Abstract
The rapid emergence of various pesticides in the market is inevitable due to the demands from agriculture industries and domestic needs to control nuisance pests and to sustain green resources worldwide. However, long-term exposure to pesticide has led to adverse effects on male fertility. Organophosphate diazinon (O,O-diethyl-O-[2-isopropyl-6-methyl-4-pyrimidinyl] phosphorothiote) is an often abusively used pesticide, as it is effective and economical. This study is to determine the adverse effects of low-dose diazinon exposure on the male reproductive system. In this study, 72 Sprague–Dawley rats were segregated into 1, 2, and 8 weeks of exposure groups and further sub-grouped (n = 6) to receive 0, 10, 15, and 30 mg/kg body weight diazinon treatment. Rats were gavaged orally with diazinon and sacrificed under anaesthesia the day after the last exposure. Our results showed that consistent diazinon exposure decreased glutathione and catalase, and increased lipid peroxidation which together lead to diazinon-mediated oxidative stress. Additionally, diazinon increased serum lactate dehydrogenase and decreased serum testosterone, which may have caused sperm and histopathological anomalies. In conclusion, exposure to diazinon caused changes in lipid peroxidation and sperm, and these two effects might be causally linked.
Introduction
The emergence of various pesticides in the market is due to the crucial need in the modern community to control invasive, nuisance, and destructive pests. The synthetic organophosphate (OP) pesticide diazinon (O,O-diethyl-O-[2-isopropyl-6-methyl-4-pyrimidinyl] phosphorothiote) was established as a warfare agent.Citation1 Even though diazinon (DZN) was officially banned in 2004,Citation1 it is still, illegally, widely used in the agricultural and domestic fieldCitation2–Citation4 due to its effectiveness and comparatively cheap cost.Citation5 DZN is well known as an acetylcholinesterase inhibitor as evidenced by the accumulation of acetylcholine at cholinergic synapses, which leads to activation of nicotinic and muscarinic receptors.Citation6
DZN also acts as an oxidative stressor, affecting overall health. Recently, Ogutcu et al.Citation7 reported that Wistar rats treated with 10 mg/kg body weight (b.w.) DZN orally, increased malondialdehyde (MDA), the end product of lipid peroxidation (LPO), in heart tissue after 1, 4, and 7 weeks of treatment. The significant increase in MDA might have been caused by DZN inducing LPO or an increase in reactive oxygen species (ROS).Citation8
DZN exposure shows a positive correlation between MDA level and biochemical and histopathological changes.Citation9 Numerous studies have shown that DZN alters liver transaminases activity,Citation10,Citation11 serum amylase, and lipase.Citation10 It was proven that DZN affects the sex hormones in femalesCitation2 and testosterone in males.Citation12 The biochemical changes paralleled evidence of histopathological changes. According to Videira et al.Citation13 histopathological damage is associated with internal changes of the lipid constituents of phospholipid membranes. The modification of membrane permeability allows DZN to penetrate and affect the intracellular components. This physicochemical change of lipid is facilitated by the autocatalytic process of LPO, which is caused by the presence of excessive free radicals.Citation8
The low amount of DZN in the environment is unnoticed over time, becoming a silent genotoxic agent. Over the past five decades, male fertility has halved and has been correlated substantially to pesticides. There has been little evidence, however, of the reproductive toxicity of DZN.Citation14,Citation15 Testis is highly sensitive to a variety of stressors,Citation16 which may intensify the negative manifestations caused by DZN exposure. This may lead to the disruption of spermatogenesis.Citation15
In a study of liver toxicity, oral DZN exposure caused pathological and biochemical changes although the dose was only 10 mg/kg b.w., which is below the agent's LD50.Citation11 A low level (0.25 mg/l) of DZN in the aquatic environment has a significant effect on the reproduction and development of carp.Citation3 It was reported that, although insecticides do not necessarily kill the fish, they affect the reproductive system, hence limiting the number of offspring the fish can produce.Citation17
Therefore, in this study we investigated the effects of low-dose DZN in male fertility and the influence of LPO in antioxidant defence. This may help to create awareness about the deleterious effect of DZN exposure thus alleviating the fecundity index of humans in the future.
Materials and methods
Chemicals
DZN was obtained from Devidayal (Sales) Limited (manufacturers of pesticides), Mumbai India. The LD50 of acute oral toxicity of technical DZN (≥90% active ingredients) is >5000 mg/kg b.w. thiobarbituric acid (TBA), reduced glutathione (GSH), 5,5′-dithio-bis-2-nitrobenzoic acid (DTNB), sulfosalicylic acid (SSA), bovine serum albumin, hydrogen peroxide (H2O2) and trichloroacetic acid (TCA) were purchased from Sigma-Aldrich Corporation, St Louis, Missouri, USA. All other solvents and chemicals used were either of analytical grade or the highest purity commercially available.
Animals
Thirty adult male Sprague Dawley (3 months old) rats obtained locally from Tesjaya Laboratory Services, Penang, Malaysia were used. Animals were segregated into three groups according to the determined durations of exposure, which were 1, 2, and 8 weeks, and again segregated into subgroups (n = 6) treated with saline or 10, 15, and 30 mg/kg b.w. DZN. The animals were housed in polypropylene plastic cages and each cage was limited to three animals to prevent overcrowding. Animals were fed with standard laboratory chow and water ad libitum. The animals were treated in a humane manner, and maintained under standard ethical principles of the National Institute of Health, Malaysia, approved by Universiti Malaysia Sabah, 12:12 hours light/dark cycles, 28 ± 2°C temperature and 50 ± 5% humidity.
