Abstract
Objectives
To evaluate the correlation between reactive oxygen species (ROS) production and micronucleus formation induced by a vitamin complex in peripheral blood mononuclear cells from healthy people aged between 40 and 85 years old.
Methods
Peripheral blood mononuclear cells (PBMNCs) were purified utilizing ficoll-hypaque gradient. ROS production by PBMNCs was quantified by luminol-dependent chemiluminescence in the presence or in the absence of the vitamin complex. DNA damage in PBMNC by the vitamin complex was detected by the micronucleus technique. Statistical analyses were made with the Student's ‘t’ test and the Pearson correlation. P < 0.05 was considered significant.
Results
The vitamin complex induced MN formation in PBMNC but did not augment ROS production. There was no correlation between ROS production and MN formation either in the presence or in the absence of the vitamin complex.
Discussion
There was no increase in the ROS production in the presence of the vitamin complex. The vitamin complex induced an augmentation in the MN formation. There was no correlation between ROS production and the induction of MN formation. Since no association could be detected between ROS production and MN formation, additional studies are required in order to investigate the possible mechanism of vitamin-induced MN formation.
Introduction
Aging is associated with a progressive impairment of mitochondrial function, increased oxidative stress, reduced antioxidant defenses, immune activation, and an increase in DNA damage and DNA mutation.Citation1 Experimental evidence has demonstrated that a low dose supplementation with vitamins and minerals normalizes the biological nutrient status as early as 6 months of treatment. In addition, the antioxidant defense in elderly subjects was improved with low doses of vitamin C, vitamin E, and beta carotene.Citation2 However, a pro-oxidant effect, characterized by the increased incidence of lung cancer, was described in smokers taking high (pharmacological) daily doses of beta-carotene for 6 months.Citation3 Many authors have shown that these vitamins might act as oxidants or pro-oxidants under certain conditions.Citation4–Citation6 It has also been suggested that pro-oxidant compounds increase the production of free radical species, neutralizing antioxidant defenses and causing cell membrane, protein, and DNA damage.Citation7 Antioxidants are reactive oxygen species (ROS) scavengers, but the exact role for antioxidants in aging is controversial. Several authors have reported that individual antioxidants act as pro-oxidants.Citation8–Citation10 In contrast, Niki et al.Citation11 have demonstrated that the antioxidants vitamin C, vitamin E, and beta-carotene act not only individually but also cooperatively and in some cases synergistically. Despite the vast literature concerning vitamins, the mechanism in through which these compounds might act as pro-oxidants, inducing DNA damage, remains unknown. ROS and micronuclei are biomarkers for oxidative stress and DNA damage, respectively.Citation12,Citation13 The micronucleus (MN) index is a reliable indicator and a well-accepted biomarker for oxidative stress-induced DNA damage, as this marker reflects whole chromosome loss or chromosomal breakage.Citation13 Different cytogenetic methods exist to analyze DNA damage, but the MN assay is a simple, cheap, and informative cytogenetic method used to detect genotoxic-related markers.Citation14 In addition to oxidative stress, MN might also be induced through exposure to toxic agents and genetic defects in the cell cycle.Citation15 Thus, it is possible to envisage an association between DNA damage and increased ROS generation. The aim of the present study was to evaluate the potential association between the ROS generation from the peripheral blood mononuclear cells (PBMNCs) of aged healthy people and MN formation during ‘in vitro’ exposure to an antioxidant vitamin complex comprising beta-carotene, alpha-tocopherol, and ascorbic acid.
Methods and materials
Subjects
The Ethical Committee of the Federal University of Minas Gerais approved this study. A detailed medical history, complete physical examination, and laboratory data for each subject were recorded before entering the study. Appropriate informed consent was obtained from each participant. The individuals excluded from this study had one or more of the following features: infections, inflammation, malignancy, lymphoproliferative disorders, arteriosclerosis, cardiac insufficiency, hypertension, and/or dementia. In addition, individuals who were smokers, pregnant, had a history of alcoholism and drug abuse, and/or were taking drugs that directly influence the immune function were excluded. Dr Edgar Nunes de Moraes, Dr Rodrigo Santos, and Dr Marco Túlio Gualberto Cintra selected the subjects (Reference Center of Age – Professor Caio Benjamin Dias from HC-UFMG). The subjects live in Belo Horizonte. The characteristics of the population are shown in (n = 40).
