absorbs at 515 nm, but upon reduction by an antioxidant (AH) or a radical species (R•), the absorption disappears.Citation18
The interaction of a potential antioxidant with DPPH depends on its structural conformation. Certain compounds react very rapidly with the DPPH reducing a number of DPPH molecules corresponding to the number of available hydroxyl groups. Nevertheless, for the majority of the compounds tested, the mechanism is more complex.
It was determined that the reduction of DPPH by standard antioxidants was completed after 30 minutes; however, kinetic measurements showed that DPPH reduction by blood plasma was not complete by this time.Citation19
Total antioxidant status in plasma was determined by the modified method of BliosCitation20 using a stable free radical DPPH at a concentration of 0.04 mM in methanol. In short, the method was applied as follows: 0.180 ml of plasma was deproteinated by the addition of 1.620 ml of methanol, vortexed for 30 seconds then centrifuged at 10 000 RPM for 30 minutes to separate the proteins. To 0.04 ml of clear supernatant, 0.2 ml of DPPH solution was added, mixed thoroughly and absorbence was read at 540 nm against blank, prepared in an identical way, but without plasma addition. Decreased absorbance of the reaction mixture indicated the increased capacities of plasma components to scavange stable radical DPPH. The total antioxidant activity of plasma was expressed as percentage of absorbance inhibition after 30 and 60 minutes of reaction with DPPH.
Measurement of propofol, thiopental, and bupivacaine antioxidant activity
The ability of free radical scavenging of propofol, thiopental, and bupivacaine was measured by using DPPH based on the method described by Shimada et al.,Citation21 with slight modifications. In short, 0.04 mM solution of DPPH in methanol was prepared and then 0.2 ml of the solution was added to 0.04 ml of propofol, thiopental, or bupivacaine solutions in methanol at different concentrations. The mixture was shaken vigorously and left in a dark place at room temperature for 30 minutes. Then the absorbance was measured at 540 nm against blank (without anesthetic) using the Thermo Labsystems Multiskan Ascent ELISA reader. Antioxidant activity of an anesthetic was expressed as percentage of absorbance inhibition after 30 minutes of reaction with DPPH.
Statistical analysis
Data were expressed as the mean ± SD. Within- and between-group differences were tested using the Student's t-test. A P value of less than 0.05 was considered to be statistically significant. The parameters were correlated using the simple linear regression test.
Results
Patient demographics
There were no significant differences between the groups in age, weight, height, and tourniquet time. There were not any significant between group differences in upper extremity and lower extremity surgery distribution ().
Table 1. Demographic characteristics of the patients and tourniquet time
Estimation of total antioxidant capacity of plasma
In this study plasma TAC was determined 30 and 60 minutes after the reaction with DPPH.
Twenty minutes after the tourniquet release, in patients who received propofol, the increase of TAC was noticed in comparison with the basal value as well as the value before the tourniquet release (). The 20-minute ATR30 value was significantly higher in group T in comparison with plasma TAC in groups S and R measured at the same time point.
Table 2. Total antioxidant capacity of plasma 30 minutes after the reaction with 1,1-diphenyl-2-picrylhydrazyl
The same pattern was also noticed 60 minutes after the reaction with DPPH ().
Table 3. Total antioxidant capacity of plasma 60 minutes after the reaction with DPPH
Determination of in vitro antioxidant activity of propofol, thiopental, and bupivacaine
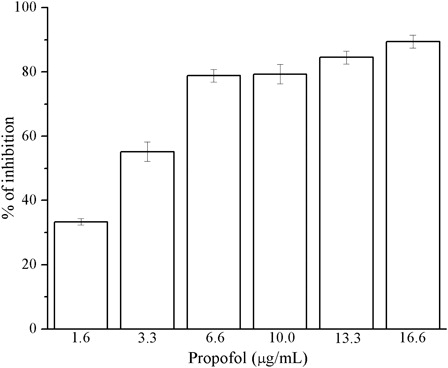
At tested concentrations, within the range known to be achieved in plasma, propofol possesses significant antioxidant activity in the reaction with DPPH• radical (). The mean value (±SD) of absorption inhibition was 70.09% ± 21.53%. The highest percentage of inhibition (89.41%) was noted at the concentration of 16.6 µg/ml. The antioxidant activity of propofol increased with increasing concentration.