Body weight and testis weight
Each animal was weighed before, during and after the last treatment to monitor their general health by using a weighing scale (Mettler Toledo PB8001-S, Switzerland). Animals were sacrificed under anaesthesia at the end of the treatment and laparotomy was done to expose the reproductive system. Both testes, each with epididymis were removed and weighed. Left and right testes were subjected to biochemical and histopathology analysis respectively.
Biochemical analysis
Preparation of 10% post-mitochondrial supernatant
Testis was weighed to determine the volume of cold phosphate buffered saline (PBS, 0.1 M, pH 7.2) required to make 10% homogenate (w/v). The organ was homogenized (Ultra Turrax T25 Basic, IKA Labortechnik, Germany) for 15 seconds. The homogenate was then centrifuged (Eppendorf Centrifuge 5810R, Germany) under 1157 g at 4°C for 15 minutes followed by 12 857×g for 30 minutes. The clear supernatant was used and the sediment was discarded.
Determination of lipid peroxidation
The LPO assay was performed according to the method of Buege and Aust.Citation18 The LPO level in testis post-mitochondrial supernatant (PMS) was determined by measuring the rate of thiobarbituric acid reactive substance production (expressed as MDA equivalents). One volume (ml) of testis PMS (10% w/v) was mixed with 0.5 ml of TCA (10% w/v) and centrifuged (Eppendorf Centrifuge 5810R, Germany) at 12 857×g for 30 minutes at 4°C. Supernatant (1.0 ml) was mixed with 1.0 ml of TBA (0.67% w/v). All the tubes were placed in a boiling water bath for 30 minutes. Next, the tubes were immediately shifted to an ice bath for a few minutes. The results were expressed as the amount of MDA formed in each of the samples and was assessed by measuring the optical density of the supernatant at 535 nm using a spectrophotometer (Varian UV-visible Spectrophotometer, Germany) at 37°C and was calculated using a molar extinction coefficient of 1.56 × 105 M/cm.
Determination of reduced glutathione
Reduced glutathione (GSH) was measured according to the method of Jollow et al.Citation19 An aliquot of 2.0 ml of testis PMS (10% w/v) was precipitated with 2.0 ml of SSA (4% w/v). The samples were kept at 4°C for 1 hour and then subjected to centrifugation (Eppendorf Centrifuge 5810R, Germany) at 1157 × g for 30 minutes at 4°C. Then, the assay mixture consisted of 0.2 ml of clear aliquot, 2.6 ml of PBS, and 0.2 ml of DTNB (4 mg/ml of PBS) with a total volume of 3.0 ml. The yellow colour developed was read immediately at 412 nm on a spectrophotometer (Varian UV-visible Spectrophotometer, Germany). The result was expressed as μmol GSH/g tissue using molar extinction coefficient 13.6 × 103 M/cm.
Determination of catalase activity
The catalase (CAT) activity was measured according to the method of Claiborne.Citation20 One part of PMS was diluted in four parts of PBS. The reaction mixture contained 0.025 ml testis PMS (10% w/v) and 0.975 ml H2O2 (0.019 M) and was adjusted with 1.0 ml PBS to a total volume of 2.0 ml. The decrease in CAT absorbance was recorded at 240 nm every 30 seconds for 30 minutes using a spectrophotometer (Varian UV-visible Spectrophotometer, Germany). The result was expressed as μmol H2O2 consumed/min/mg/protein by using molar extinction coefficient of 6.4 × 103 M/cm.
Determination of serum lactate dehydrogenase
The serum lactate dehydrogenase (LDH) was determined using Biovision LDH Assay Kit (CA, USA). The detection range or sensitivity was 1–100 mIU/ml. Samples were read by using a microplate reader (SpectraMax M2 with SoftMax Pro 5, CA, USA).
Determination of serum testosterone
Serum testosterone was determined using T ELISA Assay Kit (USCN Life Science Incorporation, Wuhan, China). The detection range or sensitivity was less than 0.03 ng/ml. Samples were read by using a microplate reader (SpectraMax M2 with SoftMax Pro 5, CA, USA).
Sperm count and sperm morphology
The epididymis was cleaned and minced in 1 ml of pre-warmed (35°C) PBS and filtered through 80 µm pore nylon mesh. The filtrate was used for the evaluation of sperm parameters as follows. For sperm count, dilution of twenty times was made prior to counting. An aliquot of suspension was carefully discharged into the new improved Neubaeur's counting chamber. Total sperm was counted in eight squares (except the central erythrocyte area) of 1 mm2 under 20× to 40× objective lens. Calculation was expressed in millions/ml.Citation21 The sperm abnormality was assessed as percentage abnormality, counting the normal and all the different types of abnormal sperm based on the standard procedure described by Narayana et al.Citation22 The total percentage of abnormal sperm, which comprised head, neck, and tail defects, was expressed as the percentage of abnormality compared with the normal sperm. Two hundred spermatozoa were counted and assessed into normal and abnormal.Citation22 The total sperm abnormality was expressed as percentage incidence (%).
