Abstract
Background
Psoriasis is a chronic hyperproliferative inflammatory skin disease, characterized by a generalized redox imbalance. Anti-tumor necrosis factor (TNF)-α therapy is widely used for the treatment of this disease, but its effect on blood redox status hasn't been explored.
Objective
To investigate the effects of anti-TNF-α therapy on blood redox status in psoriatic patients.
Methods
Twenty-nine psoriatic patients (PSO) were divided into two groups: one remained untreated (NRT) and to another the anti-TNF-α therapy was prescribed (TR). The levels of main oxidative stress markers and total antioxidant capacity (TAC) in plasma, levels of total reactive oxygen species (ROS) production, lipoperoxidation, TAC, glutathione content, and activity of NADPH oxidase in white blood cells (WBC) were evaluated in PSO, in NTR and TR after 6 months of the study.
Results
Plasma levels of malondialdehyde (MDA) and protein carbonyl content (PCO), ROS production, lipoperoxidation, and glutathione content in WBC were increased, while TAC in both plasma and WBC was decreased in PSO with respect to controls. In the plasma of TR, levels of MDA and PCO were significantly lower with respect to PSO and NTR. The activity of NADPH oxidase was significantly increased in WBC of PSO and NTR but not in TR versus controls.
Discussion
Our results represent novel data about the redox status of WBC in psoriatic patients. A significant redox-balancing effect of anti-TNF-α therapy, probably associated with the normalization of NADPH oxidase activity in WBC, was demonstrated.
Introduction
Psoriasis is an inflammatory skin disease, characterized by the appearance of itchy or sore patches of thick, red skin with silvery scales. The etiology of the disease is still unknown, but T lymphocytes, dendritic cells, neutrophils, and macrophages are believed to play a relevant part in its progression.Citation1 In psoriasis, these cells infiltrate the skin and release pro-inflammatory cytokines (such as interleukin-6, inerferon-γ, and TNF-α and O2•−,Citation2,Citation3 perturbing the redox balance of the skin and inducing the progression of inflammation. Recent evidence suggests that the redox imbalance found in blood and skin of the patients plays an important role in the pathogenesis of psoriasis.Citation3 Indeed, numerous studies revealed significantly increased levels of oxidative stress markers such as malondialdehyde (MDA), nitric oxide end products,Citation3–Citation6 and 8-OHdG (8-hydroxydeoxyguanosine)Citation7 in the plasma of psoriatic patients. Likewise, decreased total antioxidant capacity (TAC), vitamin A and E levels, and decreased activity of the main antioxidant enzymes (such as erythrocyte and serum superoxide dismutase and catalase) were also found in the plasma of these patients.Citation3–Citation6,Citation8 Moreover, the ‘severity’ of plasma redox imbalance was found to be strongly correlated with the Psoriasis Area and Severity Index (PASI),Citation4–Citation8 which in turn correlates with the level of inflammatory cytokines.Citation8
The central role of TNF-α in the pathogenesis of psoriasis has been demonstrated by the clinical efficacy of TNF-α antagonists. There are now five TNF inhibitors available for the treatment of various immune-mediated and inflammatory diseasesCitation9–Citation13: Infliximab (IFX), Adalimumab, and Golimumab are full-length monoclonal antibodies; Etanercept is a fusion protein of two TNFR2 receptor extracellular domains and the Fc fragment of human immunoglobulin 1 (IgG1); and Certolizumab is a humanized Fab fragment conjugated to polyethylene glycol.Citation14,Citation15 While these drugs appear to have a similar efficacy,Citation14,Citation16 the choice of one or another TNF inhibitor is personalized for each psoriatic patient.Citation17 It is important to note that TNF-α also acts as a potent inducer of ROS generation through impairment of mitochondrial biogenesis and activation of NADPH oxidase.Citation8,Citation18–Citation23 Taken together, these data allow us to speculate that the immune system and the redox system might in some way interact synergistically, such that both mechanisms might be relevant in the pathogenesis of psoriasis.