Table 1. Characteristics of the studied group
Separation of PBMNCs
The PBMNCs were purified from 10 ml of heparinized venous blood samples obtained from the participants using a Ficoll-Hypaque gradient according to the method of Bicalho et al.,Citation16 with slight modifications. The cellular viability of each sample was consistently greater than 95%, as determined using the trypan blue exclusion test.
Preparation of the vitamin complex
The vitamin complex was obtained in capsules from a compounding pharmacy and dissolved in 10 ml of dimethyl sulfoxide (DMSO – Vetec) and subsequently diluted 10 times in phosphate-buffered saline (PBS). The DMSO concentration was limited to 0.5% in accordance with the stipulations of the International Organization for Standardization.Citation17 In all experiments, the final concentration of DMSO was never above 0.001%, and no cell toxicity or cell death was observed; thus, the addition of DMSO at this concentration did not interfere with the results. All reagents were purchased from Sigma (Labscience Instrumentos Científicos Ltda, São Paulo, SP, Brazil, USA).
Determination of ROS generation
ROS generation was determined through a quantitative chemiluminescence assay using a luminometer 1250-101 (Lumat, LB9501; EG&G Berthold, Germany). The PBMNCs (1 × 106 cells/100 µl) were transferred to an unsealed luminescence tube together with 500 µl of luminol (10−4 M) and 100 µl of the vitamin complex. In the basal production, 100 µl of PBS (pH = 7.3) was used instead of the vitamin complex. The chemiluminescence of the mixture was measured over a 30-minute reaction time. The results were expressed in RLU/minute (relative light units/minute).
MN technique
DNA damage was detected using the MN technique according to Fenech,Citation18 with slight modifications. Briefly, the PBMNCs (1 × 106 cells/100 µl) were incubated in a 24-well plate in the presence or absence of the vitamin complex, and the total volume was adjusted to 3 ml with RPMI 1640 (Sigma-Aldrich Co., Labscience Instrumentos Científicos Ltda, São Paulo, SP, Brazil) and supplemented with 15% inactivated fetal bovine serum (Gibco), antibiotic–antimycotic solution (penicillin, streptomycin, gentamicin, and amphotericin (Gibco)), and phytohemagglutinin (PHA) (20 µg/ml) (Sigma Aldrich Co.) to stimulate cell proliferation. Treatment with the test compound (vitamin complex) was initiated at 48 hours after PHA stimulation, lasting 20 hours, followed by the addition of cytochalasin B (6 µg/ml) (Sigma-Aldrich Co.). Subsequently, the cells were harvested at 96 hours after the initiation of the culture. The cells were subjected to a mild hypotonic treatment (KCl 0.075 M), fixed once with methanol:acetic acid (5:1), and twice with the same solution at 3:1. The cells were subsequently smeared on precleaned microscope slides and air-dried. The cells were stained with Giemsa (Dinâmica) to determine the micronuclei frequency. Negative controls were performed using DMSO (Vetec, Biosan, Belo Horizonte, MG, Brazil) (15 µl). Positive controls were performed using colchicine (12 µl–0.025 µg). The results are expressed as the number of micronuclei in 1000 binucleated cells.
Statistical analyses
The ROS data are expressed in terms of the mean values of RLU/minute ± standard error (SE). The micronuclei results are expressed as the number of micronuclei in 1000 binucleated cells. The comparison of the data between the groups was performed with Origin 6.0 software (Microcal Software Inc., Northampton, MA, USA), using the unpaired Student's ‘t’-test and Pearson's correlation in the analyses. In each case, P < 0.05 was considered significant.