Histopathology
Testes were fixed in Bouin's fluid. Tissue fragments were dehydrated in graded series of ethanol, embedded in paraffin and sectioned using a microtome (Leica RM2255, Germany) at 5-μm thickness. Sectioned tissues were stained with haematoxylin-eosin for light microscopic examination. The sections were examined qualitatively (signs of necrosis) and quantitatively (seminiferous tubule diameters), and photographed on a light microscope (Olympus BX41 with Automatic Photomicrograph System, Tokyo, Japan). A total of 50 seminiferous tubules were randomly measured in each animal.Citation22
Statistical analysis
Statistical analysis was done using SPSS (version 17.0, 2008) software. Data obtained were analyzed first for Normality test, then, continued with one-way analysis of variance (ANOVA) and two-way ANOVA post hoc analyses for dose and time–response relationship, respectively. All the results were shown as mean ± standard error mean (SEM). Results were considered significant if P < 0.05 (a and b), in which (a) indicates significant increase or decrease of DZN dose-dependent toxicity effect when compared to the control, and (b) indicates significant increase or decrease of DZN time-dependent toxicity effect when compared to the 1 week exposure group.
Results
Our experiments showed that DZN had deleterious effects on the exposed animals even though the doses of exposure were very minimal based on LD50. We observed that there was no significant reduction of the animals' body weights. Instead, there was a significant increase (P < 0.05) of the body weight in the 8 weeks of exposure group when compared to 1 and 2 weeks of exposure groups. The accretion of the body weight was up to 30% and considered as the normal gradual growth of the experimental animals over time. However, the weight of the animals' testis – demonstrated as testis index (organ weight/final body weight in percentage, %) – is only shown to be significant in the 30 mg/kg b.w. DZN dose after 8 weeks of exposure ().
Table 1. Diazinon toxicity impacts on body and organ weight
Oxidative stress status subsequent to DZN exposure was assessed. The levels of antioxidants – CAT and GSH – and pro-oxidants – LPO – were measured in the 10% homogenate testis. In the testis, LPO activity was increased. Significant formation (P < 0.05) of MDA was evident in 30 mg/kg b.w. DZN dose after 1 week of exposure. After 2 and 8 weeks of exposure, a significant dose-dependent increase (P < 0.05) of MDA formation was obvious in all the DZN-treated animals (10, 15, and 30 mg/kg b.w. doses) when compared to the controls. Significant increase (P < 0.05) of LPO by 32 and 55% was observed in a longer duration of DZN exposure, 2 and 8 weeks, respectively, when compared to 1 week's exposure. The provocation of LPO in the testis caused the natural defences in the body – GSH and CAT – to gradually decline by scavenging the free radicals ().
Table 2. Effects of diazinon on testicle antioxidants and pro-oxidant (lipid peroxidation) level
The decline of GSH in the testis tissues was dose-dependent. Animals exposed to DZN for 1 week and 2 weeks (15 and 30 mg/kg b.w. doses) depleted GSH activity significantly (P < 0.05), which was about 15% lower than the controls. In 8 weeks of DZN exposure (10, 15, and 30 mg/kg b.w.), GSH was greatly reduced (P < 0.05) when compared to the control. In the time–response relationship, animals of 8 weeks' exposure showed significant decrease (P < 0.05) in GSH when compared to the 1 week of exposure group ().
Generally, CAT activities were halted dose-dependently after the exposure to DZN but only animals of 30 mg/kg b.w. dose showed an exceptional decrease (P < 0.05) of CAT after 8 weeks of DZN exposure when compared to the control. In comparison with animals treated with DZN for 1 week and 2 weeks, time-dependent analysis showed a significant decrease (P < 0.05) of CAT in DZN-treated animals after 8 weeks of exposure ().
shows a dose-dependent significant increase (P < 0.05) of serum LDH after 2 and 8 weeks of DZN exposure (10, 15, and 30 mg/kg b.w. doses). The level of LDH in DZN-treated animals was 55% higher than the controls. The duration of DZN exposure critically influenced the LDH of the animals. A significant increase (P < 0.05) of LDH in time-dependent exposure was observed to be 40% higher in 2 and 8 weeks of DZN exposure than the 1 week of exposure group.
Table 3. The influence of diazinon on serum testosterone synthesis and lactate dehydrogenase activity
The serum testosterone level was significantly increased (P < 0.05) in the animals exposed for 1 week to 15 and 30 mg/kg b.w. DZN doses when compared to the control. However, after 2 weeks of exposure, the testosterone level in all the treated groups declined. However, only the 30 mg/kg b.w. DZN dose, testosterone level declined significantly (P < 0.05) when compared to the control. The decline in testosterone level was amplified (P < 0.05) in animals after 8 weeks of DZN exposure (10, 15, and 30 mg/kg b.w. doses) when compared to the untreated animals ().
The significant increase of LDH with concomitant decrease of antioxidants may indicate cytotoxicity effects of DZN. Histopathological observation of DZN-treated animals showed necrosis in the testis. The manifestations include the shrinkage and sloughing of seminiferous tubules. Shrunk seminiferous tubules were smaller than the normal tubules. However, the number of shrunk tubules was insignificant in DZN-treated animals (). Sloughing, which appears vacuole-like (halo appearance) was observed in some tubules (). Disorganized and disintegrated cells were observed with higher dose and longer duration of DZN exposure. This was characterized by the unusual migration of the secondary spermatocytes (immature cells) into the lumen, where only mature cells, the spermatozoa, generally seen. In more severe cases of DZN exposure, abnormal sperm formation in the lumen or loss of cells was observed in some of the tubules ().
