Abstract
Paroxonase 1 displays multiple physiological activities that position it as a putative player in the pathogenesis of neurological disorders. Here we reviewed the literature focusing on the role of paraoxonase 1 (PON1) as a factor in the risk of stroke and the major neurodegenerative diseases. PON1 activity is reduced in stroke patients, which significantly correlates inversely with carotid and cerebral atherosclerosis. The presence of the R allele of the Q192R PON1 polymorphism seems to potentiate this risk for stroke. PON1 exerts peroxidase activities that may be important in neurodegenerative disorders associated with oxidative stress. PON1 is also a key detoxifier of organophosphates and organophosphate exposure has been linked to the development of neurological disorders in which acetylcholine plays a significant role. In Parkinson's disease most of the studies suggest no participation of either L55M or the Q192R polymorphisms in its pathogenesis. However, many studies suggest that the MM55 PON1 genotype is associated with a higher risk for Parkinson's disease in individuals exposed to organophosphates. In Alzheimer's disease most studies have failed to find any association between PON1 polymorphisms and the development of the disease. Some studies show that PON1 activity is decreased in patients with Alzheimer's disease or other dementias, suggesting a possible protective role of PON1. No links between PON1 polymorphisms or activity have been found in other neurodegenerative diseases such as multiple sclerosis and amyotrophic lateral sclerosis. PON1 is a potential player in the pathogenesis of several neurological disorders. More research is warranted to ascertain the precise pathogenic links and the prognostic value of its measurement in neurological patients.
Introduction
Paraoxonase 1 (PON1), an enzyme carried by high-density lipoproteins (HDLs), provides antioxidant and anti-inflammatory capacities to the particle.Citation1–Citation7 It also detoxifies xenobiotics such as organophosphates.Citation8–Citation13 Its role as a protective factor against atherogenesis is becoming more apparent in recent epidemiological studies.Citation14,Citation15 Considerable attention has been paid to the putative role of PON1 activity and its polymorphisms as a risk factor for ischemic stroke, one of the main neurological diseases associated with atherosclerosis.Citation16–Citation19 Several comprehensive reviews and meta-analysis including studies up to 2011 are available.Citation16,Citation20,Citation21 Beyond stroke, oxidative stress plays a key role in many neurodegenerative diseases, although the precise pathogenic links are yet to be elucidated. Multiple studies have surfaced that focus on the links between PON1 status and the risk of developing neurodegenerative diseases.Citation22–Citation27 The aim of this review is to present an integrated summary of the available information on PON1 status and polymorphisms as a risk factor for major neurological diseases. We will first summarize PON1 biology and its implications in oxidative stress and functional properties of HDL and then offer a summary of the current findings on ischemic stroke, Alzheimer's disease (AD) and other dementias, Parkinson's disease (PD), as well as other neurodegenerative disorders. The reader is referred to specific individual reviews for further depth.
Paraoxonase 1
Human PON1 (aryldialkylphosphatase, EC3.1.8.1) is an esterase associated with apolipoprotein AI (apoAI) and clusterin (apolipoprotein J) in the HDL particles. PON1 displays both paraoxonase and arylesterase activities, since it hydrolyzes organophosphate compounds such as paraoxon, and aromatic carboxylic acid esters such as phenylacetate.Citation2,Citation4,Citation5,Citation7,Citation28,Citation29
During the past decade, PON1 has been proven to be an important contributor to the antioxidant activity of HDL as well as an independent negative risk factor for atherosclerosis in epidemiological studies.Citation2,Citation5,Citation14,Citation28 PON1 is versatile and plays a role in several pathways, many of which are protective against atherothrombosis:
It effectively hydrolyzes peroxides and lactones in low-density lipoprotein (LDL) and HDL particles as well as protects macrophages from oxidation.Citation3–Citation7,Citation28
It participates in endothelial homeostasis.Citation2,Citation6,Citation7
It is an effective xenobiotic metabolizer.Citation8,Citation9,Citation11,Citation13,Citation29,Citation30
It is a homocysteine-thiolactonase.Citation31–Citation34
PON1 evolved from bi-functional enzymes that act as homoserine lactones which are quorum-sensing molecules employed by many gram-negative bacteria. PON1 is thereby a component of the innate immunity.Citation35–Citation39 The family also contains PON3 (circulating, 100–1000 less concentrated than PON1) and PON2, a key intracellular antioxidant enzyme. In recent years, low PON1 activity has been consistently linked with an increased risk of major cardiovascular events in the setting of secondary prevention of coronary artery disease (CAD).Citation2,Citation14,Citation40
However, the mechanism of PON1's protective action and its endogenous substrate remains elusive. Evidence is accumulating indicating that the lactonizing/lactonase activity of PON1 may be physiologically the most significant. Lactonase activity is exerted on oxidized phospholipids and homocysteine-thiolactone. Hyperhomocysteinemia, encompassing also higher concentrations of homocysteine-thiolactone, may be an added risk factor for enhanced atherogenesis.Citation6,Citation33,Citation34,Citation39,Citation41
The proposed role of PON1 in neurovascular diseases, a major consequence of atherosclerosis, is illustrated in the schematic diagram in . The diagram shows how reactive oxygen species produced by leukocytes in LDL (1) or cell membranes (2) result in the formation of oxidized phospholipids (3). PON1 is synthesized in the liver in association with HDL. PON1 may act on oxidized phosphatydyl choline derived from oxidized LDL (3) or oxidized macrophage membranes to produce a lactone (lactonizing activity of PON1, (4), which is, in turn, subjected to lactonase activity (5) to yield an innocuous carboxylic acid.
Figure 1. Protective role of paraoxonase 1 in cardiovascular disease and inflammation. Ischemic stroke is a very prevalent complication of atherosclerosis. PON1 may have a protective role in this disorder as it carries both antioxidant and anti-inflammatory functional properties. In this diagram we summarize the current knowledge on the main functional activities of PON1 a protective factor vis-a-vis atherogenesis. Reactive oxygen species issued from inflammation oxidize lipids in LDL (1) or cell membranes (2) producing oxidized phospholipids (3). PON1 produced in the liver and carried by HDL (4) exerts a lactonizing and lactonase activity (5) that results in the production of a carboxylic acid (5), eliminating further damage. The lactonase activity of PON1 also permits it to detoxify homocysteine thiolactone (6). This compound is associated with increased cardiovascular risk, since homocysteinylation of proteins (coagulation factors, lipoproteins, endothelial receptors) is atherogenic (7). Homocysteine thiolactone is one of the natural substrates of PON1, which hydrolyzes it to innocuous homocysteine (8). PON1 also exerts its salutatory action on macrophages and other inflammatory cells (9), preventing cellular oxidative stress and blocking cytokine cascades that aggravate inflammation that may lead to enhanced atherogenesis and neurovascular disease (10).