In this study, we aimed to analyze the effects of anti-TNF treatment with IFX on the blood redox status in psoriatic patients. Main oxidative stress markers (MDA and protein carbonyl content (PCO)) and TAC in plasma were assessed. Particular attention was paid to the evaluation of oxidative stress markers, antioxidant capacity, and NADPH oxidase activity in WBC obtained from treated and untreated patients. The results of the current study show the presence of redox imbalance in the blood of psoriatic patients and suggest a possible relationship between TNF-α and NADPH oxidase activity in WBC of psoriatic patients.
Materials and methods
Patients
The study was approved by the Local Ethical Community and carried out according to the Helsinki Declaration. Analyses were carried out in 29 patients (20 females and 9 males) affected by moderate psoriasis (PASI = 13 ± 1 SD; the group ‘PSO’) with a mean age of 47 ± 8 SD years, and with a mean duration of disease of 17 years (from 1 to 35 years). The demographic and clinical information for each patient is summarized in . Eighteen non-stressed healthy controls (10 females and 8 males), matched for age and body mass index were also enrolled in the study. No subjects involved in the study followed any systemic therapy before the study or had a history of any disease, e.g. diabetes mellitus and atherosclerosis, which might affect the redox status.
Table 1. Demographic and clinical data of patients involved in the study
Redox status was analyzed in the blood of PSO and Controls as described below. After that, the PSO group was divided into two sub-groups: (1) was prescribed the anti-TNF-α therapy via intravenous administration of 5 mg/kg IFX every 8 weeks for 6 months (‘treated’ psoriatic patients or ‘TR’); and (2) was not prescribed any systemic treatment for the duration of the study (‘untreated’ psoriatic patients or ‘NTR’). After 6 months blood analysis was repeated and the PASI again estimated in both TR and NTR groups of patients.
Management of the blood samples
Ten milliliters of peripheral blood was collected in EDTA tubes and treated to obtain: (a) WBC by BD FACS Lysing Solution (BD Biosciences, San Jose, Canada), following the manufacturer's protocol, (b) mononuclear cells (peripheral blood mononuclear cell (PBMC)) using 1.077 g/ml Ficoll Hypaque solution (Sigma-Aldrich, Milan, Italy), again following the manufacturer's protocol, and (c) plasma by centrifugation of whole blood at 800g for 10 minutes. Plasma was analyzed at the earliest time points for levels of thiobarbituric acid reactive substances (TBARS) using a commercially available kit (Oxitek-ZeptoMetrix Corporation, Buffalo, NY, USA), for protein carbonylation (PCO) using a commercially available Protein Carbonyl Fluorometric Assay Kit (Cayman Chemical, USA), and for oxygen radical antioxidant capacity (ORAC) as described below. WBC were immediately subjected to flow cytometric analysis (FACS) and for each of three populations, namely granulocytes, monocytes, and lymphocytes (, a), levels of intracellular ROS and lipoperoxidation were estimated using the fluorescent dyes H2DCF-DA and BODIPY 581/591 C11 (both from Molecular Probes Invitrogen, Carlsbad, CA, USA), respectively.Citation24,Citation25 Total cell lysates of WBC were prepared for ORAC analysis and glutathione content using the Glutathione Assay Kit (Cayman Chemical). PBMC (1 × 106 cells/sample) were used to determine NADPH oxidase activity by lucigenin-based luminometric assay, as described below.
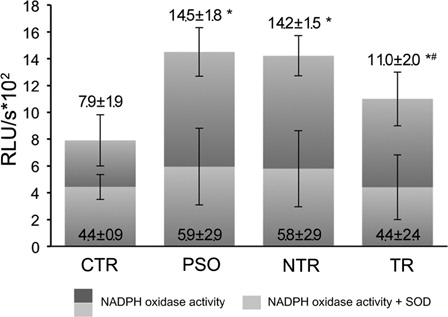
Figure 1. NADPH oxidase activity in intact PBMC, measured by lucigenin-based assay. PSO, psoriatic patients prior to anti-TNF-α therapy (n = 29); NTR, psoriatic patients without any systematic treatment after 6 months (n = 16); TR; psoriatic patients after 6 months of anti-TNF-alpha therapy (n = 13). SOD, superoxide dismutase. *P < 0.05 as compared to control; #P < 0.05 as compared to NTR.