Figure 1. Photomicrographs of testis sections of DZN-treated animals (1 week), (A) normal seminiferous tubules (L indicates lumen) in untreated animals, (B) shrinkage (*) of seminiferous tubules in 10 mg/kg b.w. DZN-treated group, (C) halo appearance or vacuoles (arrows) in the seminiferous tubules of 15 mg/kg b.w. DZN-treated group, and (D) more vacuoles (arrows) in the seminiferous tubules of 30 mg/kg b.w. DZN-treated group. Vacuoles were also observed in 2 weeks of DZN exposure – 10, 15, and 30 mg/kg groups. Slides were stained with haematoxylin-eosin dye. Scale, 200 µm.
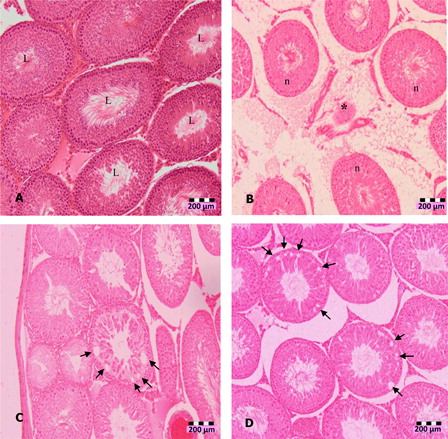
Figure 2. Photomicrographs of testis sections of DZN-treated animals, (A) in 2 weeks of DZN exposure (15 and 30 mg/kg b.w.), treated animals showed disorganization and disintegration of cell, which caused secondary spermatocyte (arrows) moved into lumen (100×), (B) shrinkage (*) of seminiferous tubules and loss of cells (lc), (C) more immature cells appeared in the lumen of 30 mg/kg b.w. DZN-treated animals (arrows), and (D) abnormal formation of spermatozoa in the lumen. Slides were stained with haematoxylin-eosin dye. Scale of (A) and (B) – 200 µm, (C) and (D) – 100 µm.
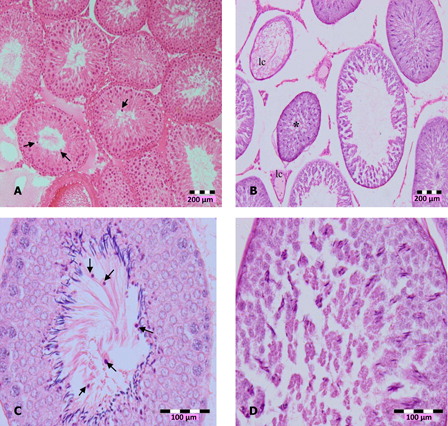
Table 4. Diazinon induced gonadotoxicity effects
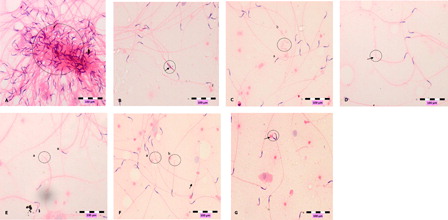
Animals with dose-dependent exposure to DZN did not have significant affects on their sperm concentration. Time–response analysis showed animals of 2 and 8 weeks of DZN exposure had a significant increase (P < 0.05) by 20% more sperm concentration than animals of 1 week of exposure (). DZN-treated animals had a significant increase (P < 0.05) in sperm abnormality after 8 weeks of exposure in a dose dependent-manner. Time-dependent analysis showed animals exposed for longer durations (2 and 8 weeks) of DZN exposure had a significant increase (P < 0.05) in sperm abnormality when compared to 1 week's exposure (). Photomicrograph of sperm abnormality is shown in (a)–3(d).
Figure 3. Photomicrographs show abnormal sperms, (A) clumping, (B) head deformity (arrow), (C) hookless (a) and coiled tail (b), (D) bent-tail (arrow, (E) broken-head (a) neck's defect (n), (F) broken head (a), broken tail (b) and bent tail (arrow), (G) double head (arrow). Slides were stained with haematoxylin-eosin dye. Scale, 100 µm.
The increase of sperm anomaly is parallel with the decrease of serum testosterone level in DZN-exposed animals (). Animals exposed to 15 and 30 mg/kg b.w. DZN doses had significant increased (P < 0.05) of testosterone when compared to the control after 1 week of exposure. However, DZN-treated animals showed a decline in testosterone after 2 weeks of exposure, with significant decrease of testosterone in 30 mg/kg b.w. DZN dose when compared to the control. A significant decrease (P < 0.05) of testosterone after 8 weeks of DZN exposure was observed in all the treated animals (10, 15, and 30 mg/kg b.w doses).
Discussion
DZN is a well-known contaminant, which is ubiquitously present in the environment. It is often found in human and animal foods and to a lesser extent in consumed water.Citation23 Its availability in the environment, especially in large scale rural farming areas, is always neglected. The severity of poisoning, however, depends on several essential factors such as dose, route of exposure, percent of absorption, physicochemical property and rate of detoxification. Due to the huge impact of DZN on human and animal health, investigation of the effects of DZN on various organs is worthwhile.Citation23 The purpose of this study was to uncover the oxidative stress status induced via LPO in DZN exposure and its influence on male fertility index.