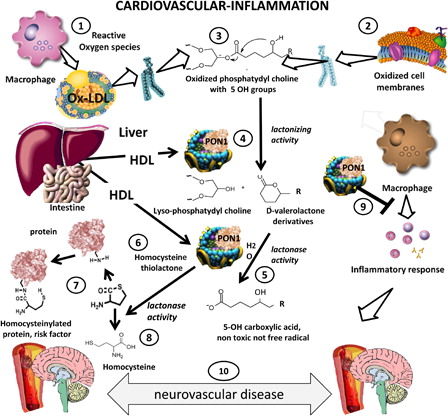
Homocysteine-thiolactone, produced in excess due to hyperhomocysteinemia (6), modifies proteins (coagulation, lipoproteins, endothelial receptors) and is a cardiovascular risk factor (7). PON1 may act on homocysteine-thiolactone (8) and detoxify it to homocysteine, as shown on the lower left of the diagram. PON1 also acts on macrophages (9), protecting them from oxidation and blunting their inflammatory cascades. Patients with lower serum lactonase activity may be more susceptible to the deleterious effects of lipid peroxidation, homocysteine-thiolactone toxicity and macrophage activation, which would increase the risk of neurovascular disease (10).
Another key role of PON1 is as a xenobiotic metabolizer. The detoxification activity of PON1 is believed to provide a significant link between environmental exposure to pesticides or pollutants and illness.Citation8–Citation11,Citation13,Citation39 PON1 received its name from its ability to hydrolyze paraoxon, the oxon stemming from parathion, but it also hydrolyzes the active metabolites of other organophosphorous (OP) insecticides. The metabolites of OP pesticides that result from PON1 action are considered biomarkers of exposure. Therefore, enzyme activity levels may be useful in the assessment of the severity of insecticide exposure. Studies in laboratory animals have demonstrated the impact of PON1 in reducing the toxicity of organophosphate pesticides.Citation8,Citation9 Clinical evidence for a role of PON1 in organophosphate toxicity also supports the role of this enzyme in pesticide-associated disease.
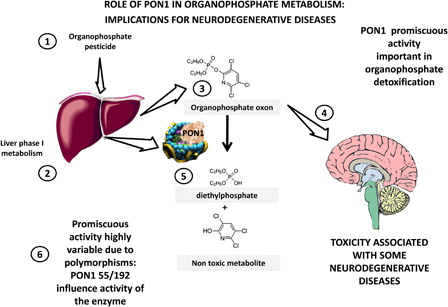
As summarized in , organophosphate pesticides (1) suffer phase-I metabolism (2) in the liver resulting in oxons (3) that are consequently hydrolyzed to specific, non-toxic metabolites by PON1 (5). Mechanistically, the link between organophosphate exposure and some neurodegenerative diseases can be ascribed to the metabolism of organophosphate pesticides as neurotoxic agents (4). Polymorphisms in PON1 precisely affect the promiscuous detoxifying activity.
Figure 2. PON1 in toxicology: implications for neurodegenerative diseases. PON1 evolved from lactonases that have a key role in natural immunity, limiting quorum sensing lactones from bacteria. In evolution it acquired promiscuous esterase activities, hydrolyzing compounds of interest in toxicology. Organophosphate pesticides (1) are metabolized to their respective oxon by phase 1 metabolism in the liver (2,3). These oxons are toxic due to their inhibition of acetylcholinesterase, which is associated with several neurodegenerative disorders (4). PON1 hydrolyzes paraoxon (oxon from parathion, from which the name derives) and oxons from many other organophosphates (5) and detoxifies them. Major polymorphisms in PON1 significantly change this activity (6) and may be associated with increased susceptibility to neurodegenerative disorders as discussed in the text.
Earlier studies showed that plasma hydrolysis of paraoxon was distributed polymorphically in human populations.Citation30 Two common polymorphisms are found in the PON1 coding sequence: a Gln(Q)/Arg(R) substitution at position 192, and a Leu(L)/Met(M) substitution at position 55.Citation1,Citation8,Citation9,Citation13,Citation30,Citation42 The gene frequency of PON1 Q192 varies from 0.75 for Northern Europeans to 0.31 for some Asian populations. Other polymorphisms have been found in the non-coding region of the PON1 gene. Over 160 single nucleotide polymorphisms (SNPs), some in the coding regions and others in introns and regulatory regions of the gene, have been identified by complete re-sequencing of PON1 from several individuals.Citation1,Citation8,Citation9,Citation13,Citation30,Citation42 Coding region polymorphisms of PON1 have been studied for effects on the catalytic efficiencies of hydrolysis of specific substrates. The L/M polymorphism at position 55 does not affect catalytic activity, but has been associated with plasma PON1 protein levels, with PON1M55 being associated with low plasma PON1.Citation1,Citation42,Citation43 This is due mostly to linkage disequilibrium with the low efficiency − 108T allele of the − 108 promoter region polymorphism. Conversely, the Q192R polymorphism considerably affects the catalytic efficiency of PON1.Citation8,Citation9,Citation30 This polymorphism is substrate-dependent. PON1 gene polymorphisms have been shown to account for more than 60% of the inter-individual variation in enzyme concentration and activity, and the 192Q isoform of PON1, Q192R, polymorphism prevents LDL oxidation in vitro more efficiently than the 192R form. PON1 and PON2 gene polymorphisms have all been implicated in a variety of human disorders, such as CAD.Citation8 Genotypic and phenotypic variations of PON1 also have been associated with specific neurological diseases that will be discussed in this work.
PON1 and ischemic stroke
Ischemic stroke ranks as the second most common cause of death across the globe and is a major cause of disability.Citation19,Citation44,Citation45 It is a classical multifactorial disease with major risk factors such as hypertension, smoking, hyperlipidemia, obesity, diabetes, and atrial fibrillation.Citation18,Citation19,Citation44,Citation46–Citation48 Nevertheless, over 40% of the risk is unaccounted for by these major factors. In this regard, studies that focus on non-traditional risk factors as pathogenic players and/or having prognostic value are important. PON1 deserves attention in this regard. In this sense, over the past decade two types of studies have surfaced: those that look for a correlation in certain polymorphisms and incidence of stroke, and those that focus on lower PON1 activity as a predictive factor for stroke and other cardiovascular events.