ORAC assay
The reaction was carried out in 96-well black microplates (Nunc, Roskilde, Denmark), with trolox (10–200 µM) used as a standard. The amount of sample applied to each well was calculated as follows: 12 µg of protein/sample for plasma and 4 µg of protein/well for total cell lysates. The final assay mixture (total volume = 200 µl) contained: 70 µl of sample diluted in 75 mM phosphate buffer (pH 7.0) and 100 µl of fluorescein reagent at final concentration of 6 nM. After 10 minutes incubation in the dark at 37°C, 30 µl of pre-heated (at 37°C) AAPH (Sigma-Aldrich Italy S.r.l.) solution (final concentration of AAPH = 127 mM) was added to each well using a multi-well channel pipette. Fluorescence was analyzed using a fluorometric microplate reader (Fluoroskan Ascent; Thermo Electron Corp., Vantaa, Finland) at 5 minutes intervals for 2 hours at excitation and emission wavelengths of 485 and 537 nm, respectively. All assays were conducted in triplicate and at least two independent tests were carried out for each sample. The area under curve was calculated for each sample by integrating the relative fluorescence curve. Regression equations obtained from net value of trolox were used to calculate the ORAC value for each assay. Final ORAC values were expressed as nmol trolox equivalent (TE) per ml (nmol TE/ml) for plasma and μmol of TE per mg of protein (μmol TE/mg) for WBC lysates.
NADPH oxidase activity luminometric assay
NADPH oxidase activity assay was performed on intact PBMC using a Lumat LB 9507 single-tube luminometer (Berthold Technologies, GmbH & Co, Bad Wildbad, Germany). After washing with phosphate-buffered saline, 1 × 106 cells were resuspended in 150 µl Krebs-HEPES buffer (99 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 1 mM KH2PO4, 1.9 mM CaCl2, 25 mM NaHCO3, 20 mM HEPES, 11.1 mM glucose, pH 7.44) and incubated for 10 minutes at 37°C and the blank value of luminescence was determined. Following this, lucigenin was added to the sample at a final concentration of 25 µM. Immediately after that a series of luminescence readings taken at 1-minute intervals for 30 minutes at 37°C was performed. We used two controls for NADPH oxidase activity: (1) by the 20th reading, the curve of NADPH oxidase activity normally reached a plateau and SOD was added to the sample at a final concentration of 450 U/ml and (2) pre-incubation with diphenyleneiodonium chloride, DPI, (20 µM) for 30 minutes at 37°C led triggered no response upon lucigenin addition. NADPH oxidase activity was expressed as relative units of fluorescence (RUL)/s.
Statistical analysis
All data are expressed as means ± SD. Comparisons between different groups were carried out using one-way analysis of variance followed by the Bonferroni t-test. A P value <0.05 was accepted as statistically significant.
Results
Redox status of plasma and WBC in PSO (T = 0 months)
Levels of oxidative stress markers, TBARS and PCO, were significantly increased and TAC significantly decreased in the plasma of PSO with respect to controls (). In WBC of PSO total ROS content and lipoperoxidation levels were also significantly higher than controls in all three populations of cells, that is, granulocytes, monocytes, and lymphocytes (). In total, WBC lysates from PSO TAC was substantially lower with respect to WBC from controls. In PSO, total glutathione levels in WBC were significantly decreased, and levels of the oxidized form increased with respect to controls ().