Results
Determination of ROS production
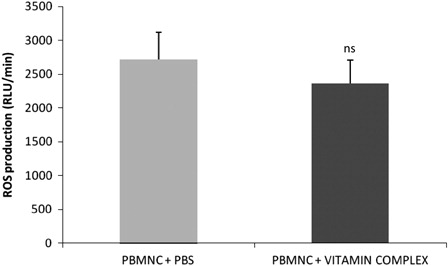
The ROS production is shown in . The vitamin complex did not alter the basal ROS production (P > 0.05). The results showed that the basal (PBMNC + PBS) ROS production was 2712 ± 401 RLU/minute, and in the presence of the vitamin complex, the ROS production was 2358 ± 359 RLU/minute.
Figure 1. Reactive oxygen species (ROS) production by PBMNC stimulated by the vitamin complex. PBMNC = peripheral blood mononuclear cells; Vitamin complex = beta carotene, ascorbic acid, and alpha tocopherol; PBS = phosphate buffer saline; ns = not significantly different compared to the basal (PBMNC + PBS).
Micronucleus formation
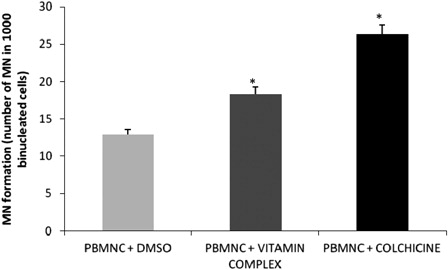
shows micronuclei formation in the presence and absence of the vitamin complex. The number of MN was significantly higher (P < 0.05) in the presence of the vitamin complex compared with the negative control. The following results, expressed as the number of MN in 1000 binucleated cells ± SE, were obtained: negative control (PBMNC + DMSO) = 12.95 ± 0.76; PBMNC + vitamin complex = 18.32 ± 0.96; positive control (PBMNC + colchicine) = 26.2 ± 1.06.
Figure 2. Micronuclei (MN) formation by PBMNC stimulated by the vitamin complex. PBMNC = peripheral blood mononuclear cells; Vitamin complex = beta carotene, ascorbic acid, and alpha tocopherol; PBS = phosphate buffer saline; DMSO = dimethyl sulfoxide; colchicine = used as positive control; * = significantly different compared to PBMNC + DMSO (negative control).
Correlation between ROS production and MN formation
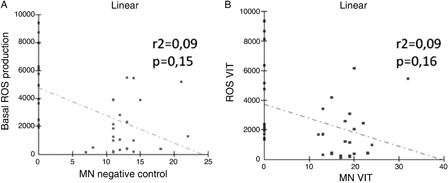
A and B shows that there was no correlation between ROS production and MN formation either in the presence or in the absence of the vitamin complex.
Figure 3. Correlation between ROS production and MN formation. (A) Correlation between nasal ROS production and MN negative control. (B) Correlation between ROS production and MN formation, both stimulated by the vitamin complex. ROS = reactive oxygen species (in relative light units/min), MN = micronucleus formation (in 1000 binucleated cells), VIT = presence of the vitamin complex.
Discussion
Vitamin complex-induced DNA damage was evaluated through MN formation in the PBMNCs from healthy people between 40 and 85 years old (), and no correlation between ROS production and MN formation was observed (). Previous studiesCitation19 have demonstrated that people aged 40 years old or older exhibit an activated oxidizing profile without a concomitant and correspondent increase in the reducing metabolic response, characterizing oxidative stress.
The vitamin complex used in the present study comprised ascorbic acid, alpha-tocopherol, and beta-carotene in previously suggested concentrations and proportions (ascorbic acid = x, alpha-tocopherol = x/2, beta-carotene = x/100).Citation20 The vitamin complex concentration used in the present study is comparable to that of Bergman et al.Citation21 and Prabhala et al.Citation22 Bergman et al.Citation21 used 0.011–1.1 µM of ascorbic acid and 0.002–0.2 µM of alpha-tocopherol in 2 × 106 PBMNCs, while Prabhala et al.Citation22 used 0.001–10 µM of beta-carotene in 1.6 × 106 PBMNCs. In this study, we used a similar vitamin complex concentration comprising 0.08 µM ascorbic acid, 0.04 µM alpha-tocopherol, and 0.0008 µM beta-carotene. Niki et al.Citation11 demonstrated that antioxidants vitamin C, vitamin E, and beta-carotene act not only individually but also cooperatively and in some cases synergistically. The advantage of using a mixture of several dietary antioxidants is reflected by the fact that some of these compounds utilize synergism and partnership to achieve protection from physical and chemical genotoxins.Citation23,Citation24 Based on these assumptions, we have examined the role of a vitamin complex.