PON genes have been extensively studied in attempts to find an association between certain polymorphisms and an increased risk for stroke.
Multiple genetic association studies observing the relationship between PON1 polymorphisms and ischemic stroke have produced contradictory or inconclusive results.Citation49–Citation66 Liu et al. in 2013 conducted and published a systematic review and meta-analysis of 28 selected studies addressing these relationships.Citation21 They found that the R allele and the RR genotype of PON1 Q192R polymorphism correlated with a higher risk for ischemic stroke in the general population.Citation21 The other genetic variants of the PON1 gene were not associated with ischemic stroke. These meta-analyses included studies conducted in populations of Caucasian, Japanese, and Chinese patients.Citation21
The other important focus of study regarding the association between PON1 and the incidence of ischemic stroke is the finding by several authors of a lower PON1 activity after the vascular event, a decrease which is maintained for a long period of time.
In 2012, Mahrooz et al. focused not only on the investigation of the polymorphisms but also in the association between the enzyme activity and vascular disease.Citation67 Their results are in agreement with the previous work by Liu et al.;Citation21 the presence of the 192 R allele potentiates the risk of stroke, especially in hypertensive subjects. They also found that patients had a significantly higher paraoxon/arylesterase ratio than control subjects and in stroke patients paraoxon/arylesterase and paraoxon/HDL ratios followed this pattern: QQ < QR < RR. They suggest that the ratio of para/aryl, para/HDL and aryl/HDL ratios may prove useful as markers for an augmented predisposition to ischemic stroke.Citation67
In 2011, Michalak et al. studied the association of PON1 triesterase (paraoxon) and arylesterase activities and conjugated dienes on stroke patient outcome during a 1-year follow-up period.Citation68 They concluded that the PON/aryl ratio is a significant predictor of ischemic stroke outcome and could be used in the clinical setting to better advantage than the paraoxon or aryl assays alone.
These findings have been confirmed by recently Sand.Citation16 They suggested that lower than expected PON1 activity within specific genotypes may be the pathogenic link to the described association between R and L alleles and the risk of acute ischemic stroke.
We are presently conducting a study on the acute changes of PON1 activity after ischemic stroke. Our preliminary data (unpublished observations) show the decrease of PON1 activity in the first days after onset followed by recovery of its activity, which is associated with clinical improvement. If these data are confirmed in a larger patient population, PON1 may prove to be useful as a marker of disease prognosis in these patients.
The impact of the different PON1 polymorphisms on the functional activity of PON1 as a preventive factor for HDL and LDL oxidation was also the subject of a 2013 study where the authors profiled PON1 polymorphisms and enzymatic activities, and evaluated atherosclerosis and particularly cerebral arteriosclerosis gravity in post-stroke patients.Citation69 They showed that stroke patients with PON1 QQ192 or MM55 genotypes displayed lower PON1 and arylesterase activities at both onset and 12 months after stroke than subjects with either RQ/RR192 or LM/LL55 genotypes.Citation69 They concluded that there are significant inverse correlations between PON1 activity and carotid atherosclerosis and cerebral arteriosclerosis in stroke patients. The lower the PON1 activity the more advanced are the atherosclerotic lesions. These findings are in agreement with several other studies addressing correlations of PON1 activity and other cardiovascular outcomes.Citation2,Citation14
PON1 and Parkinson's disease ()
PD is a neurodegenerative disorder related to the progressive degeneration of the dopamine producing neurons in the substantia nigra of the midbrain.Citation24,Citation70–Citation73 While the exact pathogenesis of the disease has not been completely elucidated, several theories implicating the association of genetic and environmental toxic elements such as exposure to pesticides or an oxidative cell environment conspire to trigger the neuron degeneration.Citation24,Citation70–Citation72,Citation74–Citation81 Because PON1 is capable of hydrolyzing toxic organophosphates and also has an important antioxidant capacity, the association of PON polymorphisms and PD has been addressed by multiple authors.
Figure 3. Putative involvement of PON1 in susceptibility to Parkinson's disease. In Parkinson's disease, due to death of degeneration in the substantial nigra there is an imbalance between acetylcholine and dopamine (1 and 2). Free radical species (3) that generate peroxide and organophosphate oxons both attack and inhibit acetylcholinesterase (4), thereby increasing acetylcholine and aggravating the imbalance. PON1, thorough its peroxidase (5) and triesterase (6) actions, blocks these effects and prevents the worsening of the imbalance. Of note, the oxonase activity of PON1 is highly dependent on the most common polymorphisms. Subjects with different phenotypes may thereby be more or less susceptible to damage to acetylcholine.
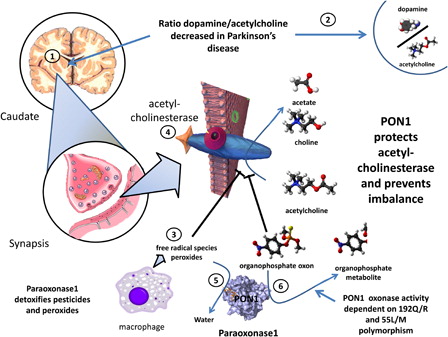
The frequency of the Met 54 allele of PON1 has been found to be significantly higher in patients with PD compared with control subjects. The relative risk of PD in the Met 54 allele carriers is 2.3-fold higher than in homozygotes for the L allele. In the subgroup of patients with early-onset PD, the Met 54 allele carries a 5-folf higher risk.Citation82 These authors conclude that the Met 54 allele may be an independent risk factor for PD. This mutation could possibly trigger PON1 lessened metabolism of environmental neurotoxins and could play a role in neurodegeneration, as we summarize in .
Other studies have yielded inconsistent results.Citation80–Citation88 Heterogeneity of the populations studied has been a major factor of possible contradictory findings among studies. A meta-analysis including both Caucasian and Asian populations, however, supported the contention that there is an association between PON1-L55M polymorphism and PD, whereas PON1-Q192R polymorphism was unlikely to be a major risk factor for susceptibility to PD.Citation83
Other studies confirm these findings in a Swedish populationCitation86 while they were not found in a Finnish population.Citation84
To shed more light on this issue, the most recent meta-analysis was performed using data from relevant studies during the past decade to assess the effects of two PON1 polymorphisms (L55M and Q192R) on PD.Citation89 The results of this meta-analysis suggested that both PON1 L55M and Q192R were not responsible for PD. This is in contradiction with the meta-analysis described earlier. The reasons for the discrepancy may lie in the heterogeneity of the studies and ethnic differences of the populations.