During the experimental period, the animals did not experience any symptoms leading to anorexia. The growth of the animals was normal and increased gradually over time. This is parallel with the previous reports, where Wistar rats and mice treated with DZN based on selected LD50 doses did not produce body weight changes.Citation2,Citation24 This indicates that body weight has no ultimate relevance in the assessment of reproductive health.Citation25 Body mass index is highly correlated with fertility. During the experimental period, animals did not experience obesity and the body weight of DZN-treated groups (1, 2, and 8 weeks) was in the range of 174 g. The normal weight range of an adult male (5–6 months old) rat is 400–700 g.
Testicular mass is a valuable index in measuring male reproductive toxicity. The testis index of the rats after DZN exposure did not decrease in either dose or time-dependent treatments. The testicle index gradually increased following the normal growth of the animals. This is consistent with previous studies, which reported that DZN did not affect the absolute weight of testis.Citation26 The time-dependent increase of body and organ weights is common regardless of the dose adopted.Citation27
The number of sperm and the size of seminiferous tubules exhibited were not due to the toxicity effect of DZN but rather to normal growth (). Previous few reports indicated DZN causes a reduction in the quality of sperm motility, sperm concentration,Citation28 and seminiferous tubule diameter.Citation14,Citation15 However, these results were inconsistent with our studies possibly due to the adoption of lesser dose levels of DZN compared to the previous reports (LD50 > 5000 mg/kg b.w.).
Previous studies have indicated that the potential risk for each pesticide is based on the measure of toxicity such as LD50. The higher the LD50, the less fatal the chemical is, as the LD50 is the value of that chemical, which is able to kill 50% of the population within the specified time. Most of the previous researchers have adopted DZN with different purities,Citation10,Citation29,Citation30 in which the LD50 was lower than the DZN dosage used in this study.Citation6–Citation8,Citation11,Citation14,Citation26,Citation29,Citation31–Citation35 Therefore, the doses used in this study were less fatal. This could be the reason for the high morbidity rate observed in this study as no death was observed during the experimental period. Though some parameters of this study are consistent with previous studies.Citation6,Citation9,Citation10 It was affirmed that the number of deaths is relatively dependent on the dose and time of exposure.Citation3
The distribution, metabolism, and elimination of DZN biologically play vital roles in determining the degree of its toxicity. DZN is rapidly absorbed in the intestine with extensive help from microvili structure.Citation34,Citation36 DZN is transported to the liver, metabolized within a few hours,Citation37 and transformed into reactive intermediates and other metabolites. DZN is widely distributed in the body by circulationCitation38 but metabolites do not significantly accumulate in the tissues as they are primarily quickly eliminated via the urine with biological half-lives from less than 30 minutes to more than 24 hours depending on the OPs and the exposure pathways.Citation39 These are the possible reasons for the high morbidity rate and the insignificant change of the body weight, organ index, sperm concentration, and the size of seminiferous tubular diameter.
However, the metabolite of DZN, diazoxon is harmful to insects and animals as it is more toxic than the parent compound.Citation1,Citation15,Citation40 In a study involving farm workers, sprayers of diazoxon experienced higher toxicity effects than DZN sprayers.Citation28 Nevertheless, the toxic effects of the parent compound or its metabolite were believed to be associated with the ineffective regulation of free radicals in the body.
The proper regulation of ROS in the body is important for the antimicrobial defenceCitation41 and phagocytosis.Citation42 However, excessive free radicals lead to oxidative stress, which is deleterious to the cells.Citation43,Citation44 Many studies have demonstrated oxidative stress induced by organophosphorous DZN pesticide in rats through LPO.Citation24,Citation45–Citation48 This is determined by the balance between the production of oxidants and the elimination of oxidants by antioxidants. In our study, rats exposed to DZN had significant increase (P < 0.05) in LPO, which denotes the capability of DZN to induce oxidative stress, and hence, retard the activity of antioxidants, CAT, and GSH, to defend the body against the toxicity effects.
The depletion of GSH may be due to the increased utilization of the same by the cells to scavenge free radicals produced by DZN. This indicates the importance of GSH as a protector to cope with oxidative substance.Citation49 In this study, the reduction of CAT in testis was corresponding to the dose and duration of exposure. This may suggest that CAT was extensively utilized to overcome the toxic effects of DZN via the catalyzation of the H2O2 to water and oxygen.Citation50
LPO is an autocatalytic process, which aggressively halts the protective effects of antioxidants and increases damages to the cell membrane components. LPO, which is caused by free radicals, is responsible for the mechanism of physicochemical change, and bio-membrane disturbances.Citation8 DZN is a lipophilic pesticide.Citation13,Citation51,Citation52 This strengthens the interaction between DZN and the bio-membrane, thus, disturbing the physical nature of the membrane and finally leading to perturbation. Consequently, DZN penetrates deeper into the intracellular and affects the cell components. The effect on mitochondria arrests adenosine triphosphate generation, which eventually shuts off the main energy source of the cell.Citation43
The dysfunction of mitochondria inactivates cell metabolism, which causes histopathological alterations. Histopathological manifestations observed in this study were mild and characterized by halo appearance, epithelium sloughing, and cell loss. The unusual migration of secondary spermatocytes into the lumen was believed to be caused by the disorganization and disintegration of cells.Citation22,Citation53 We hypothesize the degenerative changes in the testis may reasonably be caused by the indirect cytotoxic effects of DZN or its metabolites.