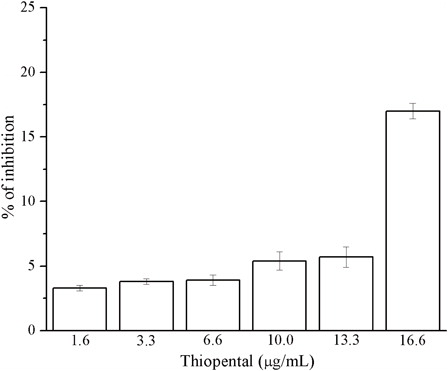
With regard to thiopental, it had little effects as an antioxidant ().
The mean value (±SD) within the range of tested concentrations was 6.50% ± SD 5.25%. The highest scavenger activity was observed at the concentration of 16.6 µg/ml (17.02%). By decreasing the concentration of thiopental, the absorption inhibition decreased too.
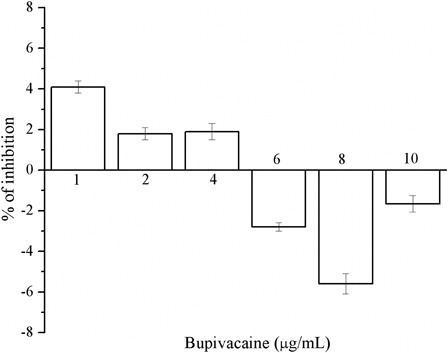
The percentage of absorbance inhibition did not linearly vary with the concentrations of bupivacaine, which we investigated in the range from 1 to 10 µg/ml; the values, even more, became negative, indicating that bupivacaine cannot trap DPPH and has no direct antioxidant activity ().
Comparison of in vitro antioxidant activity of anesthetics
We found a negative relationship between the concentration of bupivacaine and its antioxidant activity. There is a strong statistically significant correlation between propofol concentration and antioxidant property of this intravenous anesthetic ().
Table 4. Correlation between different concentrations of anesthetics and absorbance inhibition in percentage after the reaction with DPPH
Discussion
While oxidative stress is caused by an overproduction of free radicals or ROS, the accompanying lipid peroxidation in biological membranes is responsible for surgical trauma, surgery-related complications, IR injury, and cardiovascular and inflammatory damage.Citation4 In normal metabolic reactions in vivo, each biological component is naturally protected by various antioxidant defense systems.Citation22 These defense systems are very potent, but they may become insufficient in the pathological conditions. Against such conditions, the treatment with drugs possessing certain antioxidant activity might be beneficial. Although skeletal muscle is thought to be relatively insensitive to the deleterious effects of ischemia and subsequent reperfusion, injury can occur as a result of tourniquet application for limb surgery. It is important to emphasize that the pattern of ROS formation from surgical stress is different from that of reperfusion injury. The release of a tourniquet causes an abrupt, massive production of ROS, and starts oxidative damage.Citation23 The potential for protection may exist, however, in general anesthetics including inhalational agents (such as isoflurane, sevoflurane, desflurane), intravenous agents (such as ketamine, thiopental, propofol) and regional anesthesia techniques (such as peripheral nerve blocks, central nerve blocks and local infiltration).
Numerous experimental studies suggest that volatile anesthetics may protect, in addition to the heart, various tissues and organs subjected to ischemic insult.Citation24 The mechanism by which potent inhalation anesthetics may inhibit free radicals is not known, but may involve the reduction of intracellular calcium concentrations and enhanced availability of interstitial glycolysis metabolites (glucose, lactate, and pyruvate) in the skeletal muscle during ischemia and reperfusion.Citation25
The antioxidant property of propofol has been proposed because of its structural resemblance to α-tocopherol and the hypothetic participation in ascorbate-driven recycling system of α-tocopherol.Citation26 Propofol also reacts with peroxynitrite.Citation27 Since propofol has a great affinity for lipid bilayers, it is preferentially distributed in liposomal and cellular membranes, reaching a much higher concentration in membranes than in blood.Citation28 Propofol anesthesia has also been suggested to increase the capacity of plasma to inhibit lipid peroxidation and enhance the antioxidant properties of different tissues and erythrocytes.Citation29,Citation30 The commercial form of propofol (Recofol®, Schering AG., Germany: propofol 10 mg/ml) is formulated in intralipid, a lipid vehicle emulsion (10 % soya bean emulsion, egg phosphatides, and glycerol). Intralipids could also act directly on cell membranes, and induce structure alterations, leading to a decrease of ROS release in the extracellular medium.Citation31
Regional anesthesia cases (group R) were included in our study to investigate whether the effects of peripheral nerve blocks on tourniquet-induced IR injuries are mediated by antioxidant effects of bupivacaine or influenced by other mechanisms such as sympathetic blockade or anti-inflammatory effects of local anesthetics.Citation32
Our results showed that propofol enhanced TAC of plasma in children who underwent tourniquet-induced IR injury during extremity operations. More precisely, the obtained results indicated that 20 minutes after the beginning of reperfusion, TAC was increased only in patients who received propofol, in comparison to the baseline and BTR value. Furthermore, measured at the same time point, this value was statistically higher compared with plasma TAC in patients anesthetized with sevoflurane and also the patients in whom peripheral nerve blocks were performed. These findings support our previously published resultsCitation33 that 5 minutes after the tourniquet release the lowest plasma xanthine oxidase (XO) level was noticed in propofol group and that 20 minutes after reperfusion plasma malondialdehyde (MDA) concentration in patients anesthetized with sevoflurane was significantly higher in comparison with two other groups. In the same paperCitation33 we reported more significantly increased plasma protein carbonyl groups (CO) concentration and higher plasma nitrites and nitrates (NOx) values after reperfusion in sevoflurane group only.