Other studies have focused on the combined effects of toxic exposure and PON1 polymorphisms. The role of PON1 in increased susceptibility to PD associated with exposure to pesticides is more clear and better documented.Citation78–Citation80,Citation85,Citation87,Citation88,Citation90 Many studies have consistently revealed a close relationship between exposure to pesticides and PD.Citation78–Citation80,Citation85,Citation87,Citation88,Citation90 The putative pathogenic links are summarized in .
In a recent study the authors found that the carriers of the MM PON1-55 genotype exhibited a greater than 2-fold increase in PD risk when exposed to organophosphates, compared with subjects who had the wild type or heterozygous genotype and no exposure. These findings highlight the importance of bearing in mind these predisposition factors when examining environmental exposures in PD.Citation78
A review of the literature thus shows inconclusive evidence on the relationship between a genetic predisposition to develop PD in patients with different PON1 polymorphisms, supported by many of the meta-analysis available up to date. Conversely, there seems to be more solid evidence suggesting that when there is exposure to a toxic environment there is a correlation between the presence of certain genotypes and the development of the disease.Citation24,Citation70,Citation73–Citation76,Citation79,Citation91
PON1, Alzheimer's disease, and other dementias
Across the world, the prevalence of dementia is believed to be 24 million, and is expected to double every 20 years until 2040.Citation26 AD is the foremost cause of dementia starting with compromised memory.Citation25,Citation26,Citation92–Citation95 The pathognomonic traits of AD comprise diffuse and neuritic extracellular amyloid plaques in brain tissue, which are often encircled by dystrophic neurites and intraneuronal neurofibrillary tangles. The etiology of AD remains uncertain, but it is believed to stem from both genetic and environmental factors.Citation25,Citation26,Citation92–Citation95
Given the role played by oxidative stress in AD many studies have addressed the putative link between PON1 activity, its polymorphisms and AD.
A study included 120 patients with dementia: 51 with AD, 28 with dementia of vascular origin, 41 with mixed dementia, 45 with mild cognitive impairment, and 61 age and sex matched controls.Citation96 PON1 activity was decreased in patients with AD or mixed dementia as compared with control subjects. In both types of dementia homocysteine levels also were increased. In AD PON1 activity was negatively correlated with homocysteine levels. These results point to a significant role of oxidative stress in the forms of dementia with prevalent neurodegeneration.Citation96 Similar findings were obtained in a study that included patients with either AD or vascular dementia.Citation97 PON1 activity did not differ significantly in the patient groups, but compared to the healthy control subjects, it was significantly lower in both patient groups. The results suggest that the impaired PON1 activity plays a role in the pathogenesis of AD and vascular dementia.Citation97
Multiple studies have addressed the association between PON1 polymorphisms and AD.Citation91,Citation98–Citation105 In a study with 756 AD subjects, the authors found no association between the two major PON1 polymorphisms and AD in African Americans or Caucasians.Citation91 The authors conclude that either these functional PON1 polymorphisms are not associated with AD, or that the relative risk is small.Citation91 However, another study from China that included over 1000 subjects (patients and controls) indicated that Q192R polymorphism in the PON1 gene is associated with AD, and that the PON1 R allele might be a protective factor for AD in a Chinese Han ethnic population.Citation102
A more recent meta-analysis conducted in China showed that there was no significant association between PON1 Q192R polymorphism and AD risk in all comparison models.Citation98 For the PON1 L55M polymorphism, lack of an association was also found. On subgroup analysis by ethnicity, similar results were found. This meta-analysis showed that PON1 gene polymorphisms (Q192R, L55M) were unlikely to contribute to AD susceptibility.Citation98
As described earlier, PON1 L55M and Q192R genetic variants might affect individual susceptibility to exposure to acetylcholinesterase inhibitors.Citation8,Citation9,Citation11,Citation29 Cholinesterase inhibitor therapy is the treatment of choice for patients with mild-to-moderate AD.Citation25–Citation27,Citation92,Citation94 In a large study with an over 1000 cohort of clinical and autopsy-confirmed AD cases and age-matched, cognitively intact controls, multiple gender-specific effects of PON1 polymorphisms on AD pathogenesis were apparent.Citation101 The L55M Met allele exerts an AD risk-enhancing effect only in men, whereas both men and women carrying the M55M/Q192Q genotype show augmented survival and later age of onset. Associations with beta-amyloid levels, senile plaque accumulation and cholinesterase activity suggest an involvement of the PON1 gene in AD pathogenesis and responses to treatment.Citation101
Regarding the possible interference between common SNPs in PON1, a recent study suggests that PON1 common SNPs do not impact on treatment response to acethylcholinesterase inhibitors in patients with AD.Citation99
PON1 and amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS) is a degenerative disease of adult-onset and fatal outcome characterized by the simultaneous loss of motor neurons in the cerebral cortex, brainstem and spinal cord. Ninety percent of the cases are sporadic with the other 10% being transmitted as an autosomal dominant trait. In the familial cases, a large number of mutations have been found, the most prominent of which is the mutation in the gene that encodes for the cytosolic enzyme superoxide dismutase (SOD1).Citation106–Citation109
Even if the etiology is unknown for the sporadic cases, current evidence points to an association with exposure to noxious environmental elements.Citation106–Citation109 The flavin-containing monooxygenases and PON genes encode enzymes implicated in xenobiotic detoxification and their deficits may be associated with ALS. A recent publication examined PON1 gene expression in human spinal cord, medulla, and cerebral cortex and in peripheral cells (lymphocytes, fibroblasts) in ALS patients and control subjects. PON2, not PON1 gene was down-regulated in ALS patients compared with the controls.Citation110
At least seven PON gene mutations predictive of altered PON function have been identified in genomic DNA from individuals with familial and sporadic ALS.Citation111
However, a large-scale international meta-analysis of paraoxonase gene polymorphisms in sporadic ALS showed no significant association of the disease with the PON locus. This was the largest meta-analysis of a candidate gene in ALS and the first ALS meta-analysis to contain data from whole genome association studies.Citation112
In studies showing an increase in PON1-R192 frequency in ALS patients the findings suggest that the influence of PON1 polymorphisms on ALS susceptibility is not due to the ability of the enzyme to catalyze organophosphate hydrolysis.Citation113
PON1 and multiple sclerosis
Multiple sclerosis (MS) is a chronic, multifactorial disease characterized by axonal demyelination in the central nervous system (CNS) leading to multiple motor and sensory symptoms. The disease progresses through remissions and relapses.Citation114–Citation117 Oxidative stress is a critical factor in MS pathogenesis as it promotes leukocyte migration, participating in oligodendrocyte damage and axonal injury. Reactive oxygen species and reactive nitrogen species are produced in the CNS of MS patients chiefly by activated macrophages and microglia and could account for demyelinization and axonal disruption, the hallmarks of the disease.Citation114–Citation117
In this regard a few studies have addressed the possible correlation of PON1 polymorphisms with MS and all coincide in not finding evidence for this type of association.Citation118–Citation121
In a longitudinal study, PON1 activity showed no changes in the course of the stable and progressive type of MS and it decreased in the course of MS relapses.Citation122
Conclusion
Paroxonase 1, and enzyme associated with HDL particles, displays multiple physiological activities that position it as a putative player in the pathogenesis of neurological disorders. PON1 has lactonase, antioxidant and endothelial protective activities considered anti-atherogenic and multiple studies suggest that PON1 plays a protective role in cardiovascular disease. Since ischemic stroke is one of the major complications of atherosclerosis, we focused our attention on surveying the present knowledge about the potential role of PON1 in this disorder.