The cytotoxicity effect of DZN on testis is further correlated with the significant dose- and time-dependent increase of serum LDH activity in this study. LDH serves as a good indicator of cytotoxicity of tissues and cells as it occurs naturally in cytosol of cells. In a study of DZN exposure, LDH activity was significantly raised in the DZN treated rats,Citation54 which this is consistent with our result. This implies that a low dose of DZN exposure produces cytotoxic effects, which are principally reflected in the histopathological changes.
Testosterone is a steroid hormone from the androgen group that is secreted by the testes and is responsible for male growth and development. It is also a key hormone in the regulation of spermatogenesis.Citation55 Rats treated with malathion have significantly lower levels of plasma hormones, including leutenizing hormone, follicle-stimulating hormone, and testosterone.Citation12 We believe that the decrease in testosterone level is closely related to the retardation of Leydig cell (interstitial cell) functionCitation15,Citation53 as it is a major source of androgen, testosterone, and other varieties of steroids.Citation55 It is postulated that the adverse effect of DZN resulting in the decline of testosterone production is due to the damage of the Leydig cells and pituitary gland. Our observation is in accordance with previous studies of other OP insecticides.Citation56–Citation59
Sperm is a progressive motile cell with a vast number of mitochondria. Lipophilic DZN crosses the bio-membrane and intracellular components of spermatozoa, and in addition to low antioxidant defence is deleterious to the fertility index. WHOCitation21 reported that a normal and healthy man contains minimal sperm abnormality. However, the significant high percentage of sperm abnormality in DZN-treated animals in our experiment denotes the detrimental effect of DZN exposure. There are two possibilities of DZN exposure's contribution to sperm abnormality: (1) direct effect of DZN on spermatozoa and (2) direct effect of DZN on testosterone synthesis, which may lead to disruption of spermatogenesis, which then influences the formation of normal sperm morphology.Citation12 Spermatogenesis and fertility are critically dependent upon the maintenance of adequate levels of testosterone. Thus, the effects of DZN on fertility in this study may be attributed to its ability to reduce serum testosterone synthesis and to increase abnormal sperm formation.
The mechanism of action of DZN shows the severity of its impact on exposed organisms and we have correlated that DZN causes LPO, which may be the alternative key to biochemical, antioxidant, hormonal, and histopathological changes in our study. In conclusion, the insecticide DZN is detrimental to health even at low levels of exposure. Prolonged exposure to DZN without proper safety measures can lead to a decline in fertility index. The changes in LPO and sperm might be causally linked, which could be further clarified by antioxidants study. A low dose of DZN up to 8 weeks duration has few sperm-toxic effects from oxidative stress, which adversely affects the target organs directly. More studies considering longer durations of exposure to low doses of DZN are required to conclusively confirm its deleterious effects on the reproductive system.
Acknowledgement
The authors gratefully acknowledge the assistance of the Ministry of Higher Education, Malaysia for supporting this study under the Fundamental Research Grant Scheme (Grant number FRG-165-SP-2008). The authors also acknowledge the support of the authorities of the School of Medicine and Biotechnology Research Institute, UMS.
References
- Agency for Toxic Substances and Disease Registry (ATSDR). Draft toxicological profile for diazinon. Public Health Service, United State Department of Health and Human Services; 2006.
- Johari H, Shariati M, Abbasi S, Sharifi E, Askari HR. The effects of diazinon on pituitary-gonad axis and ovarian histological changes in rats. Iranian J Reprod Med 2010;8(3):125–30.
- Aydin R, Koprucu K. Acute toxicity of diazinon on the common carp (Cyprinus carpio L.) embryos and larvae. Pestic Biochem Physiol 2005;82:220–5.
- Eisler R. Diazinon hazards to fish, wildlife and inverterbrates: a synoptic review. Contaminant hazard reviews. Patuxent Wildlife Research Center 1986;9:1–35.
- Zeits P, Kakolewski K, Imtiaz R, Kaye W. Methods of assessing neurobehavioral development in children exposed to methyl parathion in Mississippi and Ohio. Environ Health Perspect 2002;110(6):1079–83.
- Kalender Y, Uzunhisarcikli M, Ogutcu A, Acikgoz F, Kalender S. Effects of diazinon on pseudocholinesterase activity and haematological indices in rats: The protective role of vitamin E. Environ Toxicol Pharmacol 2006;22:46–51.
- Ogutcu A, Uzunhisaraikli M, Kalender S, Durak D, Bayrakdar F, Kalender Y. The effects of organophosphate insecticide diazinon on malondialdehyde levels and myocardial cells in rat heart tissue and protective role of vitamin E. Pestic Biochem Physiol 2006;86:93–8.
- Akturk O, Demirin H, Sutcu R, Yilmaz N, Koylu H, Altuntas I. The effects of diazinon on lipid peroxidation and antioxidant enzymes in rat heart and ameliorating rate of vitamin E and vitamin C. Cell Biol Toxicol 2006;22:455–61.
- El-Shenawy NS, Al-Eisa RA, El-Salmy F, Salah O. Prophylactic effect of vitamin E against hepatotoxicity, nephrotoxicity, haematological indices and histopathology induced by diazinon insecticide in mice. Curr Zooly 2009;55(3):219–26.