Antioxidant vitamins A, C, and E protect against many diseases and conditions associated with aging.Citation25 Thus, we examined the association among ascorbic acid, alpha-tocopherol, and beta-carotene as potential oxidants or pro-oxidants on PBMNCs obtained from an aged population.
The results demonstrated that this vitamin complex did not significantly alter ROS production in PBMNCs (P > 0.05) (). These results are consistent with those of Fusco et al.,Citation26 who demonstrated that only specific populations with low antioxidant profiles and/or high oxidative stress levels might benefit from antioxidant supplementation. Notably, under conditions in which high levels of ROS (oxidative stress) are detected, cellular and genome damage have been reported.Citation27 Thus, it is reasonable to suggest that an antioxidant vitamin complex would prevent MN formation in PBMNCs.
MN frequency was evaluated in the presence of the vitamin complex, and the results in show that the vitamin complex activated MN formation (P < 0.05), suggesting a pro-oxidant effect. The literature concerning this subject is controversial. Previous studies using single antioxidants (vitamin C aloneCitation28 or vitamin E aloneCitation29) showed no significant increase in the micronuclei frequency. In contrast, Anderson et al. (1994)Citation30 reported that vitamin C causes a dose-dependent increase in DNA damage. A variety of ‘in vivo’ studies have shown that multiple antioxidants might act together to reduce micronuclei frequency.Citation31–Citation33 However, these studies are not directly comparable to the present study because we assayed an antioxidant mixture ‘in vitro’. The ‘in vivo’ assays used a different antioxidant mixture comprising succinate and zinc,Citation31 folic acid and rutin,Citation34 and selenium,Citation33 ascorbic acid, beta-carotene, and alpha-tocopherol. It has been suggested that under certain conditions, vitamins A, C, and E might have pro-oxidant effects.Citation20,Citation35,Citation36 Vitamin C induces an increase in the formation of oxidative DNA damage in cultured lymphocytes.Citation8,Citation10,Citation30 This ‘in vitro’ effect, mediated through vitamin C, corresponds to the same effect as observed with the concentrations used ‘in vivo’ via supplementation.Citation30,Citation37–Citation39 A similar effect was observed with high doses of E vitamin ‘in vitro’.Citation40 This effect reflects vitamin-induced oxidative damage and many authors have shown that ROS induces DNA damage.Citation41,Citation42 Weitberg and Weitzman (1985)Citation39 observed variable effects in the induction of sister chromatid exchanges in Chinese hamster ovary cells incubated with an enzymatic oxygen radical generating system (xanthine oxidase plus hypoxanthine) and ascorbate, as a protective effect, is observed in some cases, whereas in other cases, the induction of sister chromatid exchanges was observed.
The results () obtained in the present study showed no significant correlation between ROS production and the induction of MN formation. It is reasonable to suggest that MN formation is a complex phenomenon with intricate mechanisms. In addition to increased ROS production, there are other potential agents and mechanisms involved in MN formation. The results obtained in the present study clearly demonstrated the absence of a direct or indirect association between ROS production and MN formation. Thus, the exact role of vitamins on MN formation needs further study to elucidate the mechanisms underlying this function.
Conclusion
Vitamin complexes induce micronuclei formation in healthy people between the ages of 40 and 85 years old in a ROS-independent manner.
Acknowledgements
The authors thank Gláucia A A Carvalho for her excellent technical assistance. This work was supported by grants from FAPEMIG, CNPq, CAPES, and UFMG.