PON1 also exerts peroxidase activities that may be important in neurodegenerative disorders associated with oxidative stress.
PON1 is a key detoxifier of organophosphates and organophosphate exposure has been linked to the development of neurological disorders where acetylcholine plays a significant role.
In terms of ischemic stroke, the literature shows that PON1 activity is reduced in stroke patients. There are significant inverse correlations between PON1 activity and carotid and cerebral atherosclerosis in stroke patients. The Q192R PON1 polymorphism has been linked to the risk of stroke: the presence of the R allele potentiates this risk, particularly in hypertensive patients.
In neurodegenerative diseases the role of PON1 is more controversial. In PD most of the studies suggest no participation of either L55M or the Q192R polymorphisms in the pathogenesis of PD, although contradictory results have been published. However, many studies suggest that the MM55 PON1 genotype is associated with a higher risk for PD in individuals exposed to organophosphates.
In AD most studies failed to find any association between PON1 polymorphisms and the development of the disease. Some studies show that PON1 activity is decreased in patients with AD or other dementias, suggesting a possible protective role of PON1 in these disorders.
No links between PON1 polymorphisms or activity have been found in other neurodegenerative diseases such as MS and ALS.
PON1 is certainly a potential player in the pathogenesis of neurological disorders and more research is warranted to ascertain the precise pathogenic links and the prognostic value of its measurement in neurological patients.
Acknowledgements
We are grateful to Ms. Mallory Davis for editorial help. This work was funded in part by Touro University California.
References
- Mackness B, Mackness MI, Arrol S, Turkie W, Durrington PN. Effect of the human serum paraoxonase 55 and 192 genetic polymorphisms on the protection by high density lipoprotein against low density lipoprotein oxidative modification. FEBS Lett 1998;423:57–60.
- Aviram M, Vaya J. Paraoxonase 1 activities, regulation, and interactions with atherosclerotic lesion. Curr Opin Lipidol 2013;4:339–44.
- Mackness M, Boullier A, Hennuyer N, Mackness B, Hall M, Tailleux A, et al. Paraoxonase activity is reduced by a pro-atherosclerotic diet in rabbits. Biochem Biophys Res Commun 2000;269:232–6
- Aviram M, Rosenblat M, Bisgaier CL, Newton RS, Primo-Parmo SL, La Du BN. Paraoxonase inhibits high-density lipoprotein oxidation and preserves its functions. A possible peroxidative role for paraoxonase. J Clin Invest 1998;101:1581–90.
- Aviram M, Rosenblat M. Paraoxonases and cardiovascular diseases: pharmacological and nutritional influences. Curr Opin Lipidol 2005;16:393–9.
- Aviram M. Review of human studies on oxidative damage and antioxidant protection related to cardiovascular diseases. Free Radic Res 2000;33 (Suppl):S85–97.
- Mackness M, Mackness B. Targeting paraoxonase-1 in atherosclerosis. Expert Opin Ther Targets 2013;17:829–37.
- Furlong CE, Suzuki SM, Stevens RC, et al. Human PON1, a biomarker of risk of disease and exposure. Chem Biol Interact 2010;187:355–61.
- Costa LG, Giordano G, Cole TB, Marsillach J, Furlong CE. Paraoxonase 1 (PON1) as a genetic determinant of susceptibility to organophosphate toxicity. Toxicology 2013;307:115–22.
- Richter RJ, Jarvik GP, Furlong CE. Paraoxonase 1 (PON1) status and substrate hydrolysis. Toxicol Appl Pharmacol 2009;235:1–9.
- Richter RJ, Jarvik GP, Furlong CE. Paraoxonase 1 status as a risk factor for disease or exposure. Adv Exp Med Biol 2010;660:29–35.
- Ticozzi N, LeClerc AL, Keagle PJ, et al. Paraoxonase gene mutations in amyotrophic lateral sclerosis. Ann Neurol 2010;68:102–7.
- Costa LG, Li WF, Richter RJ, Shih DM, Lusis A, Furlong CE. The role of paraoxonase (PON1) in the detoxication of organophosphates and its human polymorphism. Chem Biol Interact 1999;119–120:429–38.
- Tang WH, Hartiala J, Fan Y, et al. Clinical and genetic association of serum paraoxonase and arylesterase activities with cardiovascular risk. Arterioscler Thromb Vasc Biol 2012;32:2803–12.
- Huang Y, Wu Z, Riwanto M, et al. Myeloperoxidase, paraoxonase-1, and HDL form a functional ternary complex. J Clin Invest 2013;123(9):3815–28.
- Sand PG. Paraoxonase genes and the susceptibilty to ischemic stroke. Int J Stroke 2013;8:E39.
- Rothstein L, Jickling GC. Ischemic stroke biomarkers in blood. Biomarkers in Medicine 2013;7:37–47.
- Yamamoto FI. Ischemic stroke in young adults: an overview of etiological aspects. Arquivos de Neuro-Psiquiatria 2012;70:462–6.
- Xing C, Arai K, Lo EH, Hommel M. Pathophysiologic cascades in ischemic stroke. Int J Stroke 2012;7:378–85.
- Zhang G, Li W, Li Z, et al. Association between paraoxonase gene and stroke in the Han Chinese population. BMC Med Genet 2013;14:16.
- Liu H, Xia P, Liu M, et al. PON gene polymorphisms and ischaemic stroke: a systematic review and meta analysis. Int J Stroke 2013;8:111–23.