Table 2. Redox markers in plasma of psoriatic patients and controls
Table 3. Redox markers and glutathione content in WBC of psoriatic patients and controls
We hypothesized that NADPH oxidase might be responsible for ROS overproduction in PBMC from psoriatic patients. Indeed, the results of NADPH oxidase activity measured in PBMC (a fraction of WBC lacking granulocytesCitation26) revealed increased activity of the enzyme in PBMC from PSO compared to controls (). Interestingly, following SOD addition to the sample, the residual (NADPH oxidase) activity in PSO PBMC was not significantly different from the one in control PBMC.
Redox status of plasma and WBC in TR and NTR (T = 6 months)
After 6 months, the PASI and redox status of plasma and WBC was re-evaluated in both groups of psoriatic patients (TR and NTR); significant differences in levels of oxidative stress markers were found between TR and NTR. PASI showed a significant clinical improvement in TR and a slight decrease, albeit not significant, in NTR in comparison to PSO (). TBARS and PCO were still significantly increased and TAC significantly decreased in both TR and NTR with respect to controls. However, levels of TBARS and PCO in TR were significantly lower than in NTR and PSO. Plasma TAC was not significantly different in TR compared to NTR.
Assessment of oxidative stress markers in WBC revealed tendencies similar to those observed for plasma. The total ROS content in WBC was significantly increased in both TR and NTR with respect to controls in all three populations of WBC (). However, levels of ROS production in WBC from TR were lower with respect to WBC from NTR. Interestingly, levels of lipid peroxidation were not significantly different between TR and controls, but significantly lower in TR compared to NTR. TAC in WBC from NTR was substantially lower than that in WBC of TR and controls. In contrast, no significant difference was found between WBC TAC from TR and controls. A significant decrease in total glutathione levels in WBC from both NTR and TR, with respect to controls, was still observed. Subsequently, a significant increase in oxidized/reduced glutathione ratio (GSSG/GSH) was observed in both TR and NTR compared to controls; however, the GSSG/GSH ratio was significantly lower in TR with respect to NTR ().
After 6 months NADPH oxidase activity showed no significant change in NTR with respect to PSO. Although, NADPH oxidase activity of PBMC from the TR group was significantly decreased in comparison with PSO and NTR, it was still significantly higher than the one of control PBMC. Following SOD treatment the residual (NADPH oxidase) activity in NTR and TR PBMC decreased. These values were not significantly different from the residual NADPH oxidase activity of control PBMC.
Discussion
Psoriasis is characterized by generalized oxidative stress and by augmented levels of TNF-α, a well-known inducer of ROS generation in non-phagocytic cells. Chronic inflammatory processes associated with redox imbalance in patients' skin and blood can frequently lead to premature atherosclerosis in psoriatic patients.Citation17,Citation27
IFX, a chimeric monoclonal anti-TNF-α antibody, is frequently used for treatment of psoriasis.Citation28–Citation30 While biological agents have demonstrated efficacy in patients with psoriasis and are generally considered safe and well tolerated, rare but serious safety issues (such as demyelination, infection, tuberculosis, malignancy, lymphoma, cardiovascular outcomes, and hepatitis) have been observed.Citation31–Citation35 Attention to specific aspects of patient management (e.g. pre-screening requirements, symptoms to watch for, appropriate treatment and referrals) is required to mitigate the risk.Citation36
A beneficial antioxidant effect of IFX treatment has already been shown for the skin, plasma, and erythrocytes,Citation37,Citation38 but no data about its effect on WBC, which play a prominent role in the immune profile of psoriasis, are present in the literature. In this study, we assessed the effect of IFX therapy on plasma and WBC redox status in psoriatic patients.
The results of our study confirm the presence of a redox imbalance in the plasma of psoriatic patients. In particular, we found significantly increased levels of oxidative stress markers and decreased antioxidant capacity in psoriatic patients with respect to controls. Notably, levels of oxidative stress markers decreased significantly after treatment with IFX; this finding suggests a role for TNF-α in triggering ROS generation in psoriasis and that anti-TNF-α therapy significantly reduces oxidative stress in the plasma of affected patients. In contrast, antioxidant capacity did not differ significantly in treated and untreated patients, suggesting persistent antioxidant depletion in the plasma of patients following IFX therapy. Indeed, several recent publications suggest that anti-TNF-α therapy could lead to a decrease in TAC levels.Citation38 In our case, treatment with IFX did not affect TAC levels in the plasma of psoriatic patients.