The results of this study are in accordance with the paper of Hans et al.Citation29 who concluded that in neurosurgical patients, who required cerebrospinal fluid shunting, the capacity of plasma to inhibit lipid peroxidation increased in patients during TIVA maintained with a continuous propofol infusion. In patients undergoing laparohysterectomyCitation34 propofol showed antioxidant properties, while sevoflurane and desflurane seemed to shift the redox balance towards oxidation, yet without inducing overt oxidative damage.
Concentrations at which propofol exerted a significant antioxidant activity or protection against lipid peroxidation varied considerably from less than 1 µM to over 250 µM. These significant discrepancies between studies certainly depend on the in vitro model, i.e. the reaction environment, the type of free radical and, first and foremost, the parameter used to determine the antioxidant activity.Citation35 Although this protective effect has been demonstrated at concentrations relevant to anesthesia (12.5–50 µmol−1 is eqivalent to 2.2–8.8 µgml−1) further work is required to demonstrate that this is a clinicaly significant effect. Ergun et al.Citation36 found out that even subanesthetic dosages of propofol demonstrated beneficial effects in ischemia reperfusion injuries of sceletal muscles. Our results showed that at tested concentrations, within the range known to be achieved in plasma, propofol possesses significant antioxidant activity in the reaction with DPPH• radical. The comparison of DPPH radical scavengingCitation4 indicates that the absence of a phenolic hydrohyl group and of an isopropil group at the 2-position results in an absence of antioxidant activity. The ability to scavenge free radicals is further enhanced by additional 4-isopropyl, 5-isopropyl, and 6-isopropyl groups in the increasing order of potency. Propofol (2,6 diisopropylphenol) meets best such a structural requirement.
Since general anesthesia in the sevoflurane group was induced with thiopental, we examined whether thiopental possesses antioxidant properties in vitro. We found out that thiopental has poor in vitro antioxidant activity. This result agreed with the study of Murphy et al.Citation37 On the contrary, Dogan et al.Citation38 concluded that in experimental animals, thiopental in particular, was effective in the protection against renal IR injury.
The results of this study are in keeping with previous studies claiming that bupivacaine does not provide any direct antioxidant effect per se, but is able to inhibit the increase of thiobarbituric acid reactive substances.Citation34,Citation39 These results support the idea that bupivacaine possesses indirect antioxidant properties aimed against lipid peroxidation, possibly via the mechanism of action that seems to be located at the level of inflammation-related systems.
Finally, the experimental data obtained from pure nonmembraneous conditions are not directly linked to the effects of antioxidants in real biological membranesCitation4,Citation28 but can serve to indicate the significance of chemical structure of anesthetics in terms of free radical-scavenging abilities.
Conclusion
Plasma antioxidant capacity, during tourniquet-induced IR injuries in children, is increased by continuous propofol infusion. In tested concentrations in vitro, propofol possessed significant antioxidant activity, thiopental was poorly efficient as free radical scavenger, whereas bupivacaine had no direct antioxidant activity.
Acknowledgements
This study was supported by the Serbian Ministry of Science and Technological Development within the project No. III41018.
References
- Kamat P, Juon B, Jossen B, Gajanayake T, Rieben R, Vögelin E. Assessment of endothelium and inflammatory response at the onset of reperfusion injury in hand surgery. J Inflamm 2012;9:18.