- Androutsopoulos VP, Kanavouras K, Tsatsakis AM. Role of paraoxonase 1 (PON1) in organophosphate metabolism: implications in neurodegenerative diseases. Toxicol Appl Pharmacol 2011;256:418–24.
- Bekris LM, Mata IF, Zabetian CP. The genetics of Parkinson disease. J Geriatr Psychiatry Neurol 2010;23:228–42.
- Gazewood JD, Richards DR, Clebak K. Parkinson disease: an update. Am Fam Phys 2013;87:267–73.
- Mayeux R, Stern Y. Epidemiology of Alzheimer disease. Cold Spring Harb Perspect Med 2012;2 (8).
- Selkoe D, Mandelkow E, Holtzman D. Deciphering Alzheimer disease. Cold Spring Harb Perspect Med 2012;2:a011460.
- Tanzi RE. The genetics of Alzheimer disease. Cold Spring Harb Perspect Med 2012;2 (10).
- Aharoni S, Aviram M, Fuhrman B. Paraoxonase 1 (PON1) reduces macrophage inflammatory responses. Atherosclerosis 2013;228:353–61.
- Costa LG, Giordano G, Furlong CE. Pharmacological and dietary modulators of paraoxonase 1 (PON1) activity and expression: the hunt goes on. Biochem Pharmacol 2011;81:337–44.
- Mueller RF, Hornung S, Furlong CE, Anderson J, Giblett ER, Motulsky AG. Plasma paraoxonase polymorphism: a new enzyme assay, population, family, biochemical, and linkage studies. Am J Human Genet 1983;35:393–408.
- Borowczyk K, Shih DM, Jakubowski H. Metabolism and neurotoxicity of homocysteine thiolactone in mice: evidence for a protective role of paraoxonase 1. J Alzheimers Dis 2012;30:225–31.
- Jakubowski H. Protein N-homocysteinylation: implications for atherosclerosis. Biomed Pharmacother 2001;55:443–7.
- Perla-Kajan J, Jakubowski H. Paraoxonase 1 protects against protein N-homocysteinylation in humans. FASEB J 2010;24:931–6.
- Perla-Kajan J, Jakubowski H. Paraoxonase 1 and homocysteine metabolism. Amino Acids 2012;43:1405–17.
- Afriat-Jurnou L, Jackson CJ, Tawfik DS. Reconstructing a missing link in the evolution of a recently diverged phosphotriesterase by active-site loop remodeling. Biochemistry 2012;51:6047–55.
- Bar-Rogovsky H, Hugenmatter A, Tawfik DS. The evolutionary origins of detoxifying enzymes: the mammalian serum paraoxonases (PONs) relate to bacterial homoserine lactonases. J Biol Chem 2013;288(33):23914–27.
- Ben-David M, Elias M, Filippi JJ, et al. Catalytic versatility and backups in enzyme active sites: the case of serum paraoxonase 1. J Mol Biol 2012;418:181–96.
- Draganov D, Teiber J, Watson C, et al. PON1 and oxidative stress in human sepsis and an animal model of sepsis. Adv Exp Med Biol 2010;660:89–97.
- Draganov DI. Lactonases with organophosphatase activity: structural and evolutionary perspectives. Chem Biol Interact 2010;187:370–2.
- Bhattacharyya T, Nicholls SJ, Topol EJ, et al. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA 2008;299:1265–76.
- Jakubowski H. The role of paraoxonase 1 in the detoxification of homocysteine thiolactone. Adv Exp Med Biol 2010;660:113–27.
- Mackness B, Mackness MI, Arrol S, et al. Serum paraoxonase (PON1) 55 and 192 polymorphism and paraoxonase activity and concentration in non-insulin dependent diabetes mellitus. Atherosclerosis 1998;139:341–9.
- Kim DS, Burt AA, Ranchalis JE, et al. Additional common polymorphisms in the PON gene cluster predict PON1 activity but not vascular disease. J Lipids 2012;2012:476316.
- Coplin WM. Critical care management of acute ischemic stroke. Continuum 2012;18:547–59.
- Perry JM, McCabe KK. Recognition and initial management of acute ischemic stroke. Emerg Med Clin N Am 2012;30:637–57.
- Gonzalez RG. Clinical MRI of acute ischemic stroke. J Magnc Reson Imaging 2012;36:259–71.
- Gross H, Sung G, Weingart SD, Smith WS. Emergency neurological life support: acute ischemic stroke. Neurocritical Care 2012;17 (Suppl 1):S29–36.
- Yoo AJ, Chaudhry ZA, Leslie-Mazwi TM, et al. Endovascular treatment of acute ischemic stroke: current indications. Tech Vasc Interv Radiol 2012;15:33–40.
- Schmidt H, Schmidt R, Niederkorn K, et al. Paraoxonase PON1 polymorphism leu-Met54 is associated with carotid atherosclerosis: results of the Austrian Stroke Prevention Study. Stroke 1998;29:2043–8.
- Schmidt R, Schmidt H, Fazekas F, et al. MRI cerebral white matter lesions and paraoxonase PON1 polymorphisms: three-year follow-up of the austrian stroke prevention study. Arterioscler Thromb Vasc Biol 2000;20:1811–6.
- Voetsch B, Benke KS, Damasceno BP, Siqueira LH, Loscalzo J. Paraoxonase 192 Gln–>Arg polymorphism: an independent risk factor for nonfatal arterial ischemic stroke among young adults. Stroke 2002;33:1459–64.
- Wang XY, Xue YM, Wen SJ, Zhang NL, Ji Z, Pan SY. The association of paraoxonase 2 gene C311S variant with ischemic stroke in Chinese type 2 diabetes mellitus patients. Chin J Med Genet 2003;20:215–9.
- Voetsch B, Benke KS, Panhuysen CI, Damasceno BP, Loscalzo J. The combined effect of paraoxonase promoter and coding region polymorphisms on the risk of arterial ischemic stroke among young adults. Arch Neurol 2004;61:351–6.
- Ranade K, Kirchgessner TG, Iakoubova OA, et al. Evaluation of the paraoxonases as candidate genes for stroke: Gln192Arg polymorphism in the paraoxonase 1 gene is associated with increased risk of stroke. Stroke 2005;36:2346–50.
- Aydin M, Gencer M, Cetinkaya Y, et al. PON1 55/192 polymorphism, oxidative stress, type, prognosis and severity of stroke. IUBMB Life 2006;58:165–72.
- Baum L, Ng HK, Woo KS, et al. Paraoxonase 1 gene Q192R polymorphism affects stroke and myocardial infarction risk. Clin Biochem 2006;39:191–5.