Conversely, a significant increase in TAC was observed in WBC following IFX therapy with respect to NTR and PSO. This may be partly due to the increase in reduced GSH with respect to the oxidized GSSG form. The redox GSSG/GSH ratio was much higher in untreated patients with respect to treated patients and controls; IFX treatment significantly decreased this ratio, which agrees with the findings of other authors who reported upregulation of glutathione-S-transferase and glutathione peroxidase following anti-TNF-α therapy.Citation37,Citation38
Increased TAC in WBC from treated with respect to untreated patients can be explained by the reduced consumption of low-molecular-weight antioxidants. Indeed, we have shown for the first time that ROS production in WBC of treated patients was significantly lower with respect to levels in untreated individuals, which proves that anti-TNF-α therapy has a redox balancing effect.
The results of our work reveal an upregulation of NADPH oxidase activity in PBMC from untreated patients. However, such incrimination of NADPH oxidase activity was not observed in PBMC from patients treated with IFX. This could partly explain the IFX-induced decrease in ROS production in PBMC of psoriatic patients. These findings agree with those reported by other authors, who showed that activity of NADPH oxidase can be regulated by TNF-α.Citation8,Citation18–Citation23
Indeed, a pronounced morphological change and increase in ROS production in mitochondria following TNF-α treatment has recently been shown in insulin-resistant 3T3-L1 adipocytes.Citation19 In another study, human aortic smooth muscle cells and embryonic kidney cells treated with TNF-α displayed increased superoxide production, due to upregulated expression of NOX4 and NOX2 isoforms of NADPH oxidase, respectively.Citation20 Yoshida and TsunawakiCitation21 demonstrated that, following TNF-α stimulation, coronary artery endothelial cells also show enhanced ROS-generating activity via induction of NADPH oxidase isoforms Nox2 and Nox4A. TNF-α has been also shown to activate Nox1 NADPH oxidase in mouse fibroblasts when cells undergo necrosis.Citation22 Finally, an in vitro study carried out by Cangemi et al.,Citation23 which was carried out in platelets from a subgroup of heart failure patients, showed that TNF-α (at concentrations commonly found in the peripheral circulation of heart failure patients) activates platelet NOX2. Thus, TNF-α increases ROS production and extracellular levels of NOX2.
In summary, we have for the first time shown a crucial redox imbalance in the WBC of psoriatic patients. Moreover, we provide evidence that NADPH oxidase might represent a leading source of ROS overproduction in WBC from psoriatic patients, although additional experiments should be performed to support our observation. Finally, IFX therapy displayed a significant redox balancing effect, which was associated with the modulation of NADPH oxidase activity. Taken together, our results suggest that the redox status of the blood is sensitive to treatment with IFX; this allows us to propose the assessment of blood redox status as a relevant tool to control the progression of psoriasis and to estimate the effectiveness of anti-TNF-α therapy.
Funding
Ente Cassa di Risparmio Firenze (Grant no. 2008.1597) and Abbott Italia Campoverde di Aprilia (LT).
References
- Coimbra S, Figueiredo A, Castro E, Rocha-Pereira P, Santos-Silva A. The roles of cells and cytokines in the pathogenesis of psoriasis. Int J Dermatol 2012;51(4):389–95.
- Cai Y, Fleming C, Yan J. New insights of T cells in the pathogenesis of psoriasis. Cell Mol Immunol 2012;9(4):302–9.
- Zhou Q, Mrowietz U, Rostami-Yazdi M. Oxidative stress in the pathogenesis of psoriasis. Free Radic Biol Med 2009;47:891–905.
- Gabr SA, Al-Ghadir AH. Role of cellular oxidative stress and cytochrome c in the pathogenesis of psoriasis. Arch Dermatol Res 2012;304(6):451–7.