References
- Tosato M, Zamboni V, Ferrini A, Cesari M. The aging process and potential interventions to extend life expectancy. Clin Interv Aging 2007;2:401–12.
- Girodon F, Blache D, Monget AL, Brunet-Lecompte P, Arnaud J, Richard MJ, et al. Effect of a two-year supplementation with low doses of antioxidant vitamins and/or minerals in elderly subjects on levels of nutrients and antioxidant defense parameters. J Am Coll Nutr 1997;16:357–65.
- Wolf G. The effect of low and high doses of beta-carotene and exposure to cigarette smoke on the lungs of ferrets. Nutr Rev 2002;60:88–90.
- Landmesser U, Harrison DG. Oxidant stress as a marker for cardiovascular events: Ox marks the spot. Circulation 2001;104:2638–40.
- Meagher EA, Barry OP, Lawson JA, Rokach J, FitzGerald GA. Effects of vitamin E on lipid peroxidation in healthy persons. JAMA 2001;285:1178–82.
- Neuzil J, Weber C, Kontush A. The role of vitamin E in atherogenesis: linking the chemical, biological and clinical aspects of the disease. Atherosclerosis 2001;157:257–83.
- Halliwell B. Free radicals and antioxidants: a personal view. Nutr Rev 1994;52:253–65.
- Green MH, Lowe JE, Waugh AP, Aldridge KE, Cole J, Arlett CF. Effect of diet and vitamin C on DNA strand breakage in freshly-isolated human white blood cells. Mutat Res 1994;316:91–102.
- Palozza P. Prooxidant actions of carotenoids in biologic systems. Nutr Rev 1998;56:257–65.
- Podmore ID, Griffiths HR, Herbert KE, Mistry N, Mistry P, Lunec J. Vitamin C exhibits pro-oxidant properties. Nature 1998;392:559.
- Niki E, Noguchi N, Tsuchihashi H, Gotoh N. Interaction among vitamin C, vitamin E, and beta-carotene. Am J Clin Nutr 1995;62:1322S–6S.
- Anreddy RN, Yellu NR, Devarakonda KR. Oxidative biomarkers to assess the nanoparticle-induced oxidative stress. Methods Mol Biol 2013;1028:205–19.
- Fenech M. Cytokinesis-block micronucleus cytome assay. Nat Protoc 2007;2:1084–104.
- Sinitsky MY, Druzhinin VG. The application of the cytokinesis-block micronucleus assay on peripheral blood lymphocytes for the assessment of genome damage in long-term residents of areas with high radon concentration. J Radiat Res 2013.
- Bonassi S, Znaor A, Ceppi M, Lando C, Chang WP, Holland N, et al. An increased micronucleus frequency in peripheral blood lymphocytes predicts the risk of cancer in humans. Carcinogenesis 2007;28:625–31.
- Bicalho HM, Gontijo CM, Nogueira-Machado JA. A simple technique for simultaneous human leukocytes separation. J Immunol Methods 1981;40:115–6.
- ISO ISO-. Biological evaluation of medical devices. Part 5 Test for cytotoxicity: in vitro methods 10993-5 2009.
- Fenech M. The in vitro micronucleus technique. Mutat Res 2000;455:81–95.
- Chaves MM, Rocha-Vieira E, de Lima e Silva R, Pereira dos Reis A, Nogueira-Machado JA. Host defenses in the aged: evaluation of the balance between oxidizing species generation and reducing power in phagocyting human granulocytes. Mech Ageing Dev 1998;104:103–9.
- Zhang P, Omaye ST. Beta-carotene and protein oxidation: effects of ascorbic acid and alpha-tocopherol. Toxicology 2000;146:37–47.
- Bergman M, Salman H, Djaldetti M, Fish L, Punsky I, Bessler H. In vitro immune response of human peripheral blood cells to vitamins C and E. J Nutr Biochem 2004;15:45–50.
- Prabhala RH, Maxey V, Hicks MJ, Watson RR. Enhancement of the expression of activation markers on human peripheral blood mononuclear cells by in vitro culture with retinoids and carotenoids. J Leukoc Biol 1989;45:249–54.