- Pasdar A, Ross-Adams H, Cumming A, et al. Paraoxonase gene polymorphisms and haplotype analysis in a stroke population. BMC Med Genet 2006;7:28.
- Kim NS, Kang K, Cha MH, et al. Decreased paraoxonase-1 activity is a risk factor for ischemic stroke in Koreans. Biochem Biophys Res Commun 2007;364:157–62.
- Can Demirdogen B, Turkanoglu A, Bek S, et al. Paraoxonase/arylesterase ratio, PON1 192Q/R polymorphism and PON1 status are associated with increased risk of ischemic stroke. Clin Biochem 2008;41:1–9.
- Sarkar PD, Rautaray SS. Oxidized LDL and paraoxanase status in ischemic stroke patients. Indian J Physiol Pharmacol 2008;52:403–7.
- Shin BS, Oh SY, Kim YS, Kim KW. The paraoxonase gene polymorphism in stroke patients and lipid profile. Acta Neurol Scand 2008;117:237–43.
- Xu HW, Yuan N, Zhao Z, et al. Study of the relationship between gene polymorphisms of paraoxonase 2 and stroke in a Chinese population. Cerebrovasc Dis 2008;25:87–94.
- Xiao ZJ, Chen J, Sun Y, Zheng ZJ. Lack of association between the paraoxonase 1 Q/R192 single nucleotide polymorphism and stroke in a Chinese cohort. Acta Neurol Belgica 2009;109:205–9.
- Banerjee I. Relationship between Paraoxonase 1 (PON1) gene polymorphisms and susceptibility of stroke: a meta-analysis. Eur J Epidemiol 2010;25:449–58.
- Dahabreh IJ, Kitsios GD, Kent DM, Trikalinos TA. Paraoxonase 1 polymorphisms and ischemic stroke risk: a systematic review and meta-analysis. Genet Med 2010;12:606–15.
- Lazaros L, Markoula S, Kyritsis A, Georgiou I. Paraoxonase gene polymorphisms and stroke severity. Eur J Neurol 2010;17:757–9.
- Mahrooz A, Gohari G, Hashemi MB, et al. R-carrying genotypes of serum paraoxonase (PON1) 192 polymorphism and higher activity ratio are related to susceptibility against ischemic stroke. Mol Biol Reports 2012;39:11177–85.
- Michalak S, Kazmierski R, Hellmann A, et al. Serum paraoxonase/arylesterase activity affects outcome in ischemic stroke patients. Cerebrovasc Dis 2011;32:124–32.
- Shenhar-Tsarfaty S, Waiskopf N, Ofek K, et al. Atherosclerosis and arteriosclerosis parameters in stroke patients associate with paraoxonase polymorphism and esterase activities. Eur J Neurol 2013;20:891–8.
- Dardiotis E, Xiromerisiou G, Hadjichristodoulou C, Tsatsakis AM, Wilks MF, Hadjigeorgiou GM. The interplay between environmental and genetic factors in Parkinson's disease susceptibility: the evidence for pesticides. Toxicology 2013;307:17–23.
- Bohlega SA, Al-Foghom NB. Drug-induced Parkinson's disease. A clinical review. Neurosciences 2013;18:215–21.
- Park NH. Parkinson disease. J Am Acad Phys Assist 2012;25:73–4.
- Kumar KR, Lohmann K, Klein C. Genetics of Parkinson disease and other movement disorders. Curr Opin Neurol 2012;25:466–74.
- Lee PC, Rhodes SL, Sinsheimer JS, Bronstein J, Ritz B. Functional paraoxonase 1 variants modify the risk of Parkinson's disease due to organophosphate exposure. Environ Inter 2013;56:42–7.
- van der Mark M, Brouwer M, Kromhout H, Nijssen P, Huss A, Vermeulen R. Is pesticide use related to Parkinson disease? Some clues to heterogeneity in study results. Environ Health Perspect 2012;120:340–7.
- Perfeito R, Cunha-Oliveira T, Rego AC. Revisiting oxidative stress and mitochondrial dysfunction in the pathogenesis of Parkinson disease – resemblance to the effect of amphetamine drugs of abuse. Free Radic Biol Med 2012;53:1791–806.
- Shi M, Huber BR, Zhang J. Biomarkers for cognitive impairment in Parkinson disease. Brain Pathol 2010;20:660–71.
- Manthripragada AD, Costello S, Cockburn MG, Bronstein JM, Ritz B. Paraoxonase 1, agricultural organophosphate exposure, and Parkinson disease. Epidemiology 2010;21:87–94.
- Gasser T. Molecular pathogenesis of Parkinson disease: insights from genetic studies. Expert Rev Mol Med 2009;11:e22.
- Wu YR. Pesticides and Parkinson's disease. Acta Neurol Taiwan 2005;14:38–9.
- Fong CS, Cheng CW, Wu RM. Pesticides exposure and genetic polymorphism of paraoxonase in the susceptibility of Parkinson's disease. Acta Neurol Taiwan 2005;14:55–60.
- Akhmedova SN, Yakimovsky AK, Schwartz EI. Paraoxonase 1 Met – Leu 54 polymorphism is associated with Parkinson's disease. J Neurol Sci 2001;184:179–82.
- Zintzaras E, Hadjigeorgiou GM. Association of paraoxonase 1 gene polymorphisms with risk of Parkinson's disease: a meta-analysis. J Human Genet 2004;49:474–81.
- Clarimon J, Eerola J, Hellstrom O, Tienari PJ, Singleton A. Paraoxonase 1 (PON1) gene polymorphisms and Parkinson's disease in a Finnish population. Neurosci Lett 2004;367:168–70.
- Kelada SN, Costa-Mallen P, Checkoway H, et al. Paraoxonase 1 promoter and coding region polymorphisms in Parkinson's disease. J Neurol Neurosurg Psychiatry 2003;74:546–7.
- Carmine A, Buervenich S, Sydow O, Anvret M, Olson L. Further evidence for an association of the paraoxonase 1 (PON1) Met-54 allele with Parkinson's disease. Mov Disord 2002;17:764–6.
- Taylor MC, Le Couteur DG, Mellick GD, Board PG. Paraoxonase polymorphisms, pesticide exposure and Parkinson's disease in a Caucasian population. J Neural Transm 2000;107:979–83.
- Kondo I, Yamamoto M. Genetic polymorphism of paraoxonase 1 (PON1) and susceptibility to Parkinson's disease. Brain Res 1998;806:271–3.