- Gocikmen A, Gulle K, Demirin H, Bayram D, Kocak A, Altuntas I. Effects of diazinon at different doses on rat liver and pancreas tissues. Pestic Biochem Physiol 2007;87:103–8.
- Kalender S, Ogutchu A, Uzunhisarcikli M, Acikgoz F, Durak D, Ulusoy Y, et al. Diazinon-induced hepatoxicity and protective effect of vitamin E on some biochemical indices and ultrastructural changes. Toxicology 2005;211:197–206.
- Uzun FC, Kalender S, Durak D, Demir F, Kalender Y. Malathion induced toxicity in male rats and the protective effect of vitamins C and E. Food Chem Toxicol 2009;47:1903–8.
- Videira RA, Antunes-Madeira MC, Lopes VI, Madeira VM. Changes induced by malathion, methylparathion and parathion on membrane lipid physiochemical properties correlate with their toxicity. Biochimica et Biophysica Acta 2001;1511:360–8.
- Toman R, Hluchy S, Siska B, Lukac N, Slivkova J, Golian J, et al. Computer-assisted semen analysis of rat spermatozoa after an intraperitoneal administration of insecticide diazinon. Zootehnie si Biotehnologii 2008;41(1):802–5.
- Jorsaraei SGA, Firoozjaee A, Pasha YY, Marzony ET, Sarabi E. Histopathological effects of single dose treatment of diazinon on testes structure in rat. Yakhteh Med J 2010;12(1):39–42.
- Maneesh M, Jayalekshmi H, Dutta S, Chakrabarti A, Vasudevan DM. Role of oxidative stress in ethanol induced germ cell apoptosis-An experimental study in rats. Indian J Clin Biochem 2005;20(2):62–7.
- Dutta HM, Maxwell LB. Histological examination of sublethal effects of diazinon on ovary of bluegill, Lepomis macrochirus. Environ Pollut 2003;121:95–102.
- Buege JA, Aust SD. Microsomal lipid peroxidation. In , Fleischer S, Packer L, (eds.) Methods in enzymology. New Jersey: Press; 1978. vol. 52, p. 302–10.
- Jollow DJ, Mitchell JR, Zampagilone N, Gillete JR. Bromobenzene induced liver necrosis: Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as a hepatotoxic intermediate. Pharmacol 1974;11:151–69.
- Claiborne A. Catalase activity. In: , Greenwald RA, (ed.) Handbook of methods for oxygen radical research. Boca Raton: CRC Press; 1985. p. 283–4.
- The Nordic Association for Andrology (NAFA)-European Society of Human Reproduction and Embryology (ESHRE). Manual on basic semen analysis. Final version. 2002. p. 1–34.
- Narayana K. A purine analogue-acyclovir (9-2-hydroxyethoxymethyl-9h guanine) reversibly impairs testicular functions in mouse. J Toxicol Sci 2008;33(1):61–70.
- Ali K, Reza NG, Syamak S, Aref H, Ehsan H, Leila R, et al. Protective effect of selenium on diazinon induced determination impact on the testes in mature male rats. Global Veterinaria 2011;7(4):370–80.
- El-Shenawy SN, El-Samy F, Al-Eisa R, El-Ahmary B. Ameliratory effect of vitamin E on organophosphorous insecticide diazinon-induced oxidative stress in mice liver. Pestic Biochem and Physiol 2010;96:101–7.
- Jayachandran S. Evaluation of the reproductive toxicity in male and female rat offspring exposed to their mothers throughout pregnancy and lactation. Doctor of Philosophy. Universiti Malaysia Sabah, Malaysia; 2007.
- Toman R, Hluchy S, Siska B, Golian J, Massanyi P, Lukac N. Structural changes in the rat testes caused by diazinon administration. Zootehnie si Biotehnologii 2009;42(1):295–9.
- Zain MD. The evaluation of the toxic effect of paraquat and its mechanism of action on reproductive system of male rats. Master of Science. Universiti Sains Malaysia, Malaysia; 2006.
- El-Kannishy SMH, El-Baz RM, El-Gawad SSA, Marzook HF, Hassan SA, Metwali AA. Sperm nuclear deoxyribonucleic acid denaturation in diazinon/diazoxon sprayer men. J American Sci 2011;7(6):470–5.
- Ibrahim NA, El-Gamal B. Effects of diazinon an organophosphate insecticide on plasma lipid constituents in experimental animals. J Biochem Mol Biol 2003;36(5):499–504.
- Skoulika SG, Georgiou CA, Polissiou MG. FT-Raman spectroscopy analytical tool for routine analysis of diazinon pesticide formulations. Talanta 2000;51:599–604.
- Adamkovicova M, Toman R, Cabaj M. Diazinon and cadmium acute testicular toxicity in rats examined by histopathological and morphometrical methods. Slovak J Anim Sci 2010;43(3):134–40.
- Yehia MAH, El-Banna SG, Okab AB. Diazinon toxicity affects histophysiological and biochemical parameters in rabbits. Exp Toxicol Pathol 2007;59:215–25.
- Neishabouri EZ, Hassan ZM, Azizi E, Ostad SN. Evaluation of immunotoxicity induced by diazinon in C57b1/6 mice. Toxicol 2004;196:173–9.