- Pujari VM, Suryakar AN, Ireddy S. Oxidants and antioxidant status in psoriasis patients. Biomed Res 2010;21(2):221–3.
- Kadam DP, Suryakar AN, Ankush RD, Kadam CY, Deshpande KH. Role of oxidative stress in various stages of psoriasis. Indian J Clin Biochem 2010;25(4):388–92.
- Basavaraj KH, Vasu Devaraju P, Rao KS. Studies on serum 8-hydroxy guanosine (8-OHdG) as reliable biomarker for psoriasis. J Eur Acad Dermatol Venereol 2012; Jan 14, DOI: 10.1111/j.1468-3083.2011.04441.x.
- Rocha-Pereira P, Santos-Silva A, Rebelo I, Figueiredo A, Quintanilha A, Teixeira F. Dislipidemia and oxidative stress in mild and in severe psoriasis as a risk for cardiovascular disease. Clin Chim Acta 2001;303(1–2):33–9.
- Murdaca G, Colombo BM, Barabino G, Caiti M, Cagnati P, Puppo F. Anti-tumor necrosis factor-α treatment with infliximab for disseminated granuloma annulare. Am J Clin Dermatol 2010;11(6):437–9.
- Murdaca G, Colombo BM, Puppo F. Anti-TNF-alpha inhibitors: a new therapeutic approach for inflammatory immune-mediated diseases: an update upon efficacy and adverse events. Int J Immunopathol Pharmacol 2009;22(3):557–65.
- Murdaca G, Colombo BM, Puppo F. Adalimumab for the treatment of immune-mediated diseases: an update on old and recent indications. Drugs Today (Barc) 2011;47(4):277–88.
- Murdaca G, Colombo BM, Puppo F. Emerging biological drugs: a new therapeutic approach for systemic lupus erythematosus. An update upon efficacy and adverse events. Autoimmun Rev 2011;11(1):56–60.
- Puppo F, Murdaca G, Ghio M, Indiveri F. Emerging biologic drugs for the treatment of rheumatoid arthritis. Autoimmun Rev 2005;4(8):537–41.
- Thalayasingam N, Isaacs JD. Anti-TNF therapy. Best Pract Res Clin Rheumatol 2011;25(4):549–67.
- Murdaca G, Colombo BM, Cagnati P, Gulli R, Spanò F, Puppo F. Update upon efficacy and safety of TNF-α inhibitors. Expert Opin Drug Saf 2012;11(1):1–5.
- Baker EL, Coleman CI, Reinhart KM, Phung OJ, Kugelman L, Chen W, et al. Effect of biologic agents on non-PASI outcomes in moderate-to-severe plaque psoriasis: systematic review and meta-analyses. Dermatol Ther (Heidelb) 2012;2(1):9.
- Murdaca G, Colombo BM, Contini P, Puppo F. Determination of lymphotoxin-alpha levels in patients with psoriatic arthritis undergoing etanercept treatment. J Interferon Cytokine Res 2012;32(6):277–9.
- Mariappan N, Elks CM, Haque M, Francis J. Interaction of TNF with angiotensin II contributes to mitochondrial oxidative stress and cardiac damage in rats. PLoS One 2012;7(10):e46568.
- Chen XH, Zhao YP, Xue M, Ji CB, Gao CL, Zhu JG, et al. TNF-alpha induces mitochondrial dysfunction in 3T3-L1 adipocytes. Mol Cell Endocrinol 2010;328(1–2):63–9.
- Moe KT, Aulia S, Jiang F, Chua YL, Koh TH, Wong MC, et al. Differential upregulation of Nox homologues of NADPH oxidase by tumor necrosis factor-alpha in human aortic smooth muscle and embryonic kidney cells. J Cell Mol Med 2006;10(1):231–9.
- Yoshida LS, Tsunawaki S. Expression of NADPH oxidases and enhanced H(2)O(2)-generating activity in human coronary artery endothelial cells upon induction with tumor necrosis factor-alpha. Int Immunopharmacol 2008;8(10):1377–85.