- Arnaoutoglou H, Vretzakis G, Souliotis D, Cambili M, Galaris D, Papadopoulos G. The effects of propofol or sevoflurane on free radical production after tourniquet induced ischaemia-reperfusion injury during knee arthroplasty. Acta Anaesth Belg 2007;58:3–6.
- Ozkan F, Senayli Y, Ozyurt H, Erkorkmaz U, Bostan B. Antioxidant effects of propofol on tourniquet-induced ischemia-reperfusion injury: an experimental study. J Surg Res 2012;176(2):601–7.
- Tsuchiya H, Ueno T, Tanaka T, Matsuura N, Mizogami M. Comparative study on determination of antioxidant and membrane activities of propofol and its related compounds. Eur J Pharm Sci 2010;39:97–102.
- Corbucci GG, Marchi A, Velluti C, Chelo C, Grella E, Lettieri B. Antioxidant property of propofol in the ischemic and reperfused human skeletal muscle. Minerva Anestesiol 2002;68:13–6.
- Marik PE. Propofol: an immunomodulating agent. Pharmacotherapy 2005;25 (5 Pt 2):28S–33S.
- Sánchez-Conde P, Rodríguez-López JM, Nicolás JL, Lozano FS, García-Criado FJ, Cascajo C, et al. The comparative abilities of propofol and sevoflurane to modulate inflammation and oxidative stress in the kidney after aortic cross-clamping. Anesth Analg 2008;106(2):371–8.
- Rodríguez-López JM, Sánchez-Conde P, Lozano FS, Nicolás JL, García-Criado FJ, Cascajo C. Laboratory investigation: effects of propofol on the systemic inflammatory response during aortic surgery. Can J Anaesth 2006;53(7):701–10.
- Kevin LG, Novalija E, Stowe DF. Reactive oxygen species as mediators of cardiac injury and protection: the relevance to anesthesia practice. Anesth Analg 2005;101:1275–87.
- Basu S, Meisert I, Eggensperger E, Krieger E, Krenn CG. Time course and attenuation of ischaemia-reperfusion induced oxidative injury by propofol in human renal transplantation. Redox Rep 2007;12(4):195–202.
- Zaugg M, Wang L, Zhang L, Lou PH, Lucchinetti E, Clanachan AS. Choice of anesthetic combination determines Ca2+ leak after ischemia-reperfusion injury in the working rat heart: favorable versus adverse combinations. Anesthesiology 2012;116(3):648–57.
- Kojima A, Kitagawa H, Omatsu-Kanbe M, Matsuura H, Nosaka S. Sevoflurane protects ventricular myocytes from Ca2+ paradox-mediated Ca2+ overload by blocking the activation of transient receptor potential canonical channels. Anesthesiology 2011;115(3):509–22.
- Chappell D, Heindl B, Jacob M, Annecke T, Chen C, Rehm M, et al. Sevoflurane reduces leukocyte and platelet adhesion after ischemia-reperfusion by protecting the endothelial glycocalyx. Anesthesiology 2011;115(3):483–91.
- Onishi A, Miyamae M, Kaneda K, Kotani J, Figueredo VM. Direct evidence for inhibition of mitochondrial permeability transition pore opening by sevoflurane preconditioning in cardiomyocytes: comparison with cyclosporine A. Eur J Pharmacol 2012;675(1–3):40–6.
- Saricaoglu F, Dal D, Salman AE, Doral MN, Kilinc K, Aypar U. Ketamine sedation during spinal anesthesia for arthroscopic knee surgery reduced the ischemia-reperfusion injury markers. Anesth Analg 2005;101:904–9.
- Welters ID, Menzebach A, Langefeld TW, Menzebach M, Hempelmann G. Inhibitory effects of S-(-) and R-(+) bupivacaine on neutrophil function. Acta Anaesthesiol Scand 2001;45:570–5.
- Bedirli N, Akyürek N, Kurtipek O, Kavutcu M, Kartal S, Bayraktar AC. Thoracic epidural bupivacaine attenuates inflammatory response, intestinal lipid peroxidation, oxidative injury, and mucosal apoptosis induced by mesenteric ischemia/reperfusion. Anesth Analg 2011;113(5):1226–32.
- Brand-Williams W, Cuvelier ME, Berset C. Use of free radical method to evaluate antioxidant activity. LWT-Food Sci Technol 1995;28:25–30.
- Janaszewska A, Bartosz G. Assay of total antioxidant capacity: comparison of four methods as applied to human blood plasma. Scand J Clin Lab Invest 2002;62:231–6.