- Chan AC. Partners in defense, vitamin E and vitamin C. Can J Physiol Pharmacol 1993;71:725–31.
- Xu MJ, Plezia PM, Alberts DS, Emerson SS, Peng YM, Sayers SM, et al. Reduction in plasma or skin alpha-tocopherol concentration with long-term oral administration of beta-carotene in humans and mice. J Natl Cancer Inst 1992;84:1559–65.
- Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci 2008;4:89–96.
- Fusco D, Colloca G, Lo Monaco MR, Cesari M. Effects of antioxidant supplementation on the aging process. Clin Interv Aging 2007;2:377–87.
- Thomas P, Wu J, Dhillon V, Fenech M. Effect of dietary intervention on human micronucleus frequency in lymphocytes and buccal cells. Mutagenesis 2011;26:69–76.
- Crott JW, Fenech M. Effect of vitamin C supplementation on chromosome damage, apoptosis and necrosis ex vivo. Carcinogenesis 1999;20:1035–41.
- Fenech M, Dreosti I, Aitken C. Vitamin-E supplements and their effect on vitamin-E status in blood and genetic damage rate in peripheral blood lymphocytes. Carcinogenesis 1997;18:359–64.
- Anderson D, Yu TW, Phillips BJ, Schmezer P. The effect of various antioxidants and other modifying agents on oxygen-radical-generated DNA damage in human lymphocytes in the COMET assay. Mutat Res 1994;307:261–71.
- Fenech M, Baghurst P, Luderer W, Turner J, Record S, Ceppi M, et al. Low intake of calcium, folate, nicotinic acid, vitamin E, retinol, beta-carotene and high intake of pantothenic acid, biotin and riboflavin are significantly associated with increased genome instability – results from a dietary intake and micronucleus index survey in South Australia. Carcinogenesis 2005;26:991–9.
- Schneider M, Diemer K, Engelhart K, Zankl H, Trommer WE, Biesalski HK. Protective effects of vitamins C and E on the number of micronuclei in lymphocytes in smokers and their role in ascorbate free radical formation in plasma. Free Radic Res 2001;34:209–19.
- Smolkova B, Dusinska M, Raslova K, Barancoková M, Kazimírová A, Horská A, et al. Folate levels determine effect of antioxidant supplementation on micronuclei in subjects with cardiovascular risk. Mutagenesis 2004;19:469–76.
- Gaziev AI, Sologub GR, Fomenko LA, Zaichkina SI, Kosyakova NI, Bradbury RJ. Effect of vitamin-antioxidant micronutrients on the frequency of spontaneous and in vitro gamma-ray-induced micronuclei in lymphocytes of donors: the age factor. Carcinogenesis 1996;17:493–9.
- Carr A, Frei B. Does vitamin C act as a pro-oxidant under physiological conditions? FASEB J 1999;13:1007–24.
- Miller ER, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med 2005;142:37–46.
- Cozzi R, Ricordy R, Aglitti T, Gatta V, Perticone P, De Salvia R. Ascorbic acid, beta-carotene as modulators of oxidative damage. Carcinogenesis 1997;18:223–8.
- Nowak D, Piasecka G, Antczak A, Pietras T. Effect of ascorbic acid on hydroxyl radical generation by chemical, enzymatic and cellular systems. Importance for antioxidant prevention of pulmonary emphysema. Biomed Biochim Acta 1991;50:265–72.
- Weitberg AB, Weitzman SA. The effect of vitamin C on oxygen radical-induced sister-chromatid exchanges. Mutat Res 1985;144:23–6.
- Abudu N, Miller JJ, Attaelmannan M, Levinson SS. Vitamins in human arteriosclerosis with emphasis on vitamin C and vitamin E. Clin Chim Acta 2004;339:11–25.
- de Magalhaes JP, Church GM. Cells discover fire: employing reactive oxygen species in development and consequences for aging. Exp Gerontol 2006;41:1–10.
- Gutteridge JM. Free radicals in disease processes: a compilation of cause and consequence. Free Radic Res Commun 1993;19:141–58.