- Poet TS, Wu H, Kousba AA, Timchalk C. In vitro rat hepatic and intestinal metabolism of the organophosphate pesticides chlorpyrifos and diazinon. Toxicol Sci 2003;72:193–200.
- Hamm JT, Wilson BW, Hinton DE. Increasing uptake and bioactivation with development positively modulate toxicity in early stage Medaka (Oryzias latipes). Toxicol Sci 2001;61:304–13.
- Kalipci E, Ozdemir C, Oztaz F, Sahinkaya S. Ecotoxicological effects of methyl parathion on living things and environment. Afr J Agric Res 2010;5(8):712–8.
- Busby A, Kousba A, Timchalk C. The in vivo quantitation of diazinon, chlorpyrifos, and their major metabolites in rat blood for the refinement of a physiologically-based pharmacokinetic/pharmacodynamic models. J Undergraduate Res 2004;36–40.
- Bosshard E. Diazinon (pesticides residues in food: 1993 evaluations part II toxicology): 1st draft. International Programme on Chemical Safety-INCHEM, 1993. http://www.inchem.org/documents/jmpr/jmpmono/v93pr04.htm.
- Bouvier G, Blanchard O, Momas I, Seta N. Environmental and biological monitoring of exposure to organophosphorous pesticides: Application to occupational and non-occupational exposed adult populations. J Expo Sci Environ Epidemiol 2006;16:417–26.
- Agency for Toxic Substances and Disease Registry (ATSDR). Draft toxicological profile for diazinon. Public Health Service, United State Department of Health and Human Services; 2008.
- Sanocka D, Kurpisz M. Reactive oxygen species and sperm cells (review). Reprod Biol Endocrinol 2004;2:12.
- Ford WC. Regulation of sperm function by reactive oxygen species. Hum Reprod Update 2004;10(5):387–99.
- Orrenius S, Nicotera P, Zhivotovsky B. Cell death mechanisms and their implications in toxicology. Toxicol Sci 2011;119(1):3–19.
- de Lamirande E, Jiang H, Zini A, Kodama H, Gagnon C. Reactive oxygen species and sperm physiology. Rev Reprod 1997;2:48–54.
- Fulia A, Chauhan PK, Sharma RK. Ameliorating effect of vitamin E on testicular toxicity induced by endosulphan in Capra hircus in vitro. J Pharmacol Toxicol 2011;6(2):133–40.
- Sharbidre AA, Metkari V, Patode P. Effect of diazinon on acetylcholine esterase activity and lipid peroxidation Poecilia reticulate. Res J Environ Toxicol 2011;5(2):152–61.
- Ambali SF, Abubakar AT, Shittu M, Yaqub LS, Anafi SB, Abdullahi A. Chlorpyrifos induced alteration of haematological parameters in wistar rats: Ameliorating effect of zinc. Res J Environ Toxicol 2010;4(2):55–66.
- Keramati V, Jamili S, Ramin M. Effect of diazinon on catalase antioxidant enzyme activity in liver tissue of Rutilus rutilus. J Fish Aquat Sci 2010;5(5):368–76.
- Kumar T, Muralidhara. Induction of oxidative stress by organic hydroperoxides in testis and epididymal sperm of rats in vivo. J Androl 2007;28(1):77–85.
- Agarwal A. Role of antioxidant stress in male infertility and antioxidant supplementation. Business Briefing: U.S. Kidney and Urological Disease; 2005. p. 1–8.
- Yassa VF, Girgis SM, Abumourad IMK. 2011. Potential protective effect of vitamin E on diazinon-induced DNA damage and some haematological and biochemical alterations in rats. J Mediterr Ecol 2011;11:31–9.
- Blasiak J, Walter Z, Gawronska M. The changes of osmotic fragility of pig erythrocytes induced by organophosphosphorous insecticides. Acta Biochimica Polonica 1991;38(1):75–8.
- Narayana K. An aminoglycoside antibiotic gentamycin induces oxidative stress, reduces antioxidant reserve and impairs spermatogenesis in rats. J Toxicol Sci 2008b;33(1):85–96.
- Ogutcu A, Suludere Z, Kalender Y. Dichlorvous-induced hepatoxicity in rats and the protective effects of vitamin C and E. Environ Toxicol Pharmacol 2008;26:355–61.
- Russell L, Ettlin R, SinhaHikim A, Clegg E. Histological and histopathological evaluation of the testis. Florida: Cache River Press; 1990; p. 1–5.
- Farag AT, Radwan AH, Sorour F, El-Okazy A, El-Agamy E, El-Sebae AE. Chlorpyrifos induced reproductive toxicity in male mice. Reprod Toxicol 2010;29:80–5.
- Gawish AM. The protective role of alpha-lipoic acid against pesticides induced testicular toxicity-histopathological and histochemical studies. J Aquac Res Development 2010;1(1):101.
- Choudhary N, Goyal R, Joshi SC. Effect of malathion on reproductive system of male rats. J Environ Biol 2008;29(2):259–62.
- Narayana K, Prashanti N, Nayanatara A, Bairy LK, D'Souza UJA. An organophosphate insecticide methyl parathion (o-o-dimethyl O-4 nitrophenyl phosphorothioate) induces cytotoxic damage and tubular atrophy in the testis despite elevated testosterone level in the rat. J Toxicol Sci 2006;31(3):177–89.