- Kim YS, Morgan MJ, Choksi S, Liu ZG. TNF-induced activation of the Nox1 NADPH oxidase and its role in the induction of necrotic cell death. Mol Cell 2007;26(5):675–87.
- Cangemi R, Celestini A, Del Ben M, Pignatelli P, Carnevale R, Proietti M, et al. Role of platelets in NOX2 activation mediated by TNFα in heart failure. Intern Emerg Med 2012; Jul 28, DOI: 10.1007/s11739-012-0837-2.
- Becatti M, Prignano F, Fiorillo C, Pescitelli L, Nassi P, Lotti T, et al. The involvement of Smac/DIABLO, p53, NF-kB, and MAPK pathways in apoptosis of keratinocytes from perilesional vitiligo skin: protective effects of curcumin and capsaicin. Antioxid Redox Signal 2010;13(9):1309–21.
- Becatti M, Taddei N, Cecchi C, Nassi N, Nassi PA, Fiorillo C. SIRT1 modulates MAPK pathways in ischemic-reperfused cardiomyocytes. Cell Mol Life Sci 2012;69(13):2245–60.
- Delves PJ, Martin SJ, Burton DR, Roitt IM, eds. Roitt's essential immunology. New York: Wiley-Blackwell; 2011. p. 546.
- Yiu KH, Yeung CK, Zhao CT, Chan JC, Siu CW, Tam S, et al. Prevalence and extent of subclinical atherosclerosis in patients with psoriasis. J Intern Med 2013; 273(3):273–82.
- Gniadecki R, Kragballe K, Dam TN, Skov L. Comparison of drug survival rates for adalimumab, etanercept and infliximab in patients with psoriasis vulgaris. Br J Dermatol 2011;164:1091–6.
- Warren RB, Griffiths CE. The future of biological therapies. Semin Cutan Med Surg 2010;29:63–6.
- Cantini F, Niccoli L, Nannini C, Kaloudi O, Cassarà E. Infliximab in psoriatic arthritis. J Rheumatol Suppl 2012;89:71–3.
- Perez-Alvarez R, Pérez-de-Lis M, Ramos-Casals M; BIOGEAS study group. Biologics-induced autoimmune diseases. Curr Opin Rheumatol 2013;25(1):56–64.
- De Simone C, Murri R, Maiorino A, Venier A, Caldarola G. Management of recurrent cutaneous abscesses during therapy with infliximab. Clin Ther 2011;33(12):1993–6.
- Papp KA, Dekoven J, Parsons L, Pirzada S, Robern M, Robertson L, et al. Biologic therapy in psoriasis: perspectives on associated risks and patient management. J Cutan Med Surg 2012;16(3):153–68.
- Guerra I, Algaba A, Pérez-Calle JL, Chaparro M, Marín-Jiménez I, García-Castellanos R, et al. Induction of psoriasis with anti-TNF agents in patients with inflammatory bowel disease: a report of 21 cases. J Crohns Colitis 2012;6(5):518–23.
- Alghamdi KM, Khurrum H, Rikabi A. Worsening of vitiligo and onset of new psoriasiform dermatitis following treatment with infliximab. J Cutan Med Surg 2011;15(5):280–4.
- Csiszar A, Labinskyy N, Smith K, Rivera A, Orosz Z, Ungvari Z. Vasculoprotective effects of anti-tumor necrosis factor-alpha treatment in aging. Am J Pathol 2007;170(1):388–98.
- Guzel A, Kanter M, Guzel A, Pergel A, Erboga M. Anti-inflammatory and antioxidant effects of infliximab on acute lung injury in a rat model of intestinal ischemia/reperfusion. J Mol Histol 2012;43(3):361–9.
- Campanati A, Orciani M, Gorbi S, Regoli F, Di Primio R, Offidani A. Effect of biologic therapies targeting tumour necrosis factor-α on cutaneous mesenchymal stem cells in psoriasis. Br J Dermatol 2012;167(1):68–76.