- Liu YL, Yang J, Zheng J, et al. Paraoxonase 1 polymorphisms L55M and Q192R were not risk factors for Parkinson's disease: a HuGE review and meta-analysis. Gene 2012;501:188–92.
- Belin AC, Ran C, Anvret A, et al. Association of a protective paraoxonase 1 (PON1) polymorphism in Parkinson's disease. Neurosci Lett 2012;522:30–5.
- Wingo TS, Rosen A, Cutler DJ, Lah JJ, Levey AI. Paraoxonase-1 polymorphisms in Alzheimer's disease, Parkinson's disease, and AD-PD spectrum diseases. Neurobiol Aging 2012;33:p204 e213–205.
- Zlokovic BV. Cerebrovascular effects of apolipoprotein E: implications for Alzheimer disease. JAMA Neurol 2013;70:440–4.
- Wyss-Coray T, Rogers J. Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature. Cold Spring Harb Perspect Med 2012;2:a006346.
- Masdeu JC, Kreisl WC, Berman KF. The neurobiology of Alzheimer disease defined by neuroimaging. Curr Opin Neurol 2012;25:410–20.
- Jack CR Jr. Alzheimer disease: new concepts on its neurobiology and the clinical role imaging will play. Radiology 2012;263:344–61.
- Wehr H, Bednarska-Makaruk M, Graban A, et al. Paraoxonase activity and dementia. J Neurol Sci 2009;283:107–8.
- Paragh G, Balla P, Katona E, Seres I, Egerhazi A, Degrell I. Serum paraoxonase activity changes in patients with Alzheimer's disease and vascular dementia. Eur Arch Psychiatry Clin Neurosci 2002;252:63–7.
- Pi Y, Zhang L, Chang K, et al. Lack of an association between Paraoxonase 1 gene polymorphisms (Q192R, L55M) and Alzheimer's disease: a meta-analysis. Neurosci Lett 2012;523:174–9.
- Klimkowicz-Mrowiec A, Marona M, Spisak K, et al. Paraoxonase 1 gene polymorphisms do not influence the response to treatment in Alzheimer's disease. Dement Geriatr Cogn Disord 2011;32:26–31.
- Chapuis J, Boscher M, Bensemain F, Cottel D, Amouyel P, Lambert JC. Association study of the paraoxonase 1 gene with the risk of developing Alzheimer's disease. Neurobiol Aging 2009;30:152–6.
- Leduc V, Poirier J. Polymorphisms at the paraoxonase 1 L55M and Q192R loci affect the pathophysiology of Alzheimer's disease: emphasis on the cholinergic system and beta-amyloid levels. Neuro-Degenerative Dis 2008;5:225–7.
- He XM, Zhang ZX, Zhang JW, et al. Gln192Arg polymorphism in paraoxonase 1 gene is associated with Alzheimer disease in a Chinese Han ethnic population. Chin Med J 2006;119:1204–9.
- Cellini E, Tedde A, Bagnoli S, et al. Association analysis of the paraoxonase-1 gene with Alzheimer's disease. Neurosci Lett 2006;408:199–202.
- Dantoine TF, Drouet M, Debord J, Merle L, Cogne M, Charmes JP. Paraoxonase 1 192/55 gene polymorphisms in Alzheimer's disease. Ann N Y Acad Sci 2002;977:239–44.
- Zuliani G, Ble A, Zanca R, et al. Genetic polymorphisms in older subjects with vascular or Alzheimer's dementia. Acta Neurol Scand 2001;103:304–8.
- D'Amico E, Factor-Litvak P, Santella RM, Mitsumoto H. Clinical perspective on oxidative stress in sporadic amyotrophic lateral sclerosis. Free Radic Biol Med 2013;65C:509–527.
- Evans MC, Couch Y, Sibson N, Turner MR. Inflammation and neurovascular changes in amyotrophic lateral sclerosis. Mol Cell Neurosci 2013;53:34–41.
- Kruger D. Amyotrophic lateral sclerosis. JAAPA 2012;25:53–4.
- Ludolph AC, Brettschneider J, Weishaupt JH. Amyotrophic lateral sclerosis. Curr Opin Neurol 2012;25:530–5.
- Gagliardi S, Abel K, Bianchi M, et al. Regulation of FMO and PON detoxication systems in ALS human tissues. Neurotoxicity Res 2013;23:370–7.
- Cronin S, Greenway MJ, Prehn JH, Hardiman O. Paraoxonase promoter and intronic variants modify risk of sporadic amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 2007;78:984–6.
- Wills AM, Cronin S, Slowik A, et al. A large-scale international meta-analysis of paraoxonase gene polymorphisms in sporadic ALS. Neurology 2009;73:16–24.
- Wills AM, Landers JE, Zhang H, et al. Paraoxonase 1 (PON1) organophosphate hydrolysis is not reduced in ALS. Neurology 2008;70:929–34.
- Freedman MS. Present and emerging therapies for multiple sclerosis. Continuum 2013;19:968–91.
- Katz Sand IB, Lublin FD. Diagnosis and differential diagnosis of multiple sclerosis. Continuum 2013;19:922–43.
- Miller E, Wachowicz B, Majsterek I. Advances in antioxidative therapy of multiple sclerosis. Curr Med Chem 2013.
- Popescu BF, Pirko I, Lucchinetti CF. Pathology of multiple sclerosis: where do we stand? Continuum 2013;19:901–21.
- Martinez C, Garcia-Martin E, Benito-Leon J, et al. Paraoxonase 1 polymorphisms are not related with the risk for multiple sclerosis. Neuromol Med 2010;12:217–23.
- Moghtaderi A, Hashemi M, Sharafaddinzadeh N, et al. Lack of association between paraoxonase 1 Q192R polymorphism and multiple sclerosis in relapse phase: a case-control study. Clin Biochem 2011;44:795–8.
- Sidoti A, Antognelli C, Rinaldi C, et al. Glyoxalase I A111E, paraoxonase 1 Q192R and L55M polymorphisms: susceptibility factors of multiple sclerosis? Mult Scler 2007;13:446–53.
- Zakrzewska-Pniewska B, Nojszewska M, Rog T, et al. Polymorphisms of paraoxonase 1 and 2 genes and the risk of multiple sclerosis in the Polish population. Neurologia i neurochirurgia polska 2013;47:49–52.
- Jamroz-Wisniewska A, Beltowski J, Stelmasiak Z, Bartosik-Psujek H. Paraoxonase 1 activity in different types of multiple sclerosis. Mult Scler 2009;15:399–402.