- Blios MS. Antioxidant determination by the use of stable free radical. Nature 1958;26:1199.
- Shimada K, Fujikawa K, Yahara K, Nakamura T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem 1992;40:945–8.
- Kang MY, Tsuchiya M, Packer L, Manabe M. In vitro study on antioxidant potential of various drugs used in the perioperative period. Acta Anaesthesiol Scand 1998:42:4–12.
- Cheng YJ, Wang YP, Chien CT, Chen CF. Small-dose propofol sedation attenuates the formation of reactive oxygen species in tourniquet-induced ischemia-reperfusion injury under spinal anesthesia. Anesth Analg 2002;94:1617–20.
- Minguet G, Joris J, Lamy M. Preconditioning and protection against ischaemia-reperfusion in non-cardiac organs: a place for volatile anaesthetics. Eur J Anaesthesiol 2007;24:733–45.
- Carles M, Dellamonica J, Roux J, Lena D, Levraut J, Pittet JF, et al. Sevoflurane but not propofol increases interstitial glycolysis metabolites availability during tourniquet-induced ischemia-reperfusion. Br J Anaesth 2008;100(1):29–35.
- Tsuchiya M, Asada A, Kasahara E, Sato EF, Shindo M, Inoue M. Antioxidant protection of propofol and its recycling in erythrocyte membranes. Am J Resp Crit Care Med 2002;165:54–60.
- Vasileiou I, Xanthos T, Koudouna E, Perrea D, Klonaris C, Katsargyris A, et al. Propofol: a review of its non-anaesthetic effects. Eur J Pharm 2009;605:1–8.
- Friaa O, Chaleix V, Lecouvey M, Brault D. Reaction between the anesthetic agent propofol and the free radical DPPH in semiaqueous media: kinetics and characterization of the products. Free Radic Biol Med 2008;45(7):1011–8.
- Hans P, Deby-Dupont G, Deby C. Increase in antioxidant capacity of plasma during propofol anesthesia. J Neurosurg Anesthesiol 1997;9:234–6.
- Runzer TD, Ansley DM, Godin DV, Chambers GK. Tissue antioxidant capacity during anesthesia: propofol enhances in vivo red cell and tissue antioxidant capacity in a rat model. Anesth Analg 2002;94:89–93.
- Marthy-Hartert M, Deby-Dupont G, Hans P, Deby C, Lamy M. Protective activity of propofol, diprivan and intralipid against active oxygen species. Mediators Inflamm 1998;7:327–33.
- Bedirli N, Akyu N, Kurtipek O, Kavutcu M, Kartal S, et al. Thoracic epidural bupivacaine attenuates inflammatory response, intestinal lipid peroxidation, oxidative injury, and mucosal apoptosis induced by mesenteric ischemia/reperfusion. Anesth Analg 2011;113:1226–32.
- Budic I, Pavlovic D, Kocic G, Cvetkovic T, Simic D, Basic J, et al. Biomarkers of oxidative stress and endothelial dysfunction after tourniquet release in children. Physiol Res 2011;60:S137–45.
- Cinnella G, Vendemiale G, Dambrosio M, Serviddio G, Pugliese PL, et al. Effect of propofol, sevoflurane and desflurane on systemic redox balance. Int J Immunopathol Pharmacol 2007;20(3):585–93.
- Boisset S, Steghens JP, Favetta P, Terreux R, Guitton J. Relative antioxidant capacities of propofol and its main metabolites. Arch Toxicol 2004;78(11):635–42.
- Ergun Y, Oksuz H, Atli Imrek Y, Kilinc M, Darendeli S. Ischemia-reperfusion injury in skeletal muscle: comparison of the effects of subanesthetic doses of ketamine, propofol and etomidate. J Surg Res 2010;159(1):e1–10.
- Murphy PG, Davies MJ, Columb MO, Stratford N. Effect of propofol and thiopentone on free radical mediated oxidative stress of the erythrocyte. Br J Anaesth 1996;76(4):536–43.
- Dogan Z, Yuzbasioglu MF, Kurutas EB, Yildiz H, Coskuner I, Senoglu N, Oksuz H, Bülbüloglu E. Thiopental improves renal ischemia-reperfusion injury. Ren Fail 2010;32(3):391–5.
- Leduc C, Gentili ME, Estebe JP, Le Corre P, Moulinoux JP. The effect of local anesthetics and amitriptyline on peroxidation in vivo in an inflammatory rat model: preliminary reports. Anesth Analg 2002;95:992